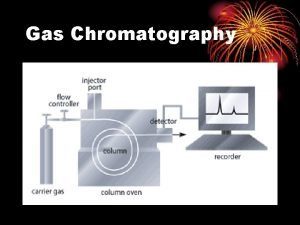
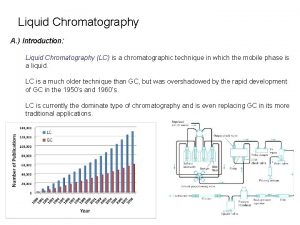
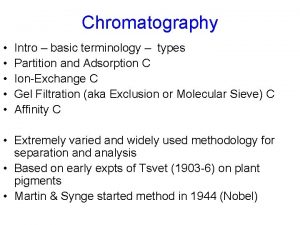
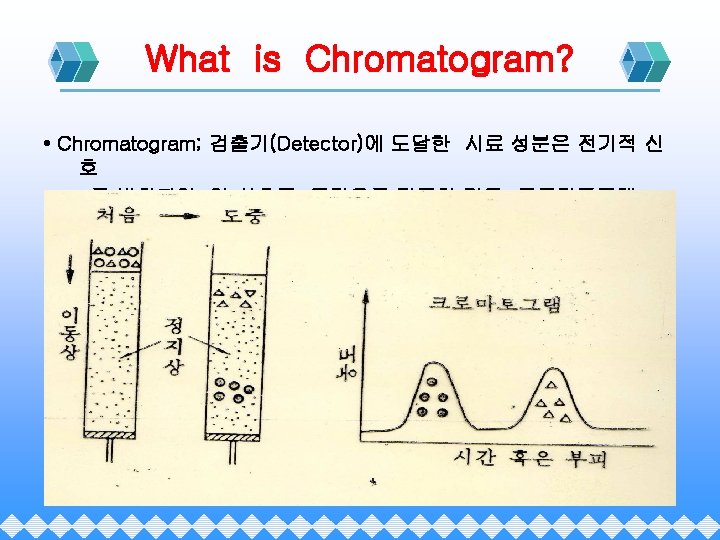
Chromatography Chromatography Gas Chromatography Adsorption Liquid Chromato Partition













