Particle modal of matter SOLID LIQUID GAS All












- Slides: 29

Particle modal of matter SOLID LIQUID GAS

• All substances are made up of matter. • All matter is made up of particles. • Atoms and molecules are regarded as these particles. • Look at the figure on the right. • Name the solids, liquids and gases. TB pg. 89

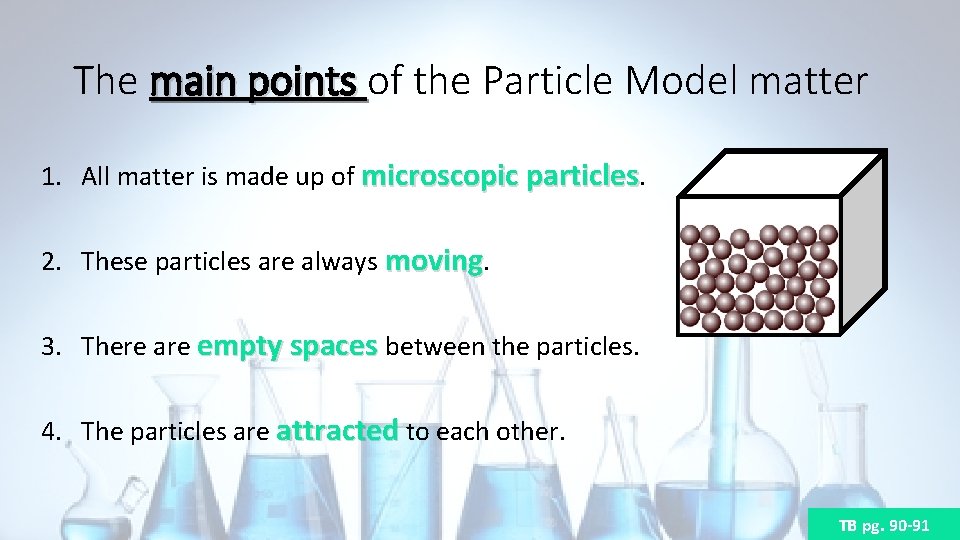
The main points of the Particle Model matter 1. All matter is made up of microscopic particles. 2. These particles are always moving. 3. There are empty spaces between the particles. 4. The particles are attracted to each other. TB pg. 90 -91

There are 3 states of matter: 1. Solid 2. Liquid 3. Gas TB pg. 92 -93

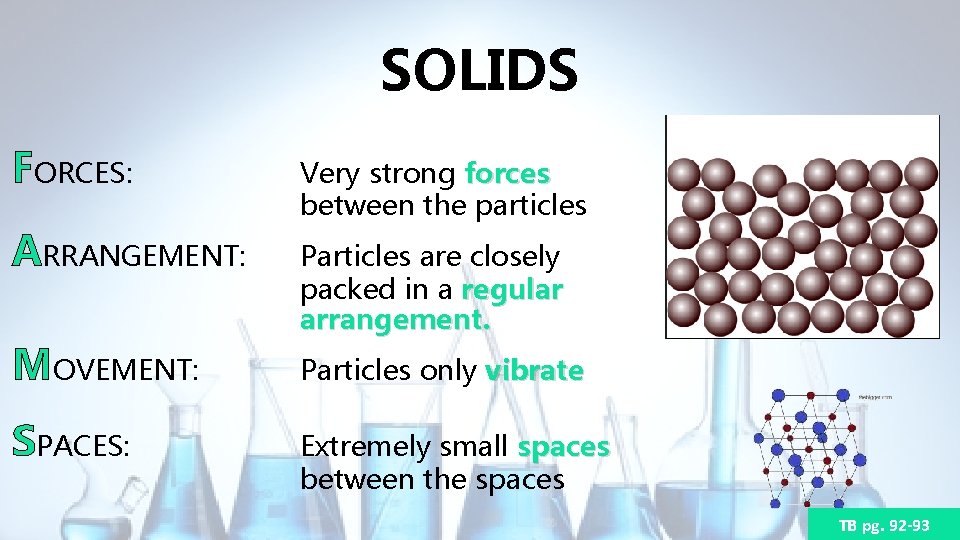
SOLIDS FORCES: Very strong forces between the particles ARRANGEMENT: Particles are closely packed in a regular arrangement. MOVEMENT: SPACES: Particles only vibrate Extremely small spaces between the spaces TB pg. 92 -93

