Thermochemistry Phase Change Review n Melting solid liquid








- Slides: 38

Thermochemistry

+ Phase Change Review n Melting: solid liquid n Vaporization: liquid gas n Evaporation: occurs only at surface of liquid n Boiling: bubbles of vapor form within the liquid when the temperature of the liquid is increased n Condensation: gas liquid n Freezing: liquid solid

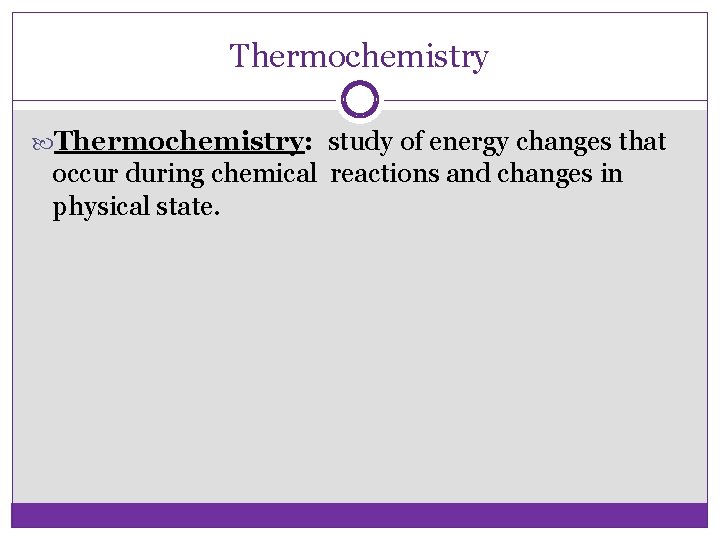
Thermochemistry: study of energy changes that occur during chemical reactions and changes in physical state.

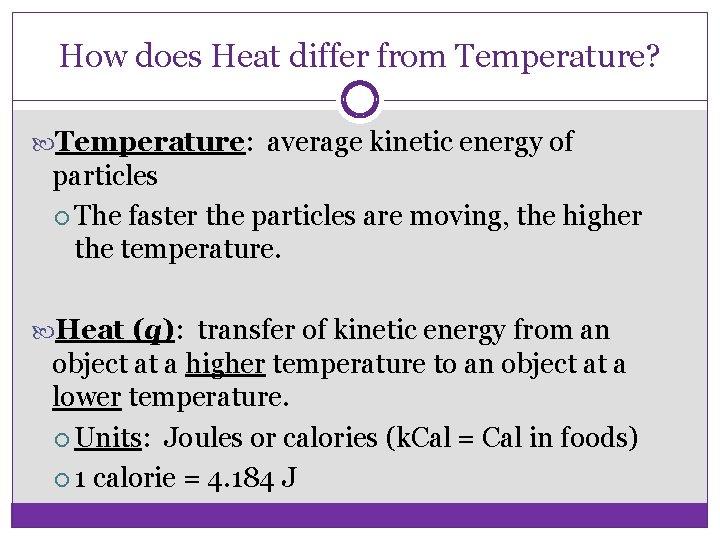
How does Heat differ from Temperature? Temperature: average kinetic energy of particles The faster the particles are moving, the higher the temperature. Heat (q): transfer of kinetic energy from an object at a higher temperature to an object at a lower temperature. Units: Joules or calories (k. Cal = Cal in foods) 1 calorie = 4. 184 J