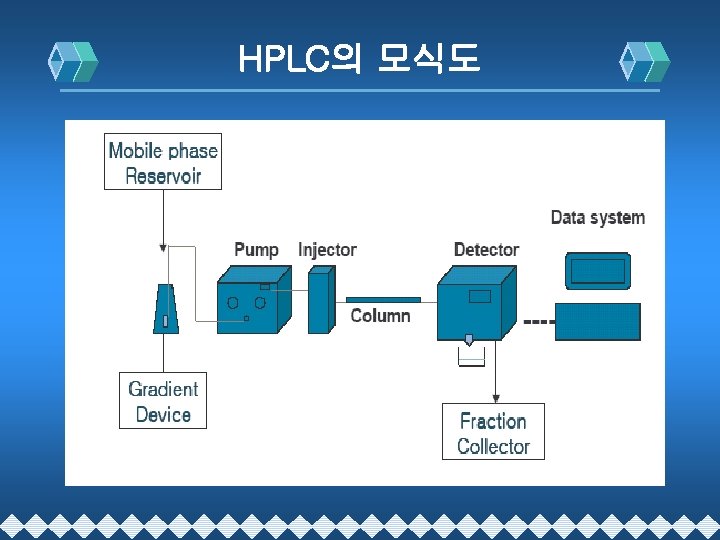













- Slides: 56









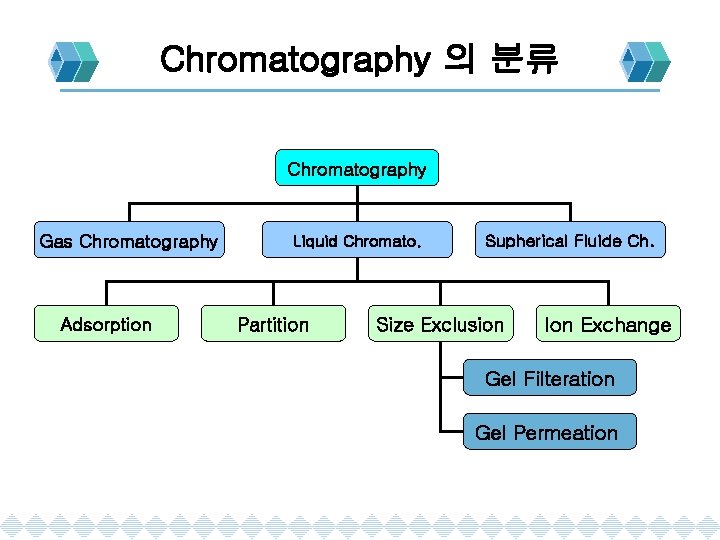
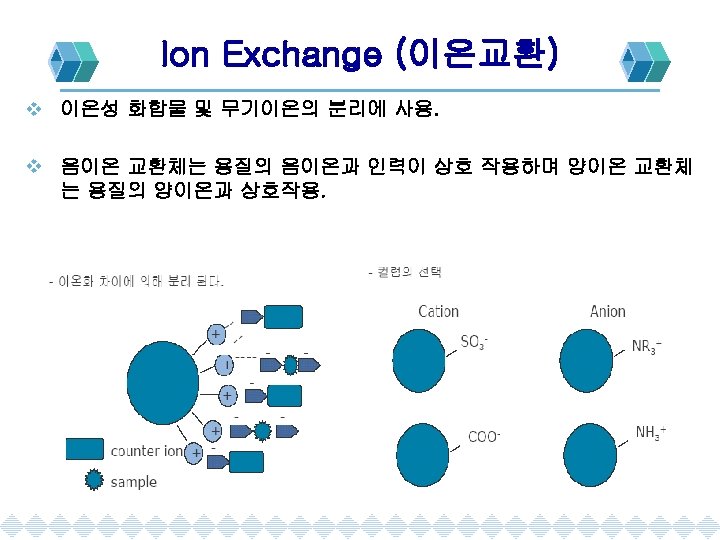
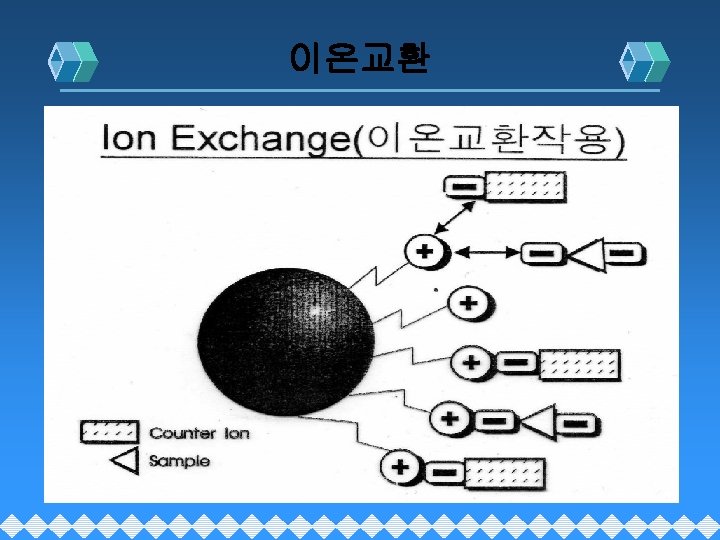
Chromatography 의 분류 Chromatography Gas Chromatography Adsorption Liquid Chromato. Partition Supherical Fluide Ch. Size Exclusion Ion Exchange Gel Filteration Gel Permeation







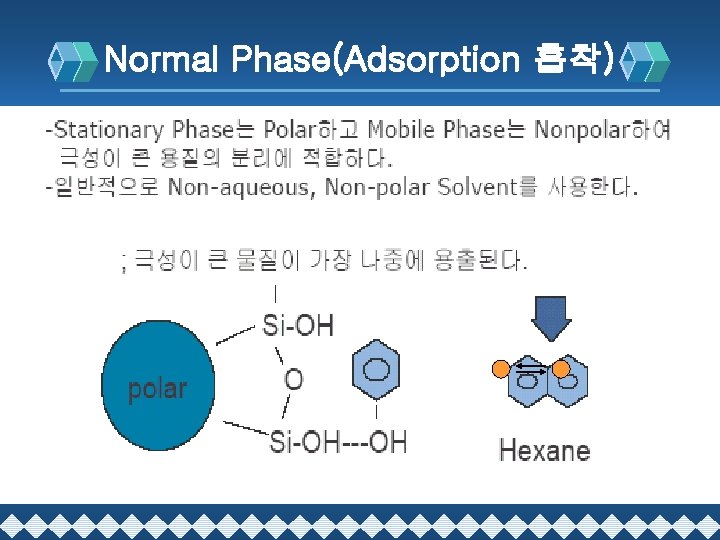
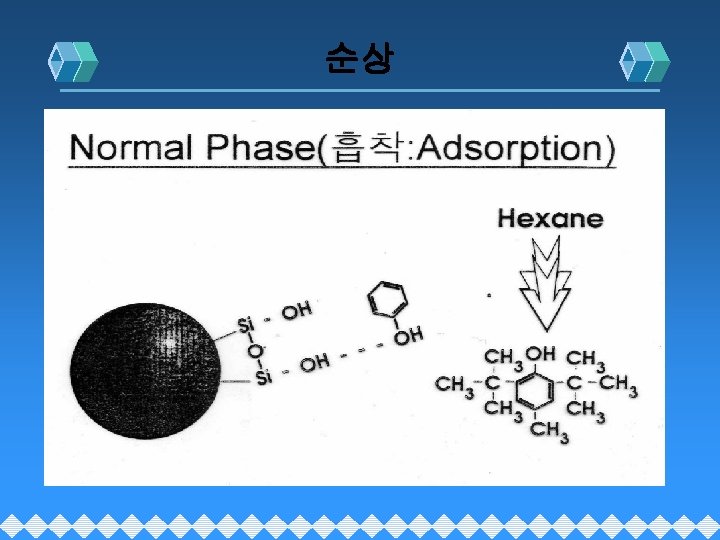
Normal Phase(Adsorption 흡착)


