Phases of Matter Overview Solid liquid gas vapor





















- Slides: 48

Phases of Matter Overview • Solid, liquid, gas (vapor) properties • Molecular motion vs. phase • Gases and pressure • Liquids, evaporation, boiling • Solids, melting

Properties of the different phases 1 -1. In your group, list the different physical properties of a) Solids b) Liquids c) Gases or vapors

Solids Fixed volume and shape Heating melting Powders vs. chunk of something Crystals

Liquids Fixed volume, but adopt shape of container “Wet”, pourable Heating vaporization (boiling) Cooling freezing

Gases or Vapors No fixed volume or shape, assume shape and volume of container Cooling condensation

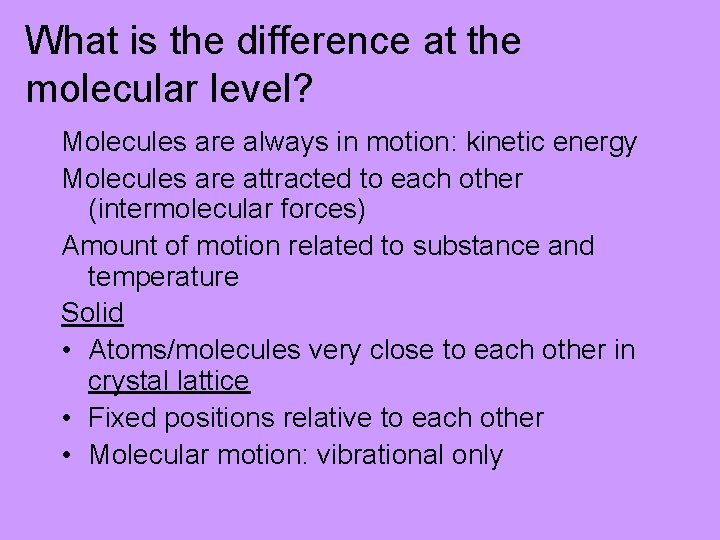
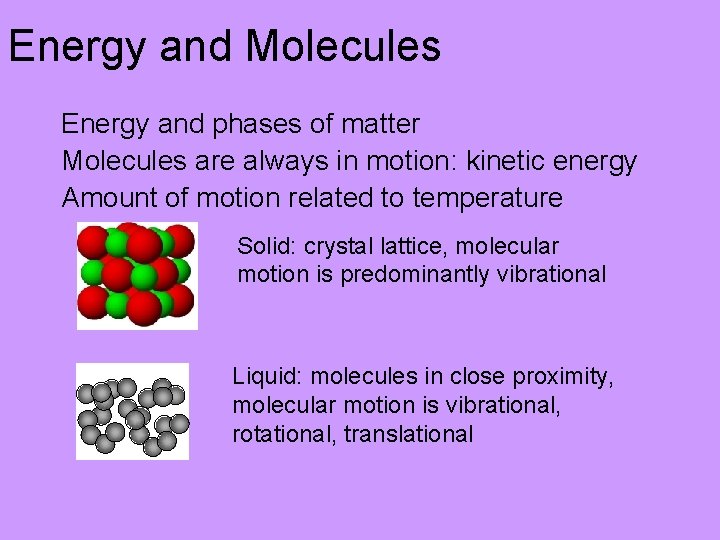
What is the difference at the molecular level? Molecules are always in motion: kinetic energy Molecules are attracted to each other (intermolecular forces) Amount of motion related to substance and temperature Solid • Atoms/molecules very close to each other in crystal lattice • Fixed positions relative to each other • Molecular motion: vibrational only