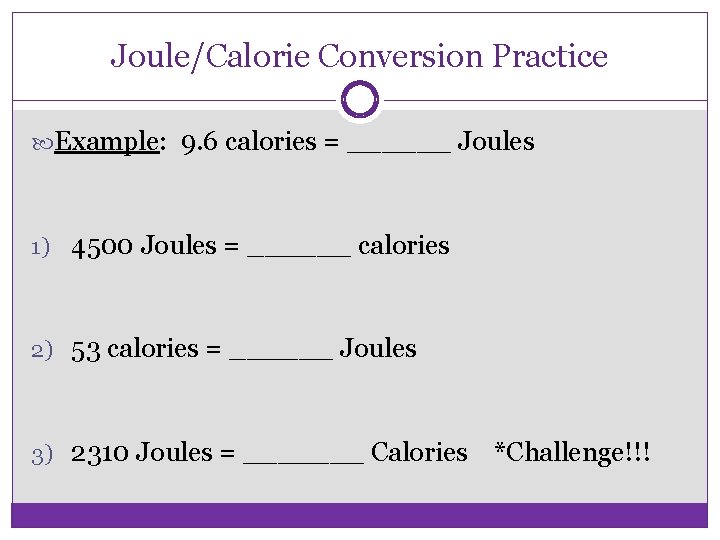
Joule/Calorie Conversion Practice Example: 9. 6 calories = ______ Joules 1) 4500 Joules = ______ calories 2) 53 calories = ______ Joules 3) 2310 Joules = _______ Calories *Challenge!!!

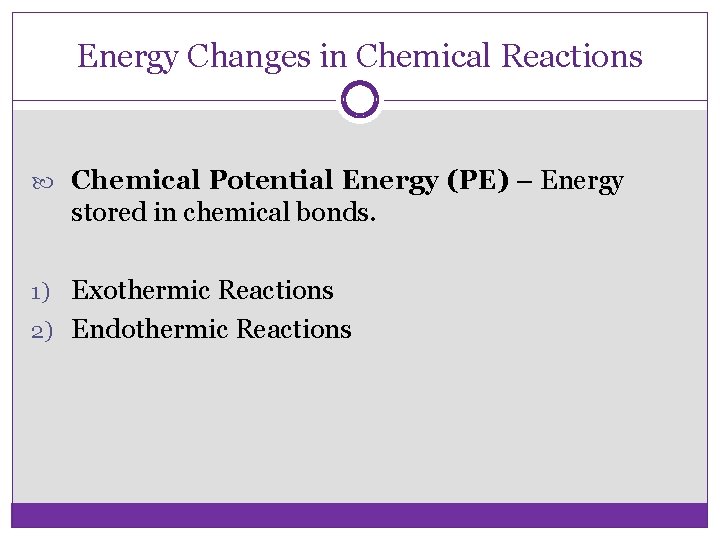
Energy Changes in Chemical Reactions Chemical Potential Energy (PE) – Energy stored in chemical bonds. 1) Exothermic Reactions 2) Endothermic Reactions

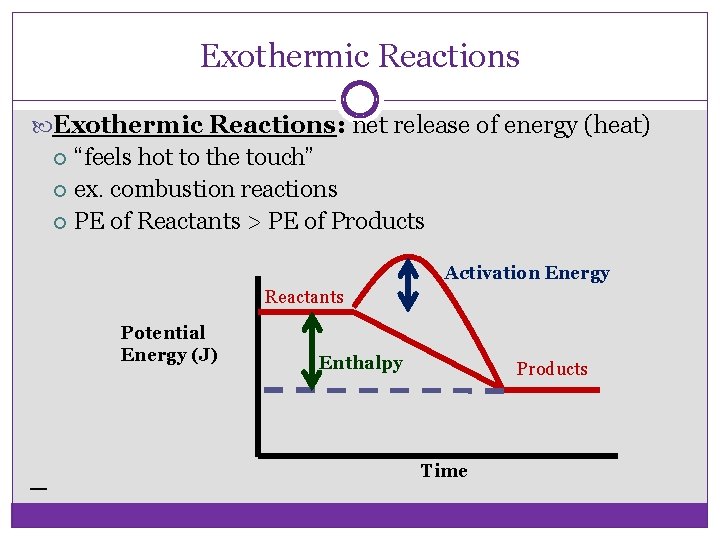
Exothermic Reactions: net release of energy (heat) “feels hot to the touch” ex. combustion reactions PE of Reactants > PE of Products Activation Energy Reactants Potential Energy (J) Enthalpy Products Time