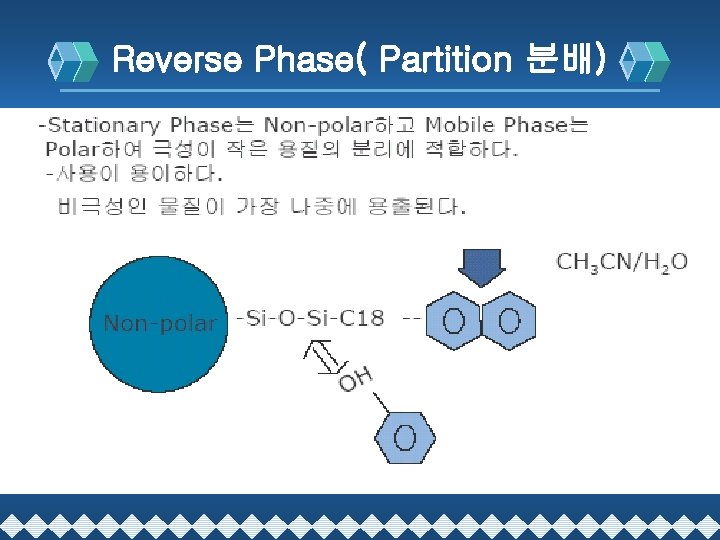
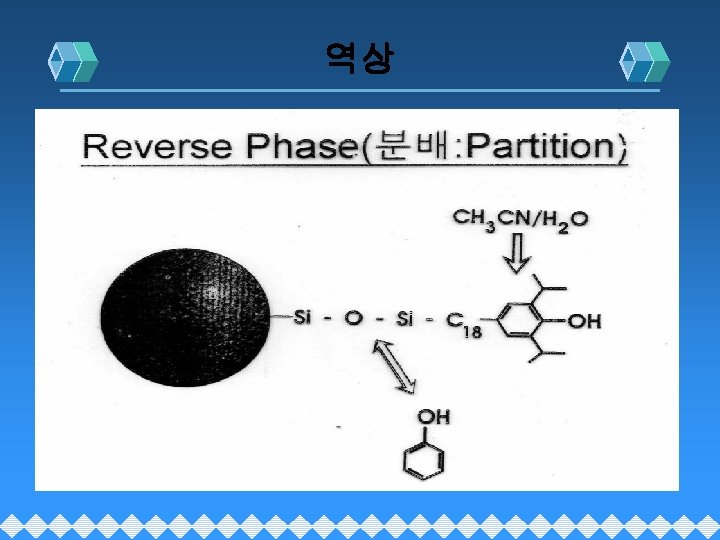
Reverse Phase( Partition 분배)


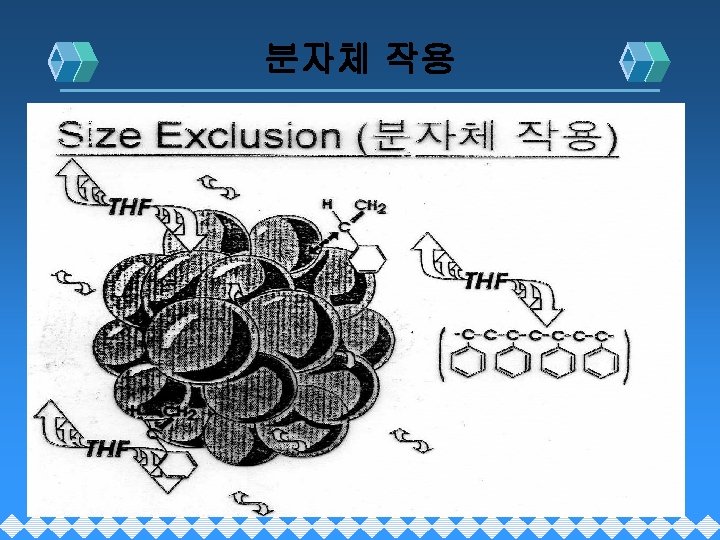
Size Exclusion (크기배제)