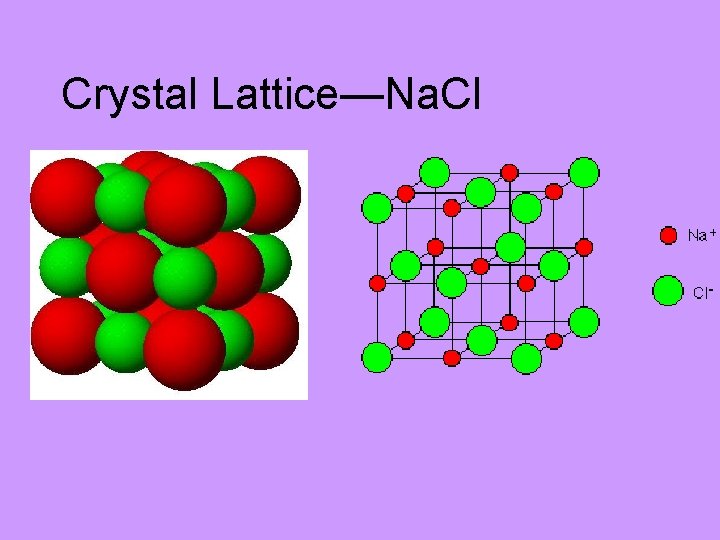
Crystal Lattice—Na. Cl

Crystal Lattice

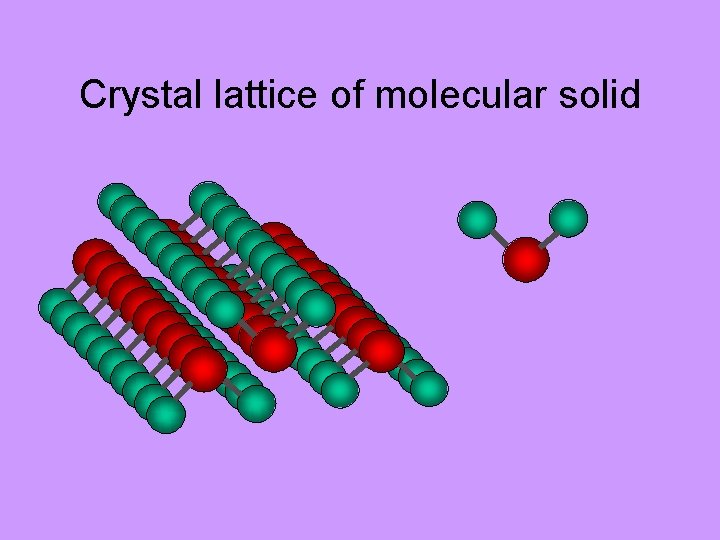
Crystal lattice of molecular solid

Water crystal lattice

Energy and Molecules Energy and phases of matter Molecules are always in motion: kinetic energy Amount of motion related to temperature Solid: crystal lattice, molecular motion is predominantly vibrational Liquid: molecules in close proximity, molecular motion is vibrational, rotational, translational

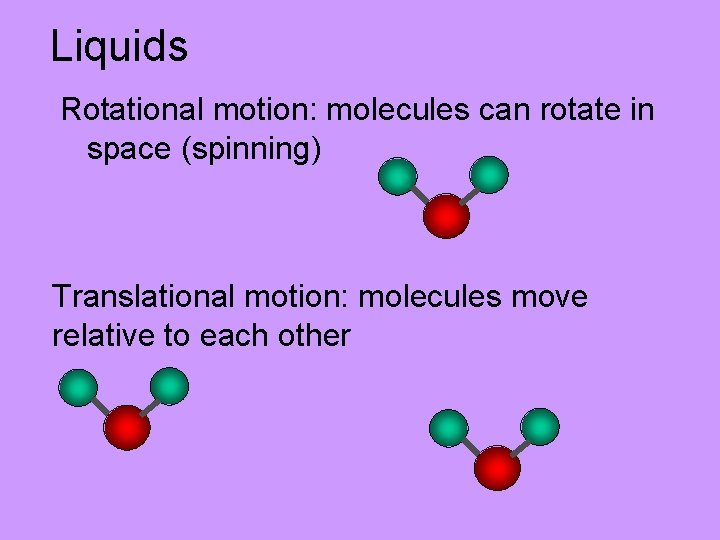
Liquids Rotational motion: molecules can rotate in space (spinning) Translational motion: molecules move relative to each other

Liquids Molecules are close together (attractive forces) but have a lot of freedom of movement. Gives rise to macroscopic properties associated with liquids: • Can pour a liquid, • Adopts shape of container • Viscosity: resistance to flow

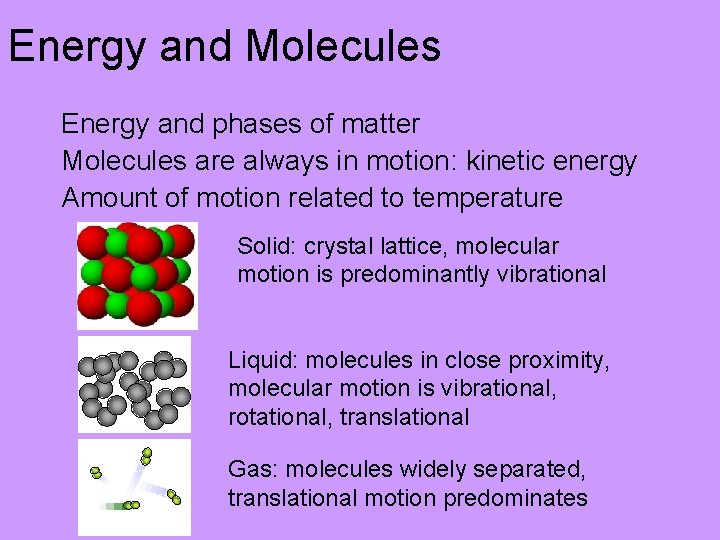
Energy and Molecules Energy and phases of matter Molecules are always in motion: kinetic energy Amount of motion related to temperature Solid: crystal lattice, molecular motion is predominantly vibrational Liquid: molecules in close proximity, molecular motion is vibrational, rotational, translational Gas: molecules widely separated, translational motion predominates