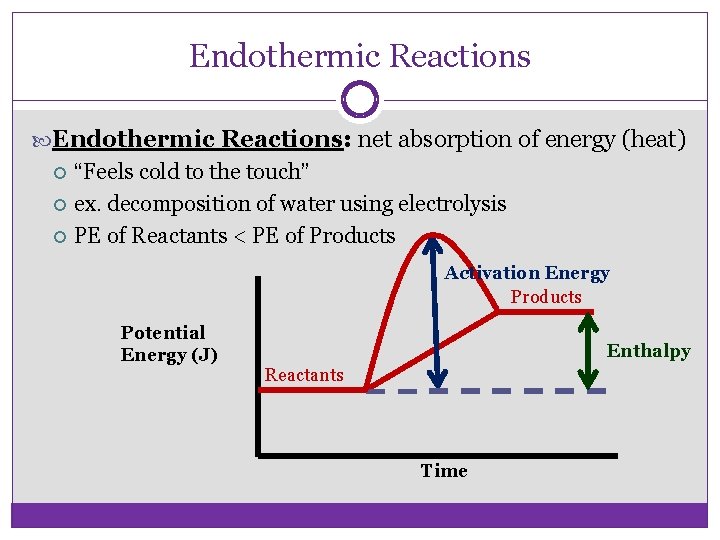
Endothermic Reactions: net absorption of energy (heat) “Feels cold to the touch” ex. decomposition of water using electrolysis PE of Reactants < PE of Products Activation Energy Products Potential Energy (J) Enthalpy Reactants Time

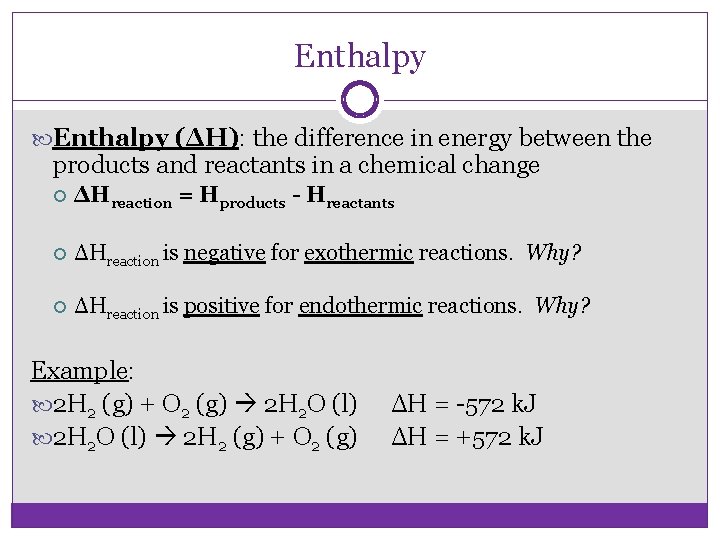
Enthalpy (ΔH): the difference in energy between the products and reactants in a chemical change ΔHreaction = Hproducts - Hreactants ΔHreaction is negative for exothermic reactions. Why? ΔHreaction is positive for endothermic reactions. Why? Example: 2 H 2 (g) + O 2 (g) 2 H 2 O (l) 2 H 2 (g) + O 2 (g) ΔH = -572 k. J ΔH = +572 k. J

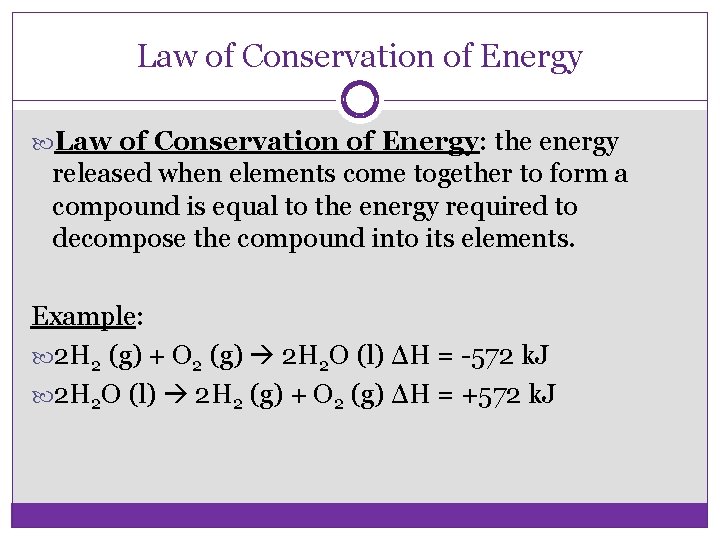
Law of Conservation of Energy: the energy released when elements come together to form a compound is equal to the energy required to decompose the compound into its elements. Example: 2 H 2 (g) + O 2 (g) 2 H 2 O (l) ΔH = -572 k. J 2 H 2 O (l) 2 H 2 (g) + O 2 (g) ΔH = +572 k. J