LIQUIDS FORCES: Have weaker forces than those of a solid. ARRANGEMENT: Loosely arranged but still MOVEMENT: Move faster than in a solid and can slide past each other SPACES: Small spaces between quite close together. them. TB pg. 92 -93

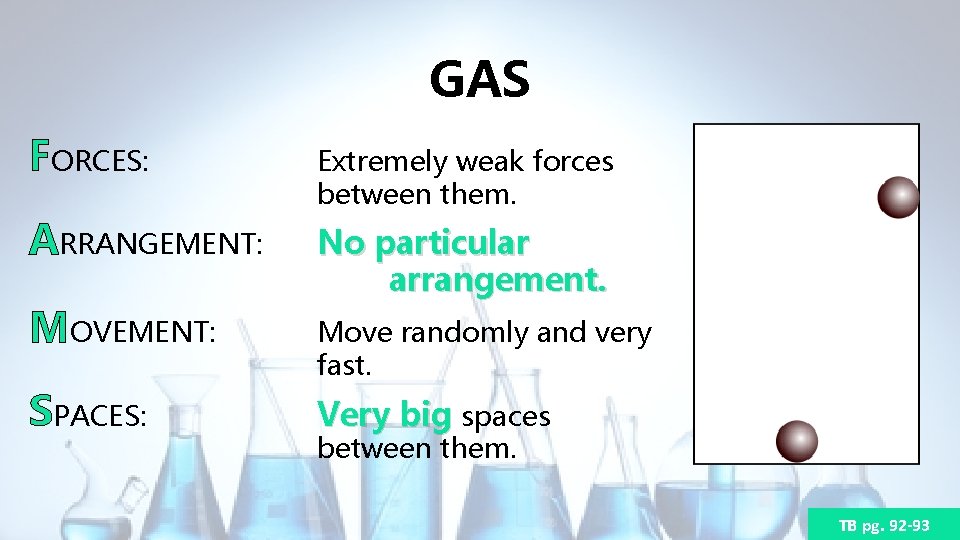
GAS FORCES: Extremely weak forces between them. ARRANGEMENT: No particular arrangement. MOVEMENT: SPACES: Move randomly and very fast. Very big spaces between them. TB pg. 92 -93

Homework Activity 3 page. 93 TB pg. 92 -93

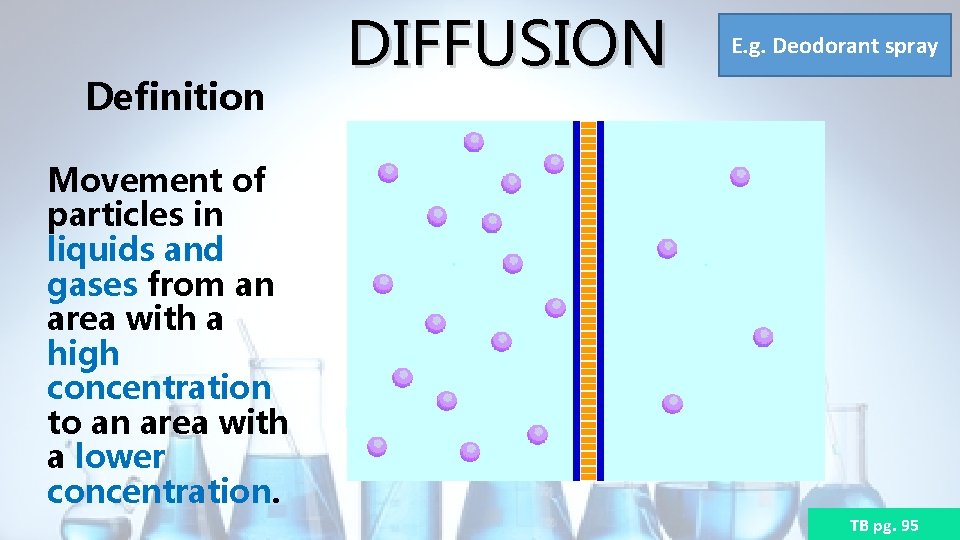
Definition DIFFUSION E. g. Deodorant spray Movement of particles in liquids and gases from an area with a high concentration to an area with a lower concentration. TB pg. 95