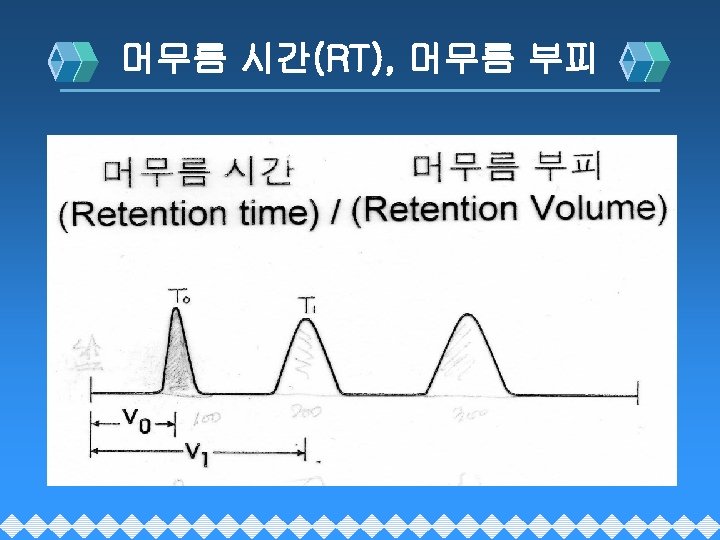
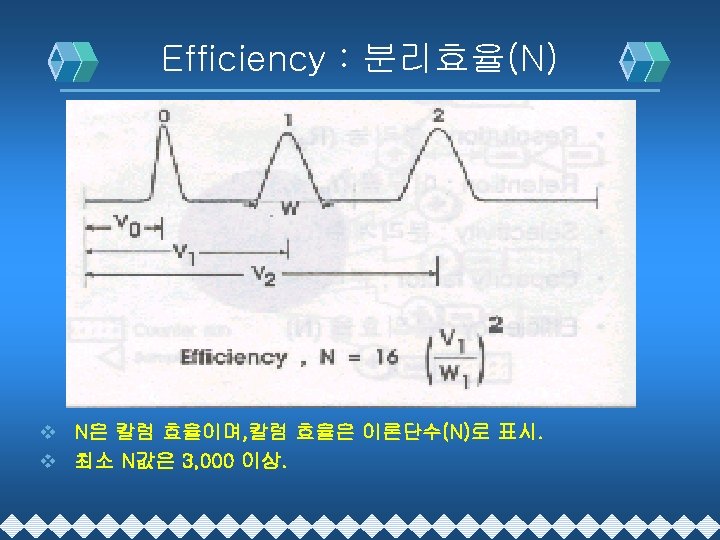
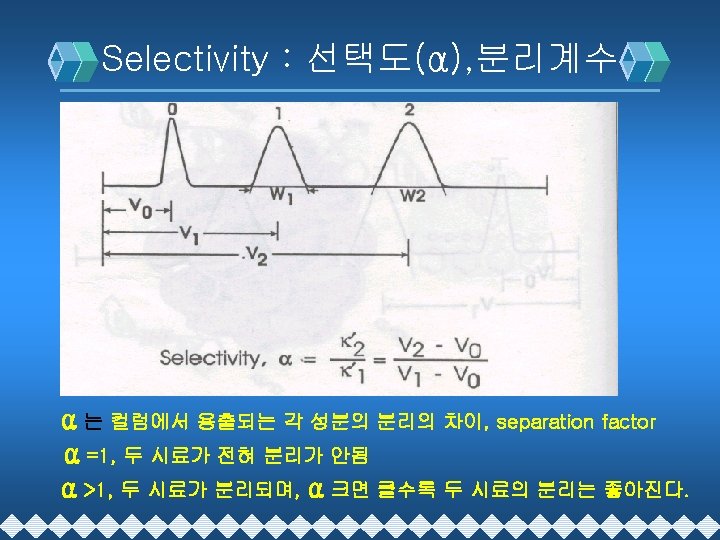
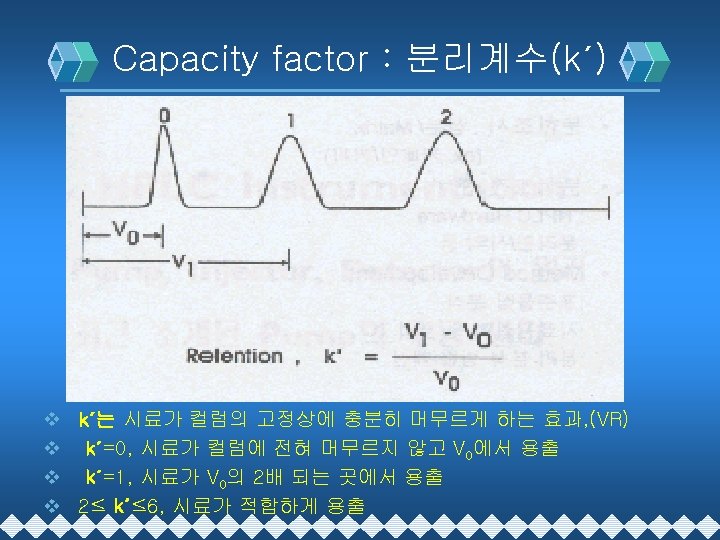
HPLC 분리이론 v Resolution : 분리능(Rs) v Retention Time : 머무름 시간(t. R), RT v Selectivity : 선택도(α) v Capacity factor : 분리계수(k´) v Efficiency : 분리효율(N) v (N=Theoretical Plate Number)