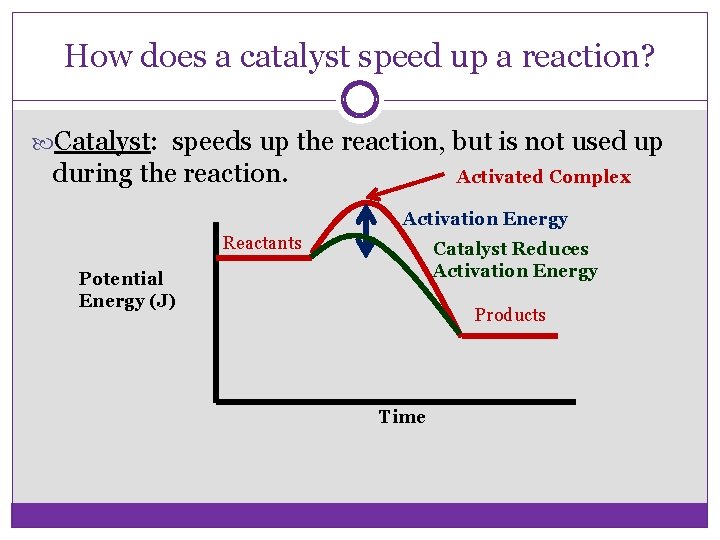
Activation Energy & Catalysts Activation energy: The amount of energy needed to get a reaction started. Required for BOTH endothermic and exothermic reactions.

How does a catalyst speed up a reaction? Catalyst: speeds up the reaction, but is not used up during the reaction. Activated Complex Activation Energy Reactants Catalyst Reduces Activation Energy Potential Energy (J) Products Time

Potential Energy Diagram WS

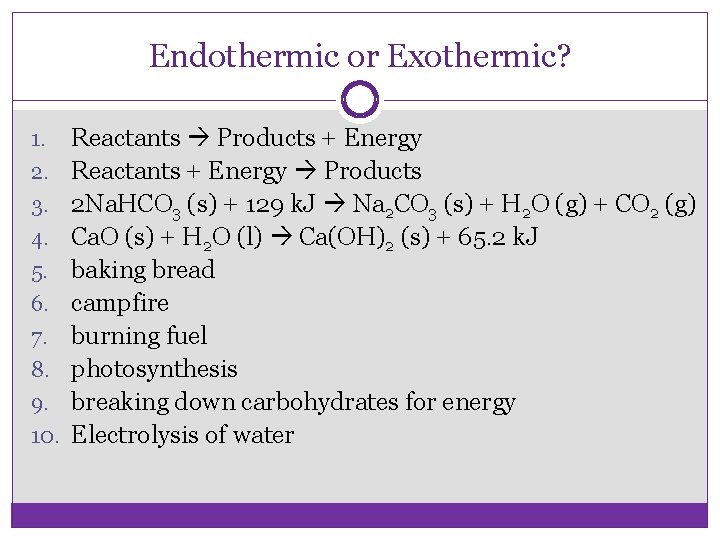
Endothermic or Exothermic? 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Reactants Products + Energy Reactants + Energy Products 2 Na. HCO 3 (s) + 129 k. J Na 2 CO 3 (s) + H 2 O (g) + CO 2 (g) Ca. O (s) + H 2 O (l) Ca(OH)2 (s) + 65. 2 k. J baking bread campfire burning fuel photosynthesis breaking down carbohydrates for energy Electrolysis of water

Endothermic or Exothermic? Phase changes are physical changes (no new substance is formed)… 1. 2. 3. 4. Melting Freezing Boiling/Evaporation/Vaporization Condensation

Thought Question Why are orange trees sprayed with water before cold nights to protect the fruit from frost damage?