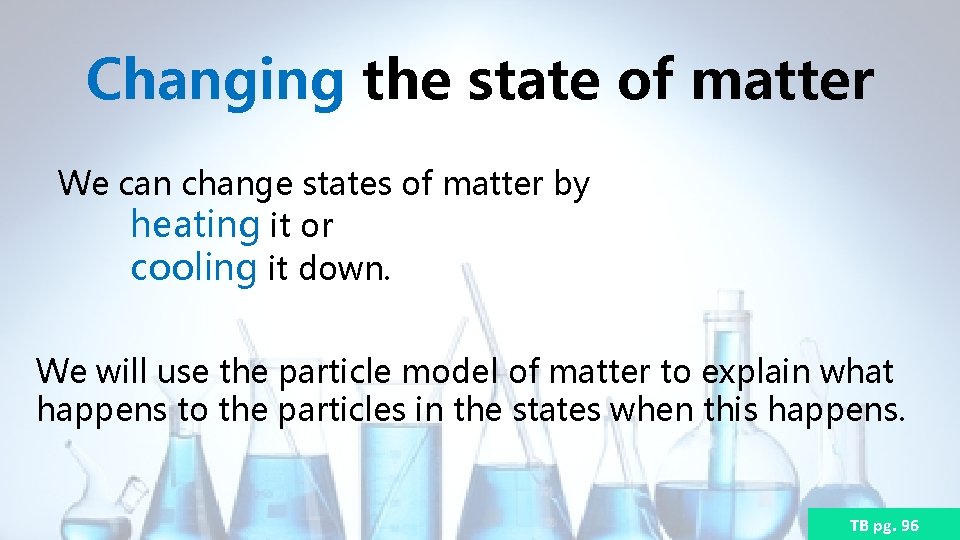
Changing the state of matter We can change states of matter by heating it or cooling it down. We will use the particle model of matter to explain what happens to the particles in the states when this happens. TB pg. 96

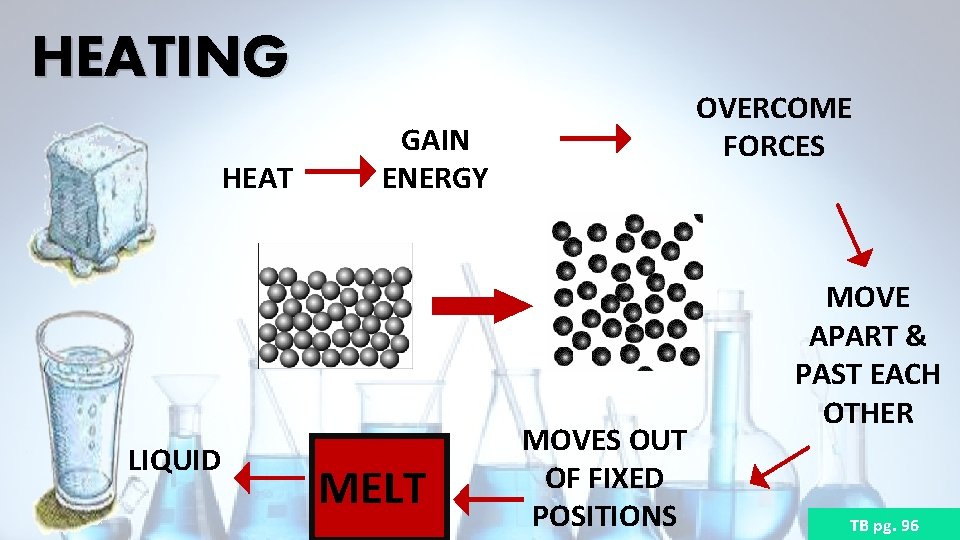
HEATING HEAT LIQUID OVERCOME FORCES GAIN ENERGY MELT MOVES OUT OF FIXED POSITIONS MOVE APART & PAST EACH OTHER TB pg. 96