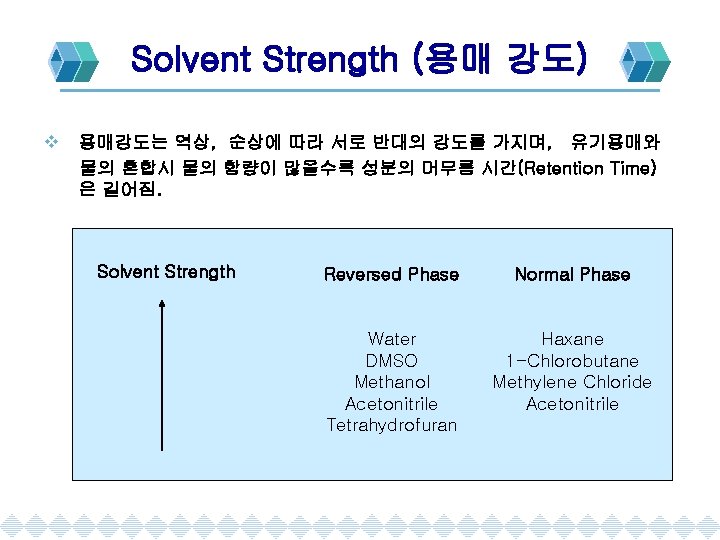
Solvent Strength (용매 강도) v 용매강도는 역상, 순상에 따라 서로 반대의 강도를 가지며, 유기용매와 물의 혼합시 물의 함량이 많을수록 성분의 머무름 시간(Retention Time) 은 길어짐. Solvent Strength Reversed Phase Normal Phase Water DMSO Methanol Acetonitrile Tetrahydrofuran Haxane 1 -Chlorobutane Methylene Chloride Acetonitrile

Solvent Delivery Pump(펌프)


Injector(주입기) Manual Injector Autosampler

Injector 의 원리

Column(고정상)

Column(고정상)

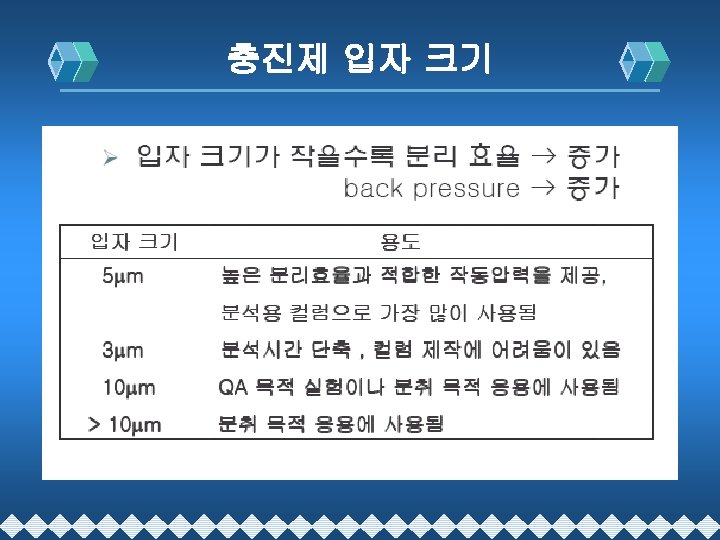
Packing Material(충진제)