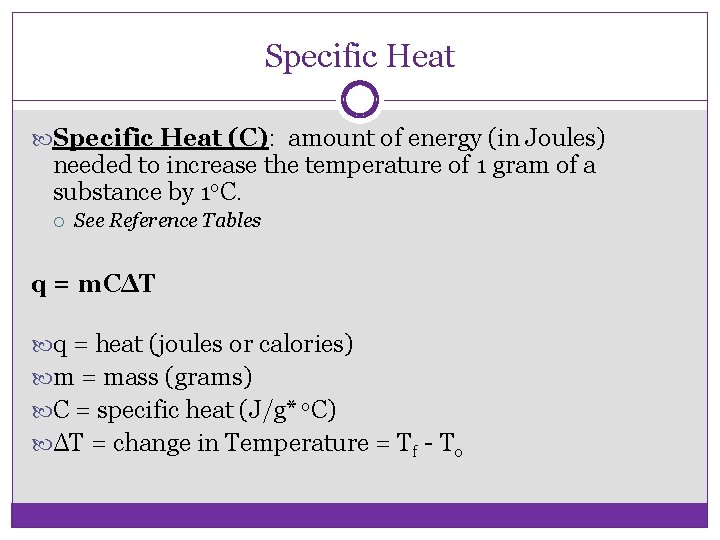
Specific Heat (C): amount of energy (in Joules) needed to increase the temperature of 1 gram of a substance by 1 o. C. See Reference Tables q = m. CΔT q = heat (joules or calories) m = mass (grams) C = specific heat (J/g* o. C) ΔT = change in Temperature = Tf - To

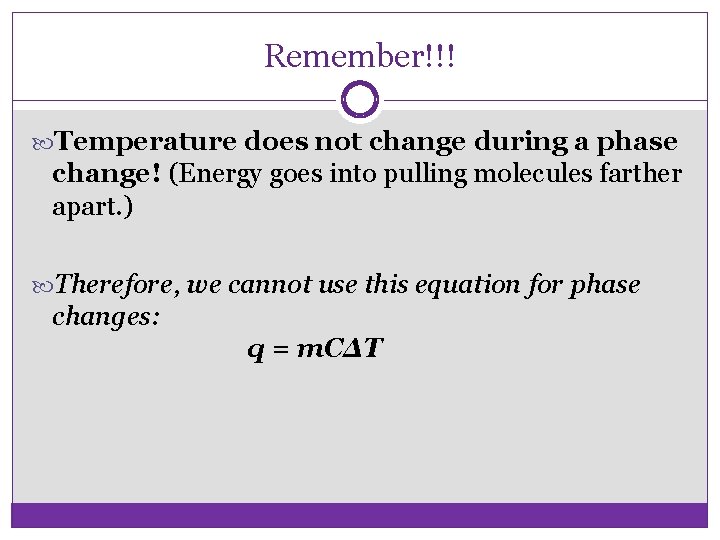
Remember!!! Temperature does not change during a phase change! (Energy goes into pulling molecules farther apart. ) Therefore, we cannot use this equation for phase changes: q = m. CΔT

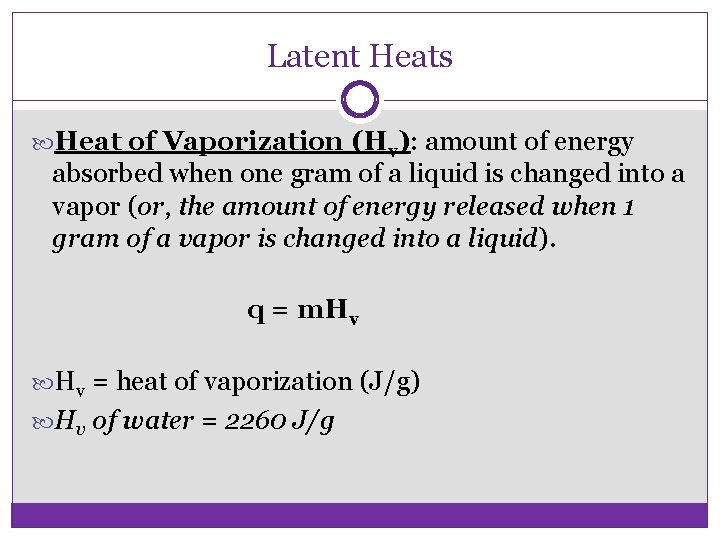
Latent Heats Heat of Vaporization (Hv): amount of energy absorbed when one gram of a liquid is changed into a vapor (or, the amount of energy released when 1 gram of a vapor is changed into a liquid). q = m. Hv = heat of vaporization (J/g) Hv of water = 2260 J/g