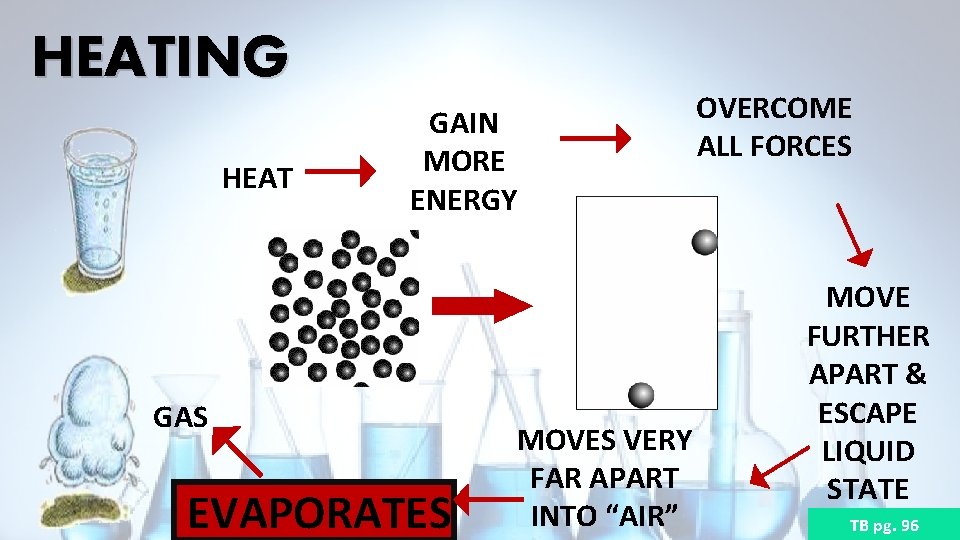
HEATING HEAT GAIN MORE ENERGY GAS EVAPORATES MOVES VERY FAR APART INTO “AIR” OVERCOME ALL FORCES MOVE FURTHER APART & ESCAPE LIQUID STATE TB pg. 96

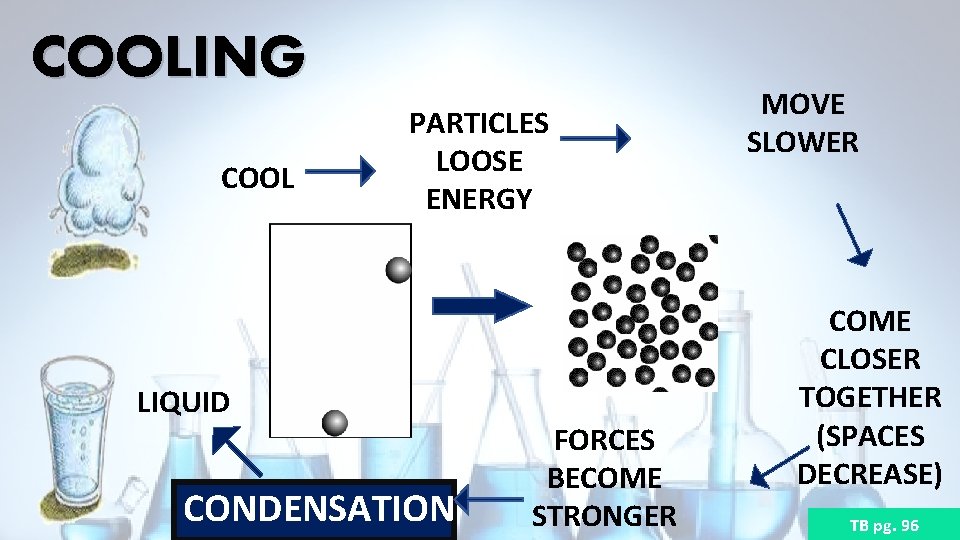
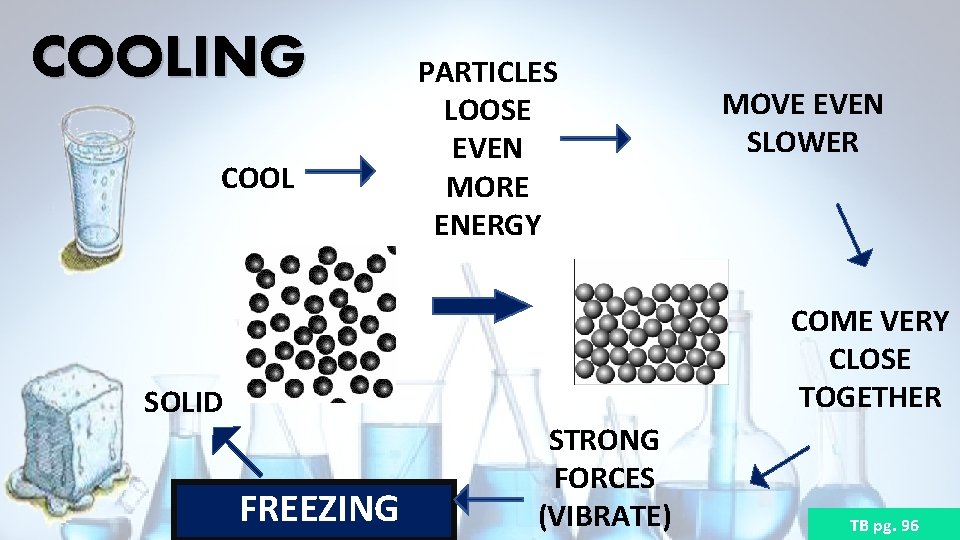
COOLING COOL PARTICLES LOOSE ENERGY LIQUID CONDENSATION FORCES BECOME STRONGER MOVE SLOWER COME CLOSER TOGETHER (SPACES DECREASE) TB pg. 96