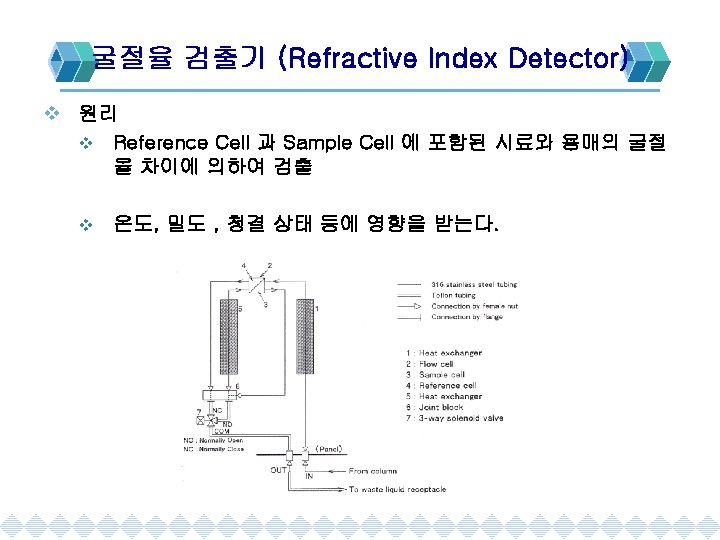
Detector(검출기)

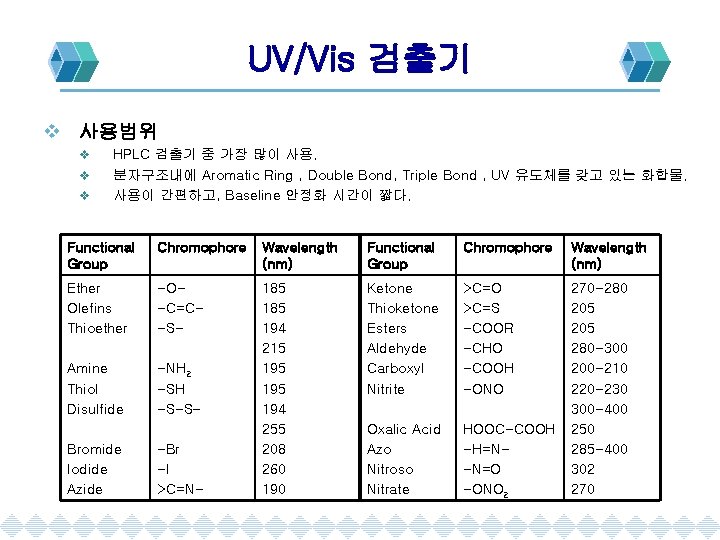
UV/Vis 검출기 v 사용범위 v v v HPLC 검출기 중 가장 많이 사용. 분자구조내에 Aromatic Ring , Double Bond, Triple Bond , UV 유도체를 갖고 있는 화합물. 사용이 간편하고, Baseline 안정화 시간이 짧다. Functional Group Chromophore Wavelength (nm) Ether Olefins Thioether -O-C=C-S- Amine Thiol Disulfide -NH 2 -SH -S-S- 185 194 215 195 194 255 208 260 190 Ketone Thioketone Esters Aldehyde Carboxyl Nitrite >C=O >C=S -COOR -CHO -COOH -ONO Oxalic Acid Azo Nitroso Nitrate HOOC-COOH -H=N-N=O -ONO 2 270 -280 205 280 -300 200 -210 220 -230 300 -400 250 285 -400 302 270 Bromide Iodide Azide -Br -I >C=N-