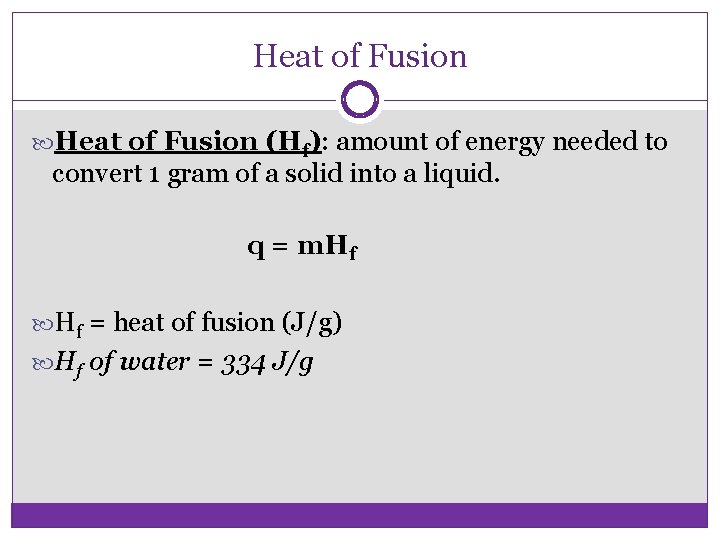
Heat of Fusion (Hf): amount of energy needed to convert 1 gram of a solid into a liquid. q = m. Hf = heat of fusion (J/g) Hf of water = 334 J/g

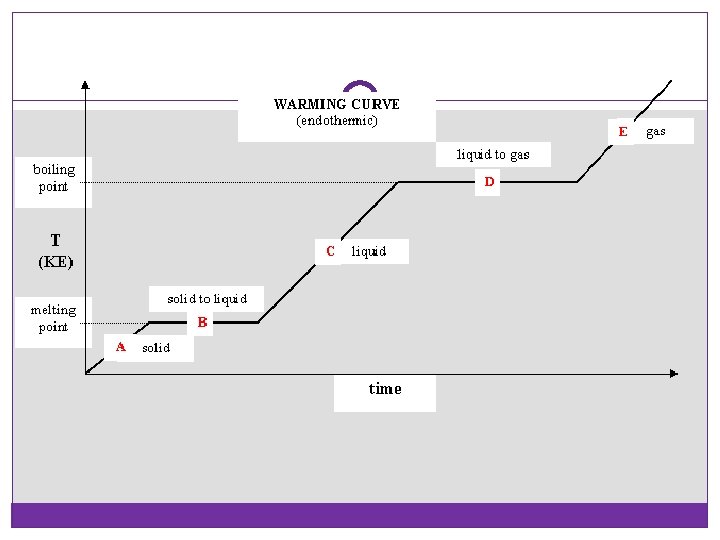
Heating and Cooling Curves Label Diagram +q heat absorbed -q heat released

Example Problems – Level One 1. How much heat is added if 100 grams of liquid water increases in temperature from 30 o. C to 70 o. C? 2. How much heat is absorbed if 200 g of ice increases in temperature from -15 o. C to -5 o. C?

(cont’d) 3. How much heat is released if 80 grams of water vapor decreases in temperature from 150 o. C to 125 o. C? 4. How much heat is absorbed when 30 g of ice is changed into liquid water at 0 o. C? 5. How much heat is released when 50 grams of water vapor is changed into liquid water at 100 o. C?

Example Problems – Level Two 1. How much heat is absorbed if 30 grams of water at -10 o. C is converted into liquid water? 2. How much heat is absorbed if 45 grams of water at 80 o. C is converted into steam at 105 o. C?