Gas or Vapor Phase • Molecules are far apart; no intermolecular forces • Molecules move independently of each other, shape and volume of container • Translational motion predominates • Elastic collisions w/ other gas molecules and with container walls • Collisions with container walls gives rise to “pressure”

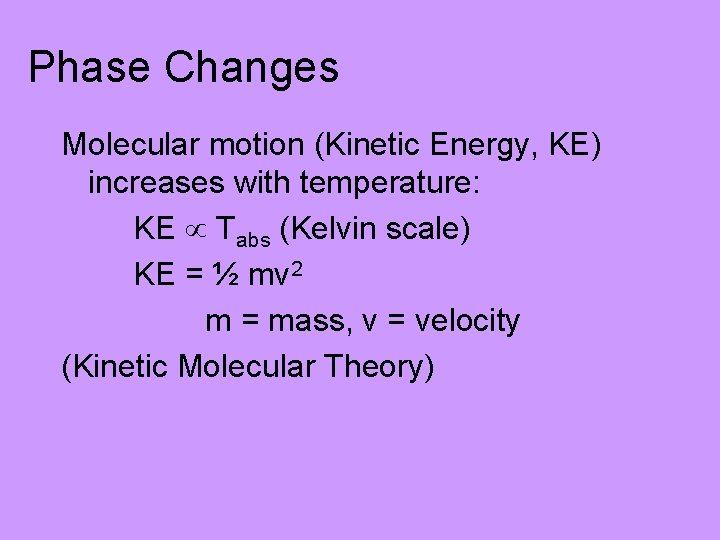
Phase Changes Molecular motion (Kinetic Energy, KE) increases with temperature: KE Tabs (Kelvin scale) KE = ½ mv 2 m = mass, v = velocity (Kinetic Molecular Theory)

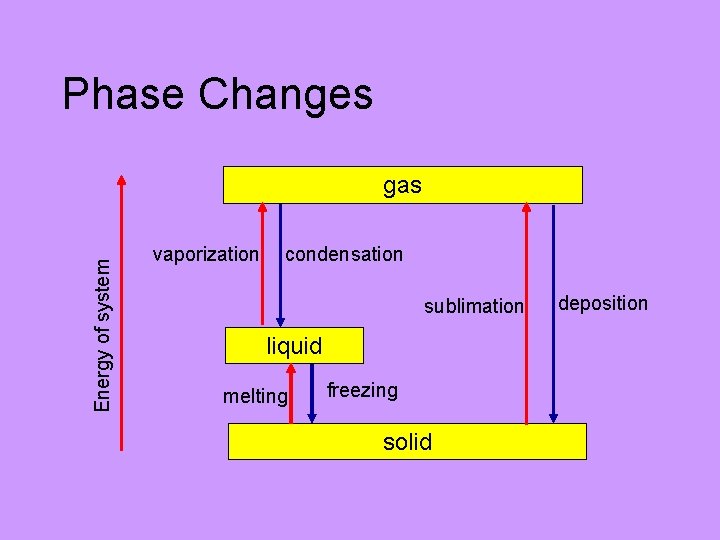
Phase Changes: Solid Liquid • Solid: vibrational motion increases with temperature until energy overcomes intermolecular forces to some extent. • Lattice collapses but molecules still in close proximity. • More molecular motion possible (rotational, translational) • Liquid ensues MELTING

Phase Changes: Liquid Gas • Liquid: motion (vibrational, rotational, translational) increases with temperature. • Molecules eventually have enough kinetic energy to completely overcome intermolecular forces. • Molecule escape into gas phase. VAPORIZATION

Phase Changes: Gas Liquid • Vapor: motion (translational) decreases with decreasing temperature. • Molecules eventually do not have enough kinetic energy to overcome intermolecular forces; stick together on collisions. • Molecules cluster and form droplets of liquid. CONDENSATION (precipitation)

Phase Changes: Liquid Solid • Liquid: motion (vibrational, rotational, translational) decreases with decreasing temperature. • Molecules stick together more and more as substance is cooled. Eventually form small crystal lattices (seed crystals, nucleation) which grow. FREEZING

Other Phase Changes Solid Vapor: sublimation (low temperature, low pressure) “dry” ice, frozen CO 2 snow disappearing below freezing temps Vapor Solid: deposition (low temperature, low pressure) frost


Phase Changes Energy of system gas vaporization condensation sublimation liquid melting freezing solid deposition