COOLING COOL PARTICLES LOOSE EVEN MORE ENERGY MOVE EVEN SLOWER COME VERY CLOSE TOGETHER SOLID FREEZING STRONG FORCES (VIBRATE) TB pg. 96

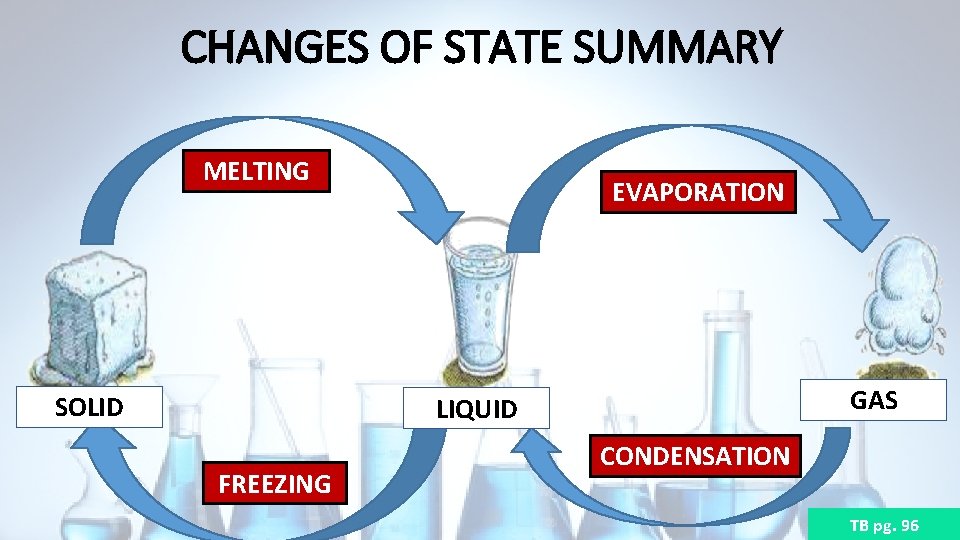
CHANGES OF STATE SUMMARY MELTING SOLID EVAPORATION GAS LIQUID FREEZING CONDENSATION TB pg. 96

DENSITY RELATIONSHIP: MASS AND VOLUME

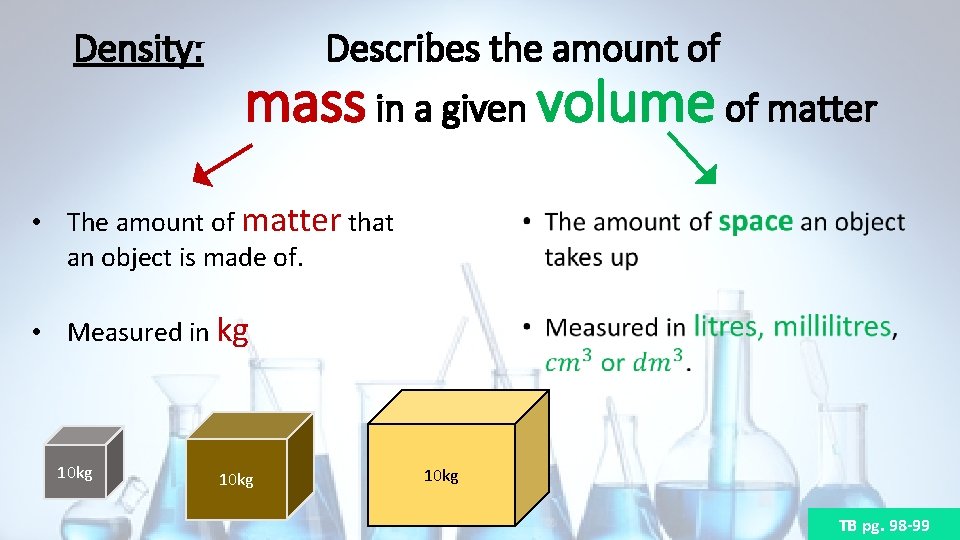
Density: Describes the amount of mass in a given volume of matter • The amount of matter that an object is made of. • Measured in kg 10 kg TB pg. 98 -99