(cont’d) 3. How much heat is released if 500 grams of vapor at 120 o. C changes to liquid water 70 o. C?

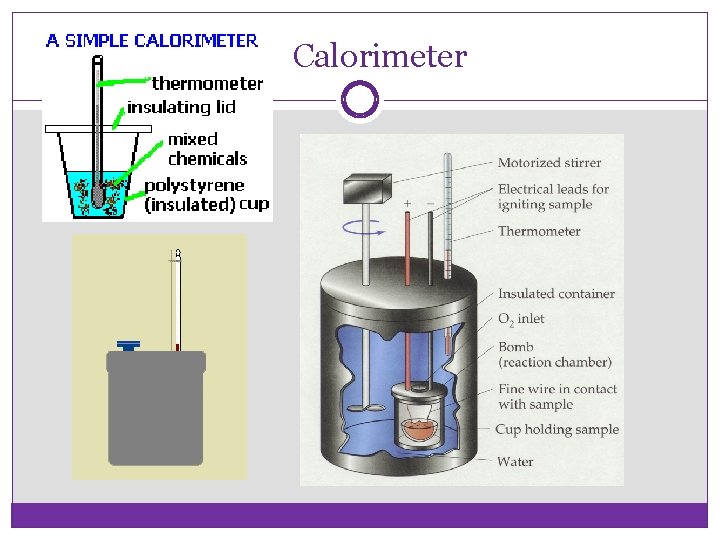
Calorimetry: the precise measurement of the heat flow out of a system for chemical and physical processes. Calorimeter: composed of a reaction chamber surrounded by water. The water supplies heat for an endothermic reaction or absorbs heat from an exothermic reaction. Use q = m. CΔT to find the amount of heat released or absorbed by the reaction.

Calorimeter

Calorimeter Ex Problems (short) NOT in packet 1. How much heat is released by the reaction if 10 grams of water is heated from 20 o. C to 60 o. C. 2. What is the final temperature of 15 g of water if the water starts at 20 o. C and loses 300 J of heat? 3. Determine the substance if a sample of 200 grams increases in temperature from 10 o. C to 28 o. C when it absorbs 1620 Joules of energy.

Calorimeter Example Problems NOT in packet 1. How much heat is released by the reaction if 10 grams of water is heated from 20 o. C to 60 o. C. 2. What is the mass of the water if a release of 650 J of heat causes the water to increase in temperature by 15 o. C?

Example Problems 3. What is the final temperature of 15 g of water if the water starts at 20 o. C and loses 300 J of heat? 4. Determine the substance if a sample of 200 grams increases in temperature from 10 o. C to 28 o. C when it absorbs 1620 Joules of energy.

Entropy (S): a measure of the disorder or randomness of the particles that make up a system. Observations: Systems tend to go from a state of high energy to a state of low energy (exothermic reactions are more likely to occur than endothermic reactions). Systems tend to become more disordered. Ex. a deck of cards, tea cup

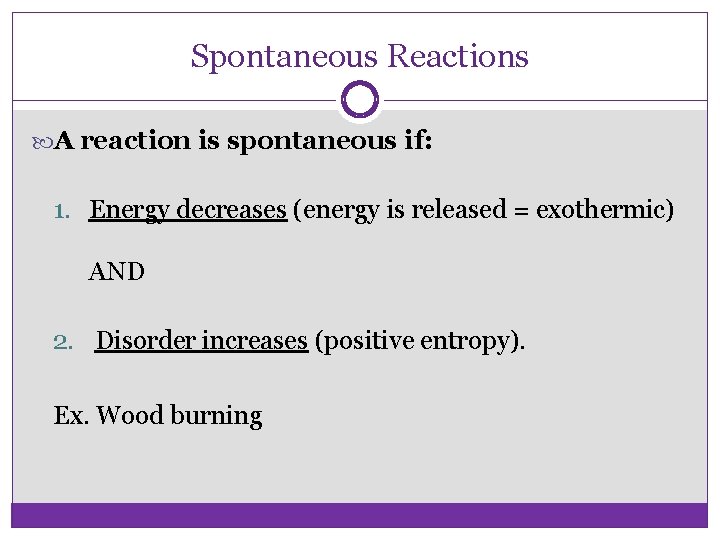
Spontaneous Reactions A reaction is spontaneous if: 1. Energy decreases (energy is released = exothermic) AND 2. Disorder increases (positive entropy). Ex. Wood burning

