Experiment Five Synthesis and purification of the analgesic











- Slides: 15


Experiment Five Synthesis and purification of the analgesic agent, Acetaminophen (Paracetamol)

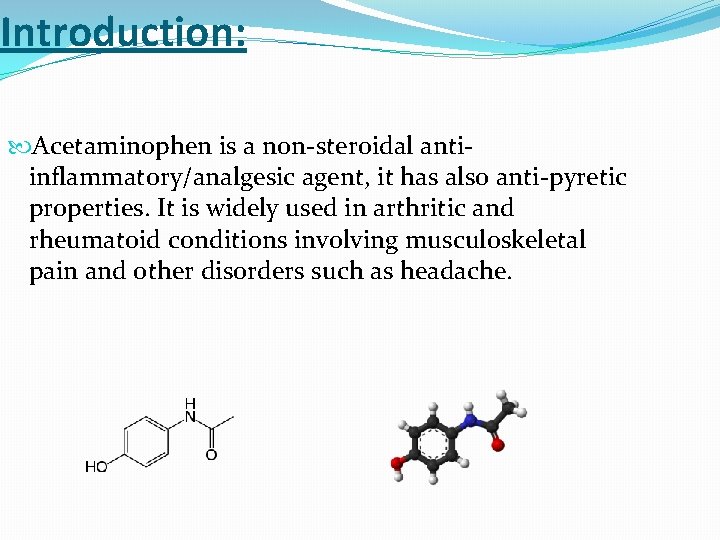
Introduction: Acetaminophen is a non-steroidal antiinflammatory/analgesic agent, it has also anti-pyretic properties. It is widely used in arthritic and rheumatoid conditions involving musculoskeletal pain and other disorders such as headache.

Mechanism of action Now, recent research (2) has shown the presence of a new, previously unknown cyclooxygenase enzyme COX-3, found in the brain and spinal cord, which is selectively inhibited by paracetamol, and is distinct from the two already known cyclooxygenase enzymes COX-1 and COX-2. It is now believed that this selective inhibition of the enzyme COX-3 in the brain and spinal cord explains the effectiveness of paracetamol in relieving pain and reducing fever without having unwanted gastrointestinal side effects.

Chemical properties: White crystalline powder. Molecular weight = 151. 17 g/mole. Melting point = 170 °C. Insoluble in water, very soluble in ethanol.

Synthetic procedure: Suspend 2. 2 g of P-aminophenol in 6 ml distilled water. Warm the mixture in a water bath until all solid dissolved. Add 2. 5 ml acetic anhydride to the hot solution, and then heat the reaction in water bath for 10 minutes. Filter while the solution is hot using Buchner filtration unit. Leave to cool down for 10 minutes. Filter the solid precipitate, and then wash it with cold water. Re-dissolve in about 20 ml hot distilled water…. filter while hot…cool in ice bath to form crystals. Collect the crystals by Buchner filtration, and then dry it in the oven.

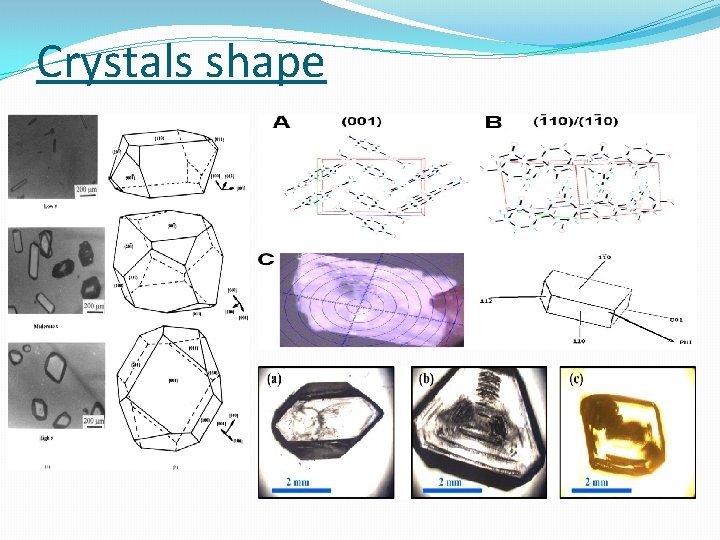
Crystals shape

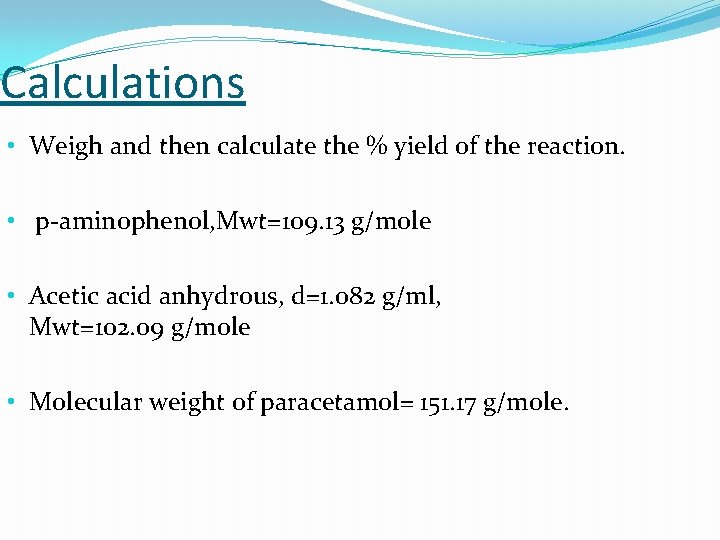
Calculations • Weigh and then calculate the % yield of the reaction. • p-aminophenol, Mwt=109. 13 g/mole • Acetic acid anhydrous, d=1. 082 g/ml, Mwt=102. 09 g/mole • Molecular weight of paracetamol= 151. 17 g/mole.

Purity Definition is the absence of impurity or contaminants in a substance

Purification technique: Crystalization Extraction Chromatography Filtration distillation

Technique to measure purity mp HPLC TLC NMR Mass spectroscopy

Identification test Test 1: Dissolve 0. 1 g paracetamol in 10 ml water Add 0. 05 ml ferric chloride solution. Violet blue color is produced Test 2: Boil 0. 1 g of paracetamol with 1 ml HCl for 3 min. Add 10 ml water & cool, no ppt produced. Add 0. 05 ml 0. 1 N potassium dichromate. A violet colour slowly develops, which don’t become red.

Assay is done usually by using: Spectrophotometric Method. HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY (HPLC).

What is the class of Paracetamol ? What is the difference between Panadol, Panadol extra, Panadol night , Panadol cold & flu , Panadol advance & Panadol joint? How to prepare acetaminophen from benzene? Why it is better to start with p-aminophenol to synthesis acetaminophen? Why acetic anhydride must not be in excess? Why the percent yield could be very low?

 Vital sign form
Vital sign form Pain score kkm
Pain score kkm Nursing management of sleep disorders
Nursing management of sleep disorders Paracetamol dose per kg
Paracetamol dose per kg Transderml
Transderml Analgesic
Analgesic What is pain
What is pain Timmitus
Timmitus Rubefacient
Rubefacient Analgesic
Analgesic Analgesic
Analgesic Analgesic classification
Analgesic classification Intrapertoneal
Intrapertoneal Protein characterization techniques
Protein characterization techniques Purification and characterization of organic compounds
Purification and characterization of organic compounds 5 elements and 5 senses
5 elements and 5 senses