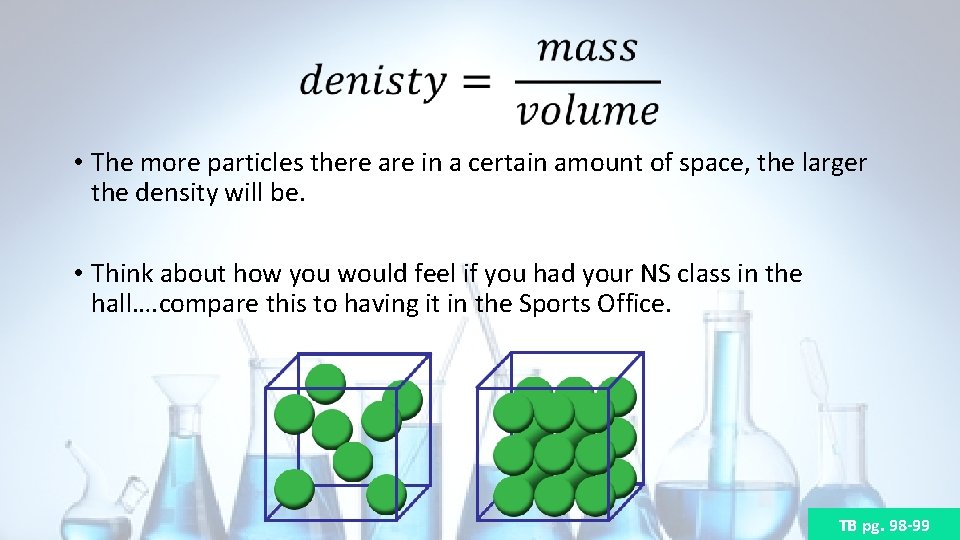
• The more particles there are in a certain amount of space, the larger the density will be. • Think about how you would feel if you had your NS class in the hall…. compare this to having it in the Sports Office. TB pg. 98 -99

Practical Activity 8 pg. 99 Using small blocks TB pg. 98 -99

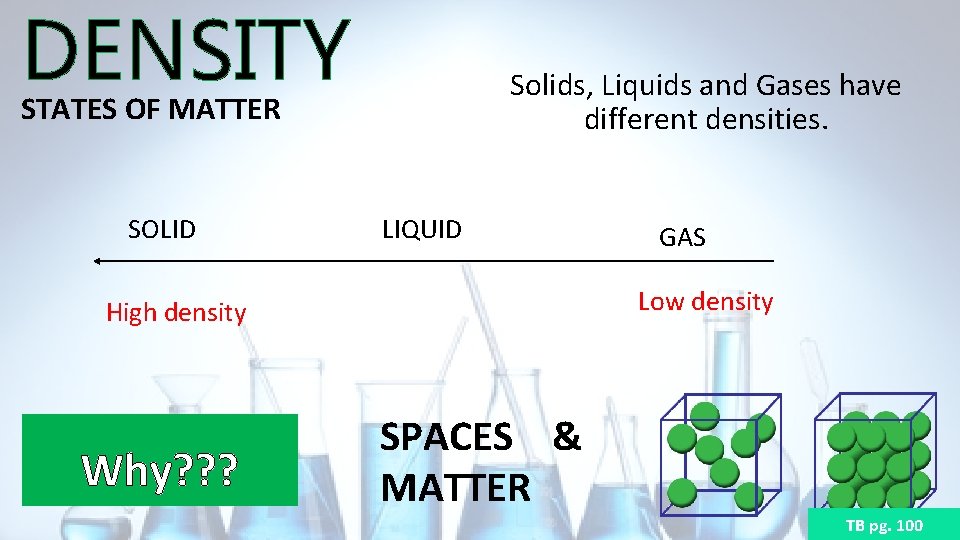
DENSITY STATES OF MATTER SOLID Solids, Liquids and Gases have different densities. LIQUID Low density High density Why? ? ? GAS SPACES & MATTER TB pg. 100