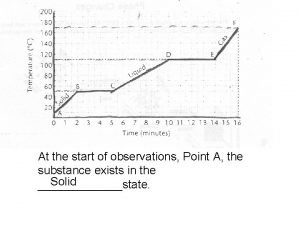
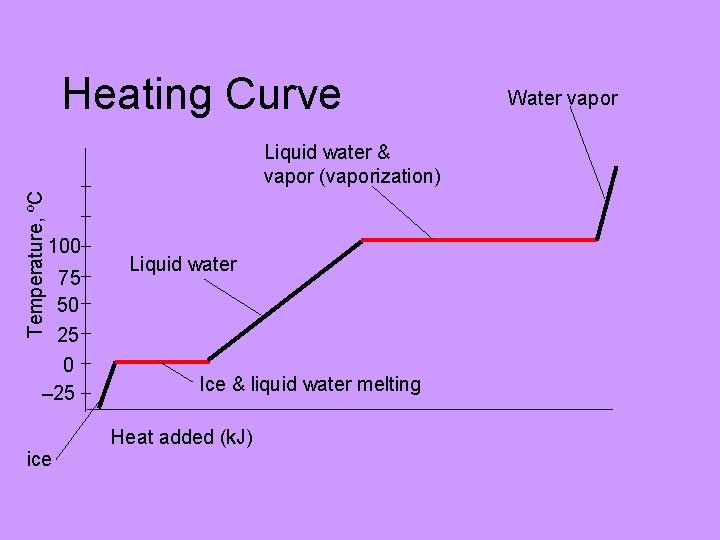
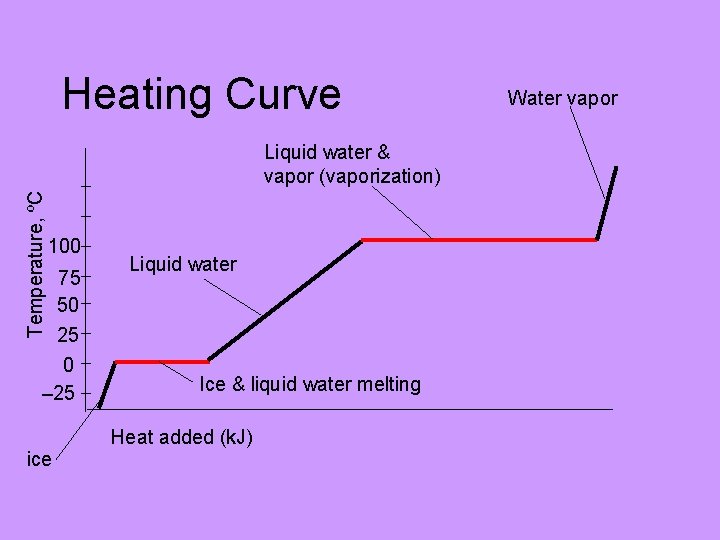
Heating Curve Temperature, ºC Liquid water & vapor (vaporization) 100 75 50 25 0 – 25 Liquid water Ice & liquid water melting Heat added (k. J) ice Water vapor

Properties of Gases (Gas Laws) • Pressure and Temperature are directly proportional • Pressure and volume are inversely proportional • Volume and temperature are directly proportional (video) • Volume and amount of a gas are directly proportional What is happening at the molecular level?

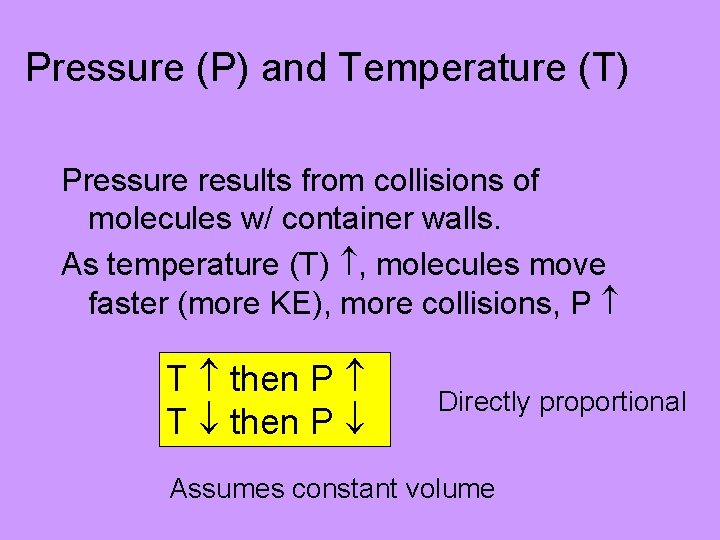
Pressure (P) and Temperature (T) Pressure results from collisions of molecules w/ container walls. As temperature (T) , molecules move faster (more KE), more collisions, P T then P Directly proportional Assumes constant volume

