Phase Changes Solid Liquid Gas Review 3 Phases














- Slides: 22

Phase Changes

�Solid �Liquid �Gas Review: 3 Phases of Matter

�Is a change from one state of matter (solid, liquid, gas) to another. �Phase changes are physical changes because: - It only affects physical appearance, not chemical make-up. - Reversible What is a Phase Change?

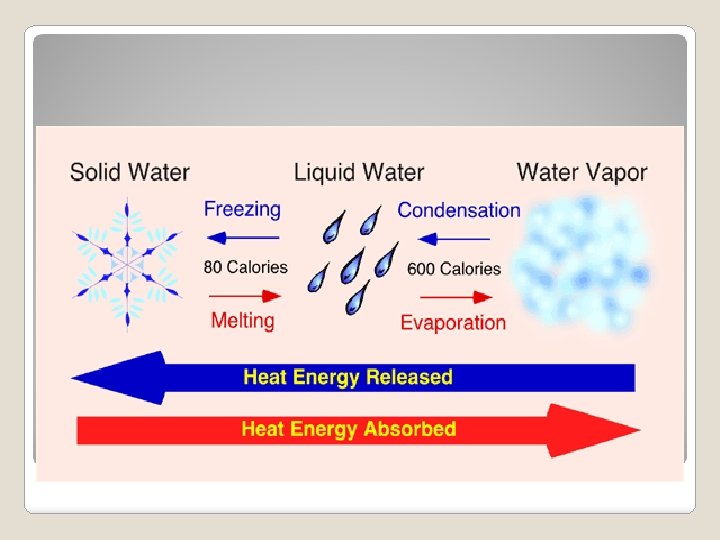
During a phase change, heat energy is either absorbed or released. Heat energy is released as molecules slow down and move closer together. What happens during a Heat energy is absorbed as phase moleculeschange? speed up and expand.

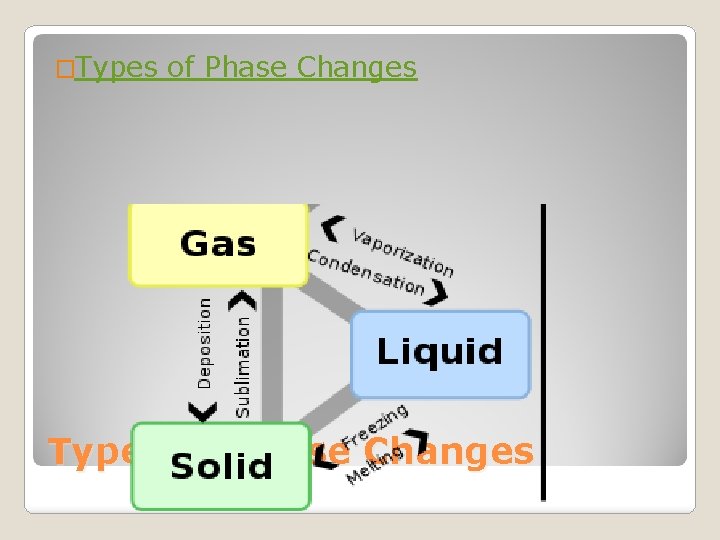
�Types of Phase Changes

Phase change from a solid to a liquid Molecules speed up, move farther apart, and absorb heat energy Melting

Phase Change from a liquid to a solid Molecule slow down, move closer together and release heat energy. Freezing

Phase change from a liquid to gas. It occurs at the boiling point of matter. Molecules speed up, move farther apart, and absorb heat energy. Vaporization (Boiling)

Phase change from a liquid to a gas on the surface of a liquid (occurs at all temperatures). Molecules speed up, move farther apart, and absorb heat energy. Evaporation

Phase change from a gas to a liquid. Molecule slow down, move closer together and release heat energy. Condensation

Phase change from a solid to a gas. Molecules speed up, move farther apart, and absorb heat energy. Sublimation