Homework Activity 9 pg. 100 TB pg. 100

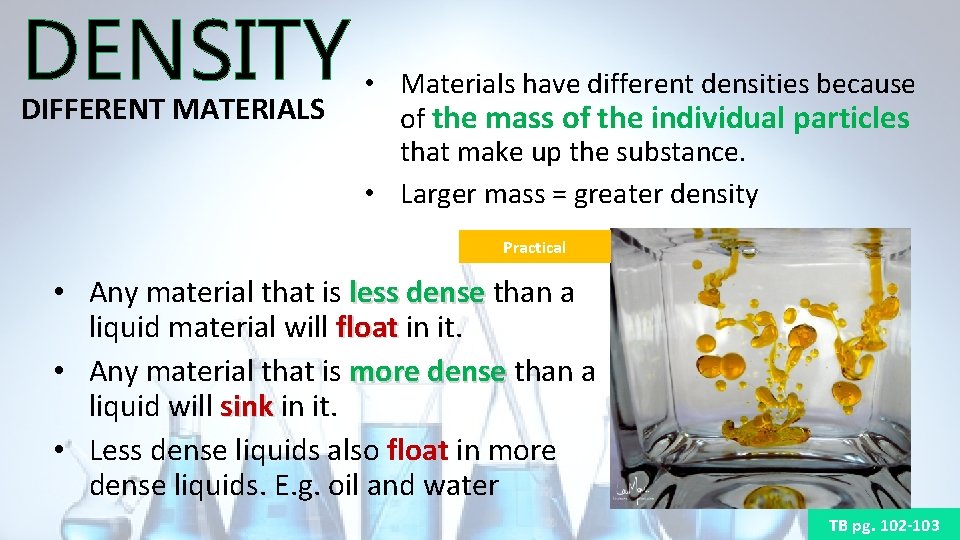
DENSITY DIFFERENT MATERIALS • Materials have different densities because of the mass of the individual particles that make up the substance. • Larger mass = greater density Practical • Any material that is less dense than a liquid material will float in it. float • Any material that is more dense than a liquid will sink in it. sink • Less dense liquids also float in more dense liquids. E. g. oil and water TB pg. 102 -103

? Expansion and Get bigger, swell up, increase in size Contraction of materials ? Get smaller, shrink TB pg. 106 -107

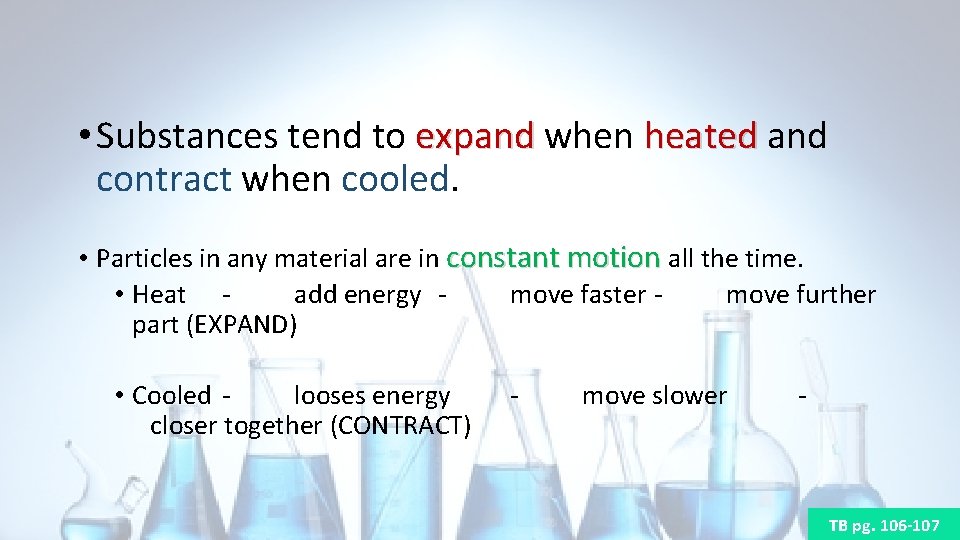
• Substances tend to expand when heated and expand heated contract when cooled. • Particles in any material are in constant motion all the time. • Heat add energy move faster move further part (EXPAND) • Cooled looses energy closer together (CONTRACT) - move slower - TB pg. 106 -107