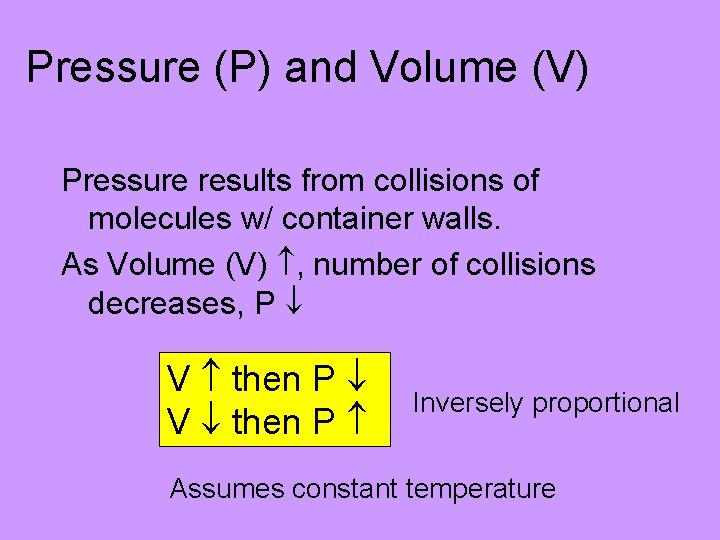
Pressure (P) and Volume (V) Pressure results from collisions of molecules w/ container walls. As Volume (V) , number of collisions decreases, P V then P Inversely proportional Assumes constant temperature

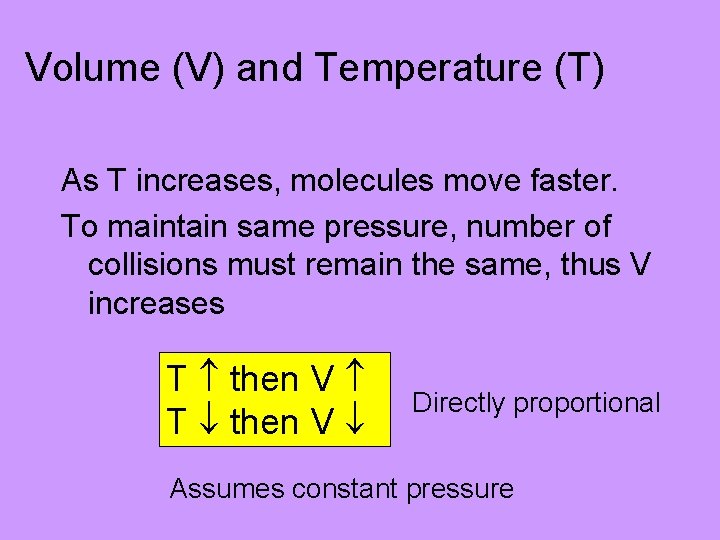
Volume (V) and Temperature (T) As T increases, molecules move faster. To maintain same pressure, number of collisions must remain the same, thus V increases T then V Directly proportional Assumes constant pressure

Volume (V) and Number of molecules Two samples of gas at the same P, T, and V: same number of collisions same number of molecules

Properties of Gases Explain each of the following: 1. Balloons hung outside in the sunshine pop. 2. A hot air balloon rises up in the air. 3. Collapsing can. 4. Balloon in liquid nitrogen (video). 5. Your water bottle shrinks when you fly to Dallas. 6. How you pull liquid up in a straw. 7. How a siphon works.

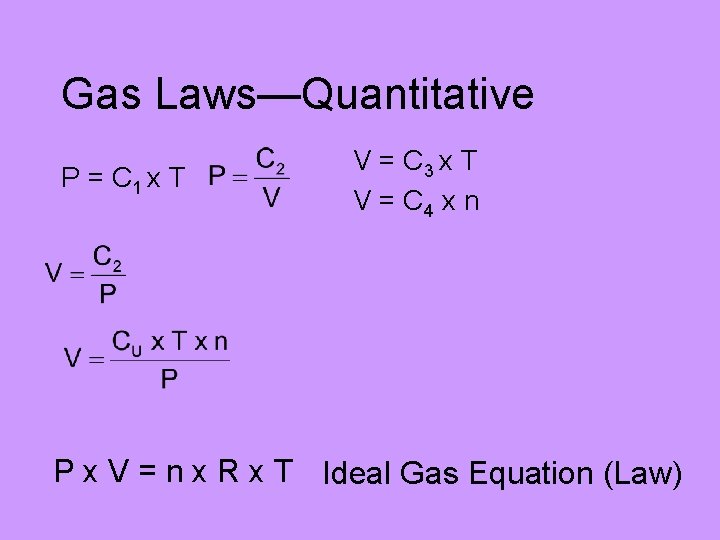
Gas Laws—Quantitative • Pressure and Temperature are directly proportional: P = C 1 x T • Pressure and volume are inversely proportional: • Volume and Temperature are directly proportional: V = C 3 x T • Volume and amount are directly proportional: V = C 4 x n

Gas Laws—Quantitative P = C 1 x T V = C 3 x T V = C 4 x n P x V = n x R x T Ideal Gas Equation (Law)