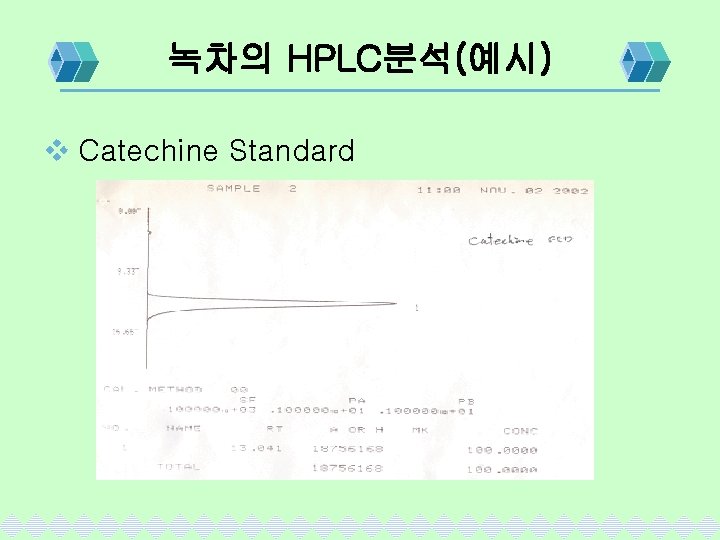
녹차의 HPLC분석(예시) v Catechine Standard

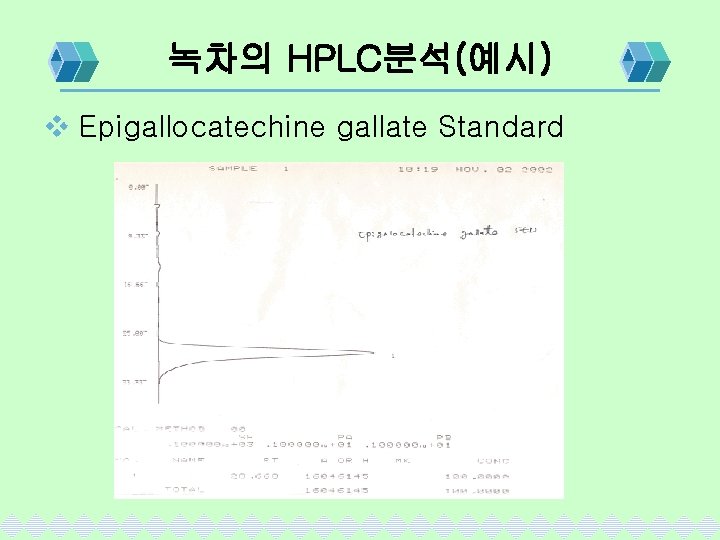
녹차의 HPLC분석(예시) v Epigallocatechine gallate Standard



Vit-C의 HPLC정량 v 표준용액의 조제(ST) Ascorbic acid 10. 0 mg H 2 O qs 100 ml 시료의 조제(SA) 비타500 5 ml H 2 O qs 100 ml