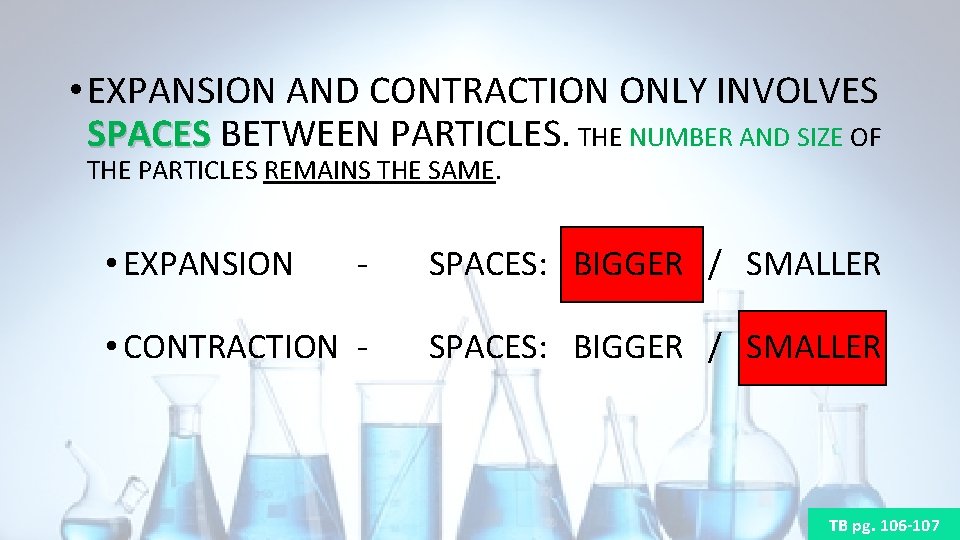
• EXPANSION AND CONTRACTION ONLY INVOLVES SPACES BETWEEN PARTICLES. THE NUMBER AND SIZE OF SPACES THE PARTICLES REMAINS THE SAME. • EXPANSION - SPACES: BIGGER / SMALLER • CONTRACTION - SPACES: BIGGER / SMALLER TB pg. 106 -107

PRESSURE TB pg. 110 -111

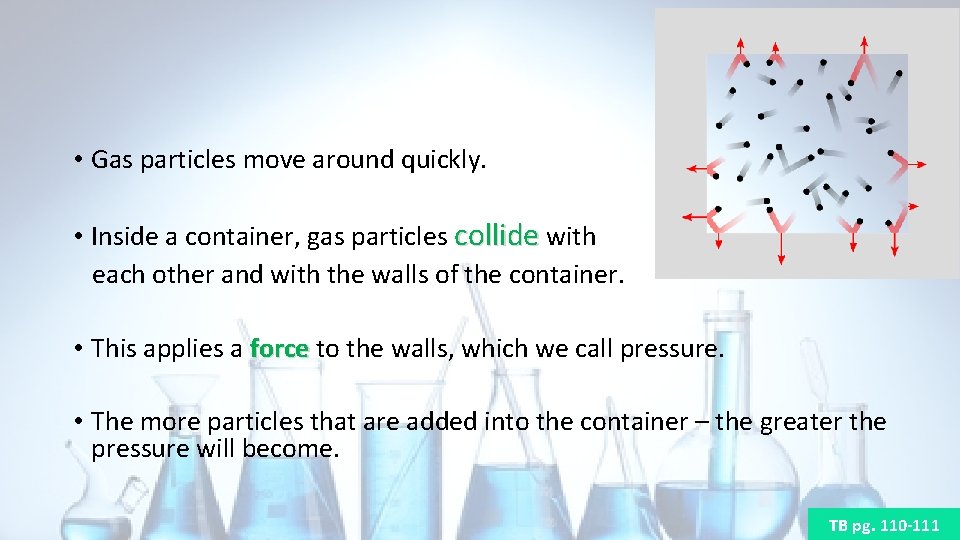
• Gas particles move around quickly. • Inside a container, gas particles collide with each other and with the walls of the container. • This applies a force to the walls, which we call pressure. force • The more particles that are added into the container – the greater the pressure will become. TB pg. 110 -111

TB pg. 110 -111

Homework TOPIC 5 REVISION PG. 114