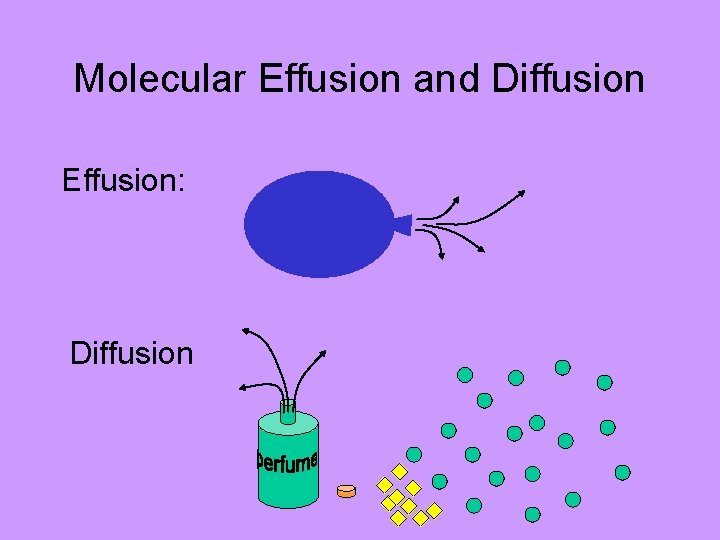
Molecular Effusion and Diffusion Effusion: Diffusion ACTIVITY: smelly balloons

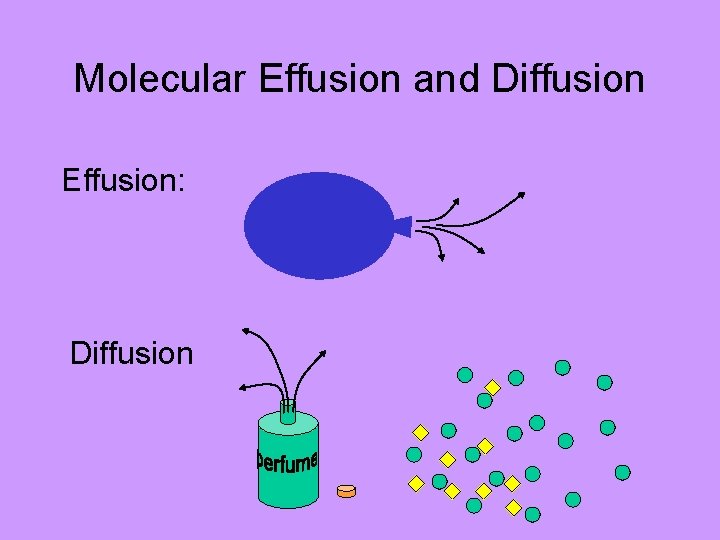
Molecular Effusion and Diffusion Effusion: Diffusion

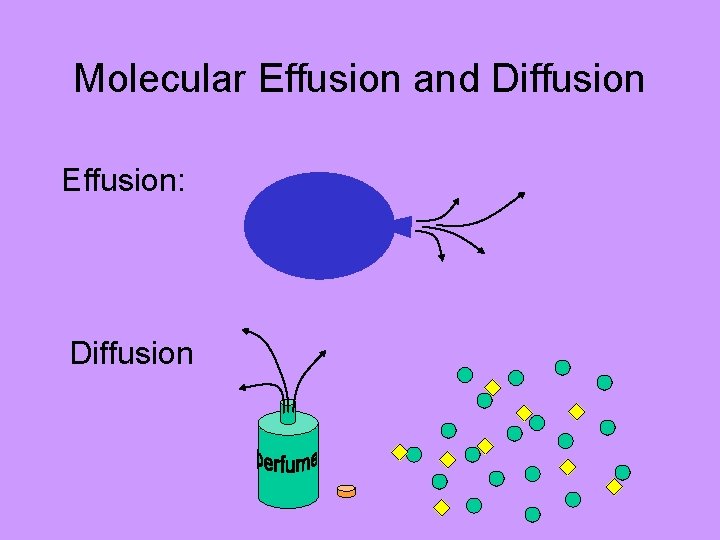
Molecular Effusion and Diffusion Effusion: Diffusion

Molecular Effusion and Diffusion Effusion: Diffusion

Molecular Effusion and Diffusion Effusion & Diffusion are dependent upon: • Temperature (hotter = faster) • Molecular Size (bigger = slower)

Properties of Liquids Intermolecular attractive forces (IMAF) Forces between molecules – “Like dissolves like. ” similar IMAF – Stronger forces • Larger molecules • Polar molecules (like water)

Properties of Liquids • Viscosity: resistance to flow – As IMAF viscosity – Viscosity as T • Surface Tension – Surface effect of stronger IMAF – As IMAF surface tension – Surface tension as T – Surfactants