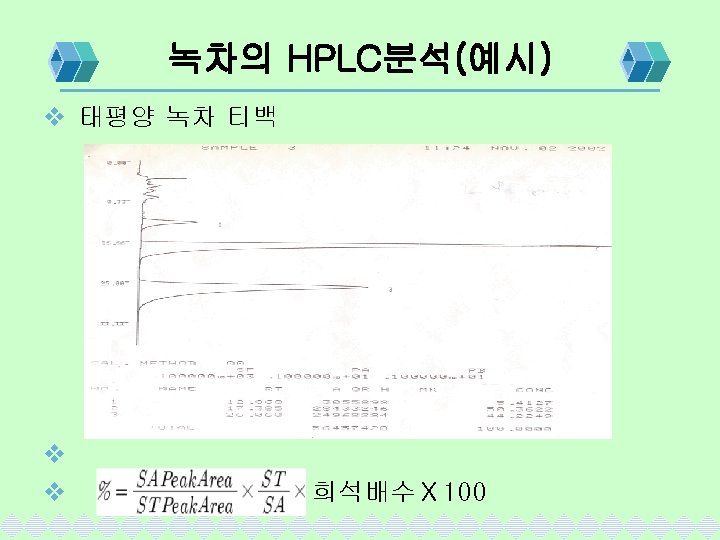
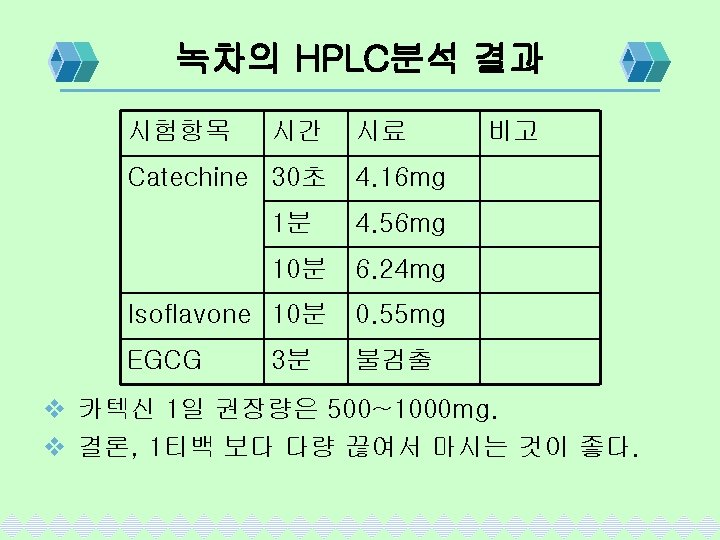
Vit-C 분석조건 및 계산 v 분석조건 Column(Stationary Phase) ; u-Bondapak C 18 Mobile phase; 0. 05 M KH 2 PO 4: ACN= 60: 40 Flow rate; 1. 0 ml/min Detector; 254 nm SA peak area ☓ ST취한량 %= ST peak area ☓ SA취한량 평균중량 ☓희석배수 ☓ ☓역가 기준량
 액체 크로마토그래피 원리
액체 크로마토그래피 원리 Gas liquid chromatography
Gas liquid chromatography Principle of gas chromatography
Principle of gas chromatography Chromatography mobile phase and stationary phase
Chromatography mobile phase and stationary phase Introduction for chromatography
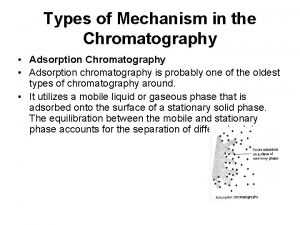
Introduction for chromatography Adsorption chromatography types
Adsorption chromatography types What is partition coefficient
What is partition coefficient Solid/gas interface example
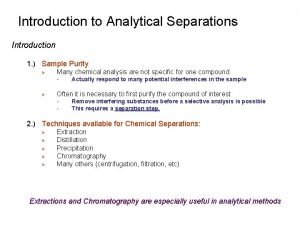
Solid/gas interface example Introduction to analytical separations
Introduction to analytical separations Difference between isocratic and gradient elution
Difference between isocratic and gradient elution Partition chromatography applications
Partition chromatography applications Definition chromatography
Definition chromatography Disadvantages of liquid chromatography
Disadvantages of liquid chromatography Oil gas partition coefficient inhaled anesthetics
Oil gas partition coefficient inhaled anesthetics Liquid liquid extraction unit
Liquid liquid extraction unit Difference between pure liquid and commercial liquid
Difference between pure liquid and commercial liquid What is void volume in gel filtration
What is void volume in gel filtration High performance liquid chromatography introduction
High performance liquid chromatography introduction High performance liquid chromatography hplc machine
High performance liquid chromatography hplc machine Limitations of liquid chromatography
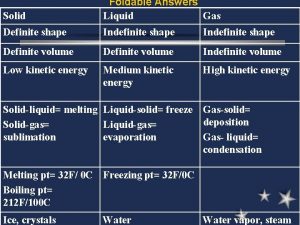
Limitations of liquid chromatography States of matter solid liquid gas
States of matter solid liquid gas Solid liquid gas particles
Solid liquid gas particles Is the volume of a liquid definite or indefinite
Is the volume of a liquid definite or indefinite Definite volume
Definite volume Why are liquids incompressible
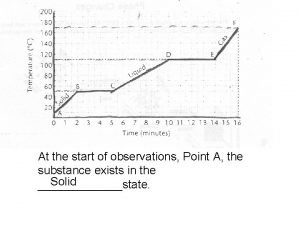
Why are liquids incompressible Water expansion temperature graph
Water expansion temperature graph Concept map of matter solid liquid and gas
Concept map of matter solid liquid and gas What are the properties of solids
What are the properties of solids Why is gas easier to compress than a liquid or a solid
Why is gas easier to compress than a liquid or a solid Mass of solid liquid and gas
Mass of solid liquid and gas Sebuah tangki berisi alkohol yang massa jenisnya 800
Sebuah tangki berisi alkohol yang massa jenisnya 800 Gas liquid
Gas liquid Multiphase reactor
Multiphase reactor Are solutions homogeneous
Are solutions homogeneous Thiele modulus equation
Thiele modulus equation Liquid to gas
Liquid to gas Solid
Solid Gas liquid
Gas liquid Solid gas liquid
Solid gas liquid Gas liquid solid
Gas liquid solid Is pepsi solid liquid or gas
Is pepsi solid liquid or gas Example of solid liquid and gas
Example of solid liquid and gas Properties of solid liquid and gas
Properties of solid liquid and gas Factors affecting on solubility
Factors affecting on solubility Solid liquid gas
Solid liquid gas Arrangement particles of liquid
Arrangement particles of liquid Gas liquid solid
Gas liquid solid Properties of matter graphic organizer
Properties of matter graphic organizer Solid to gas examples
Solid to gas examples Closely packed in an orderly manner
Closely packed in an orderly manner Chemistry
Chemistry Solid liquid gas
Solid liquid gas What is sound
What is sound Solid to gas
Solid to gas What are solids
What are solids Liquid solid gas
Liquid solid gas Solid liquid gas difference
Solid liquid gas difference