Entropy is NOT conserved. The natural tendency of entropy is to increase. In most spontaneous, naturally occurring processes, entropy increases. ΔSsystem = Sproducts - Sreactants

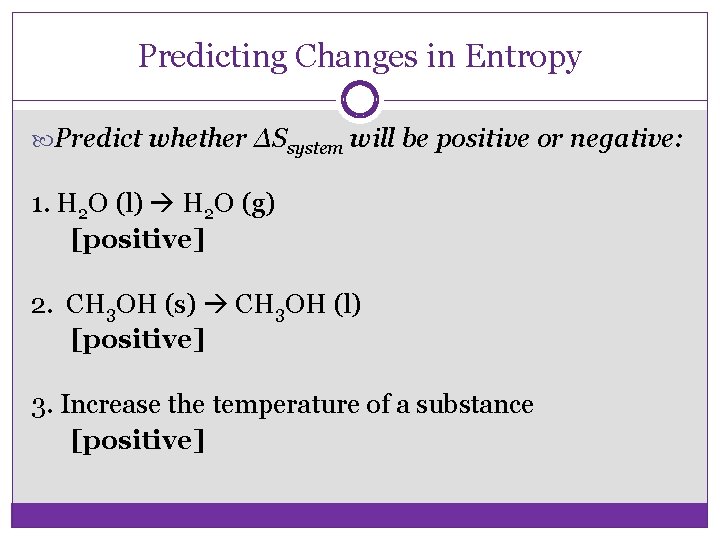
Predicting Changes in Entropy Predict whether ΔSsystem will be positive or negative: 1. H 2 O (l) H 2 O (g) [positive] 2. CH 3 OH (s) CH 3 OH (l) [positive] 3. Increase the temperature of a substance [positive]

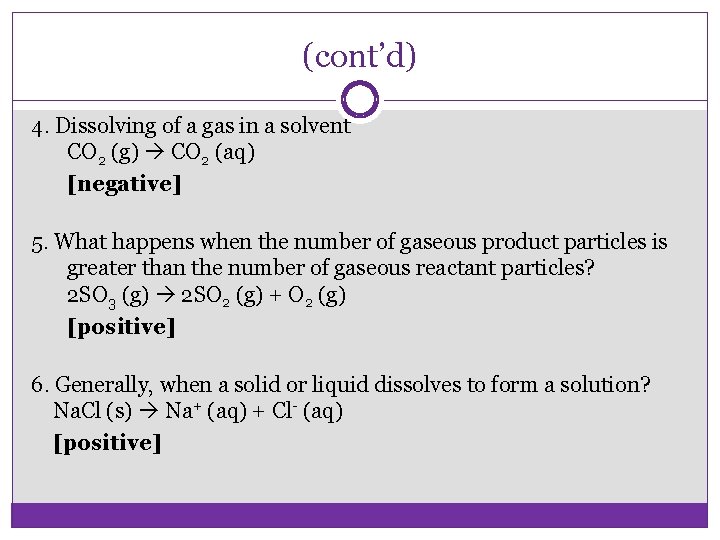
(cont’d) 4. Dissolving of a gas in a solvent CO 2 (g) CO 2 (aq) [negative] 5. What happens when the number of gaseous product particles is greater than the number of gaseous reactant particles? 2 SO 3 (g) 2 SO 2 (g) + O 2 (g) [positive] 6. Generally, when a solid or liquid dissolves to form a solution? Na. Cl (s) Na+ (aq) + Cl- (aq) [positive]

(add…. ) MP / BP chart… when is it a solid, liquid, or gas?

Bell Work for Monday, May 14 Grab a scantron, periodic table, Bohr model sheet Calculator (if needed) Scantron Name Subject: Thermochemistry Test Date Study for your test!!