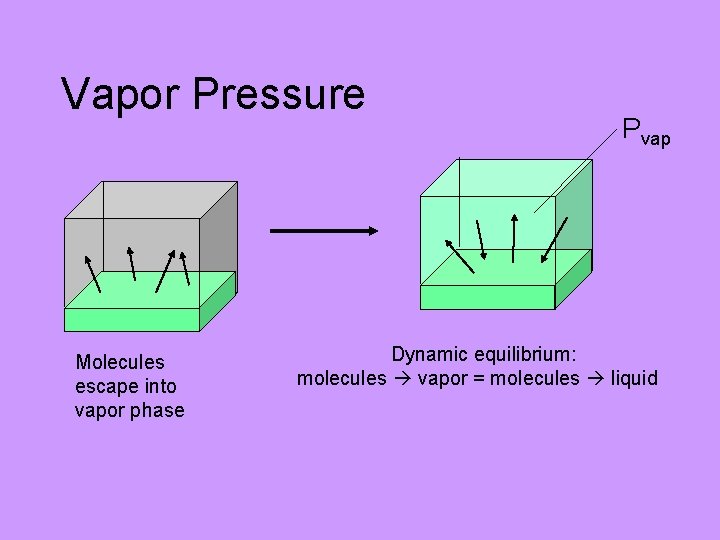
Vapor Pressure Vapor pressure: the pressure exerted by the vapor above a liquid when the liquid and the vapor are in dynamic equilibrium VERY difficult conceptually for students

Vapor Pressure Molecules escape into vapor phase Pvap Dynamic equilibrium: molecules vapor = molecules liquid

Vapor Pressure Pvap as T When Pvap = Patm: “boiling” Bubbles of gas in liquid

Explain the following… 1. How a pressure cooker works. 2. Why it takes longer to cook rice or pasta at high altitude. 3. How we were able to boil water with ice.

Heating Curve Temperature, ºC Liquid water & vapor (vaporization) 100 75 50 25 0 – 25 Liquid water Ice & liquid water melting Heat added (k. J) ice Water vapor

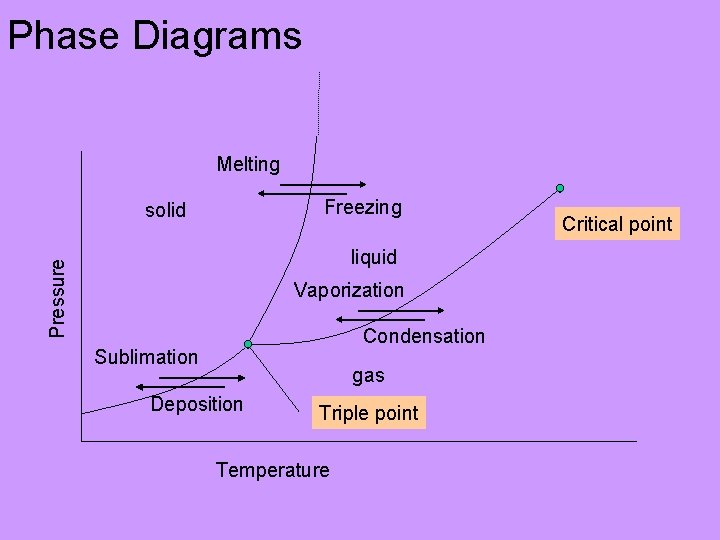
Phase Diagrams Melting Freezing solid Pressure liquid Vaporization Condensation Sublimation gas Deposition Triple point Temperature Critical point

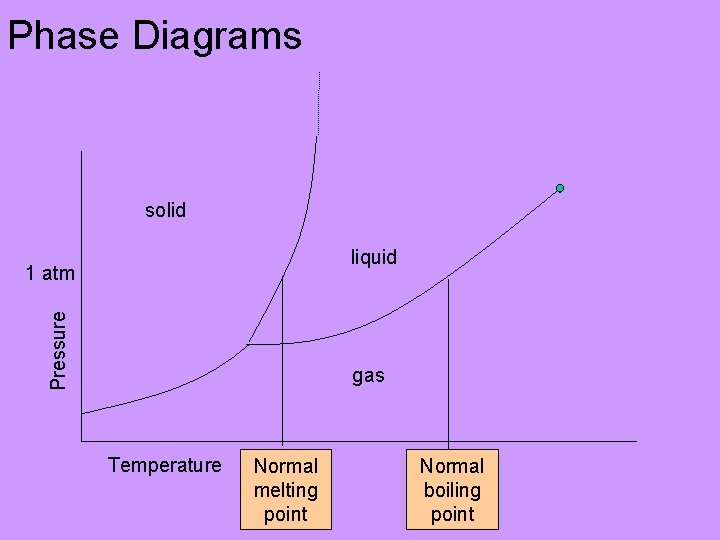
Phase Diagrams solid liquid Pressure 1 atm gas Temperature Normal melting point Normal boiling point

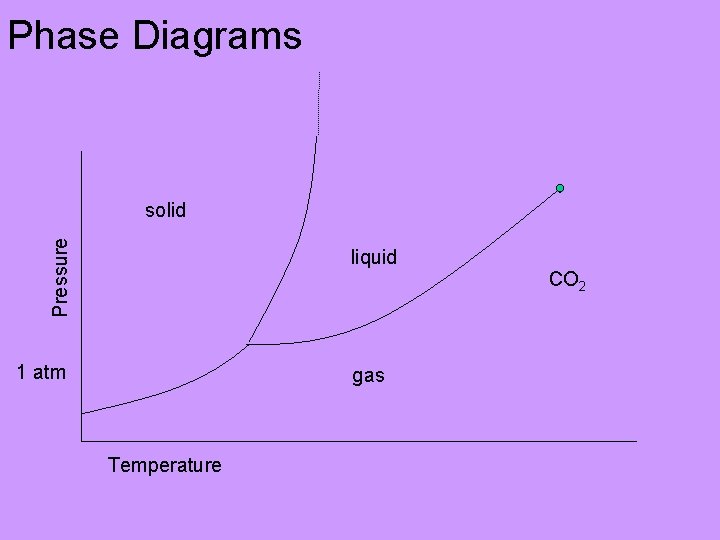
Phase Diagrams Pressure solid liquid CO 2 1 atm gas Temperature

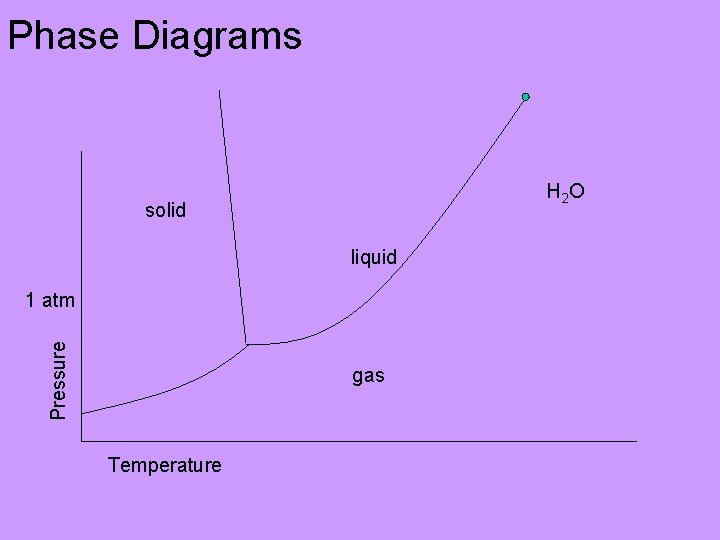
Phase Diagrams H 2 O solid liquid Pressure 1 atm gas Temperature
 Solid to plasma
Solid to plasma States of matter solid liquid gas
States of matter solid liquid gas Phase change concept map
Phase change concept map Nr-13
Nr-13 Solid shapes for kids
Solid shapes for kids Solid liquid gas plasma
Solid liquid gas plasma Solid liquid gas examples
Solid liquid gas examples Solid liquid venn diagram
Solid liquid venn diagram 20 examples of liquids
20 examples of liquids Pepsi particles candy
Pepsi particles candy Solid liquid, gas aqueous chart
Solid liquid, gas aqueous chart Why are gases easy to compress
Why are gases easy to compress Process of liquid to gas
Process of liquid to gas Solid liquid gas phase change
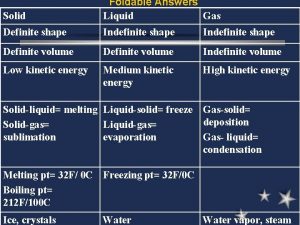
Solid liquid gas phase change Solid liquid gas foldable
Solid liquid gas foldable Graphic organizer of matter
Graphic organizer of matter Solid liquid gas concept map
Solid liquid gas concept map Gas liquid solid
Gas liquid solid Ehat is sound
Ehat is sound Solid liquid gas particles
Solid liquid gas particles Particle arrangement of a solid
Particle arrangement of a solid Solid liquid gas difference
Solid liquid gas difference Thiele modulus equation
Thiele modulus equation Gas liquid solid
Gas liquid solid Gas liquid solid
Gas liquid solid Chapter 21: temperature, heat, and expansion answer key
Chapter 21: temperature, heat, and expansion answer key Solid gas liquid
Solid gas liquid Solid liquid gas
Solid liquid gas Two film theory
Two film theory Solid liquid gas
Solid liquid gas Liquid solid gas
Liquid solid gas Solid liquid and gas
Solid liquid and gas Compressibility of solid liquid and gas
Compressibility of solid liquid and gas Solid liquid gas particle model
Solid liquid gas particle model Liquid homogeneous mixture
Liquid homogeneous mixture Mass of solid liquid and gas
Mass of solid liquid and gas Properties of solid liquid and gas
Properties of solid liquid and gas Porta test separator
Porta test separator Paint classification of matter
Paint classification of matter Four states of matter
Four states of matter Four states of matter
Four states of matter Phases changes of matter
Phases changes of matter 3 phases of matter
3 phases of matter Why isn't it a good idea to classify matter by its phases
Why isn't it a good idea to classify matter by its phases States of matter foldable
States of matter foldable Phases of matter
Phases of matter Classification of liquid dielectrics
Classification of liquid dielectrics Sieve tray tower
Sieve tray tower Osmotic pressure formula
Osmotic pressure formula