Phase change from a gas to a solid. Molecules slow down, move closer together and release heat energy. Deposition

�Number One �No video two �Number Three Cool Videos

Phases is the term scientist use to more properly define solid, liquids and gases. It means the same as the term “state” § Phase is defined as “a part of matter that has uniform properties through out the entire substance” § Phases

As scientists we can change phases. We can change a solid into a liquid and a liquid into a gas. § When two phases exist at the same time it is called equilibrium. § Equilibrium is a dynamic condition in which two opposing changes occur in equal rates in a closed system § Phases change

To have equilibrium we need to have both a temperature and pressure. § When we have both a measured temperature and pressure two or three states will exist at the same time. § Ice melting into water, water freezing into ice. § Equilibrium

Scientist have phase diagrams to show exactly the temperature and pressure must be achieved to have a solid, liquid, gas, two phases or all three phases § A phase diagram by definition is “a relationship between physical states that deals with temperature and pressure. ” § Phase Diagrams

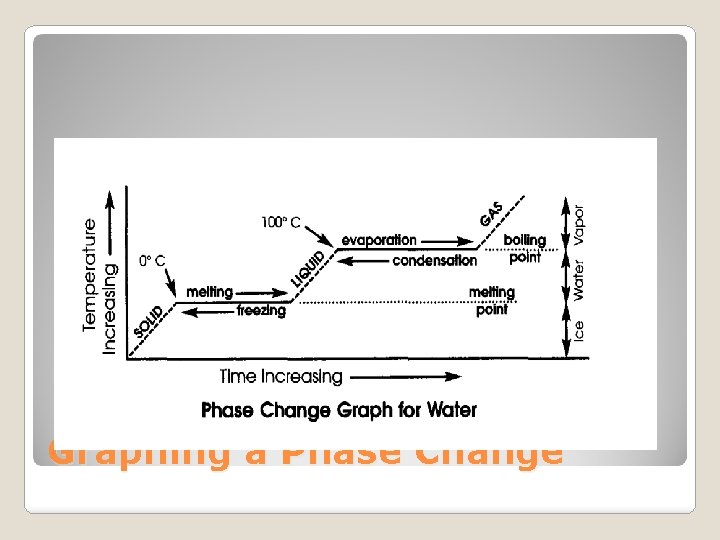
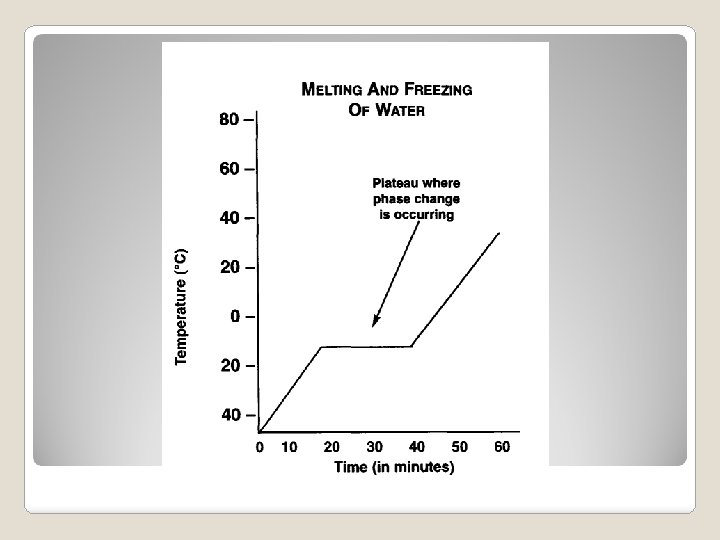
Graphing a Phase Change


�Melting Point: The temperature at which a solid changes into a liquid. �Boiling Point: The temperature at which a liquid changes into a gas. �What is a Freezing point? Compare the freezing and melting points of water. Melting & Boiling Points

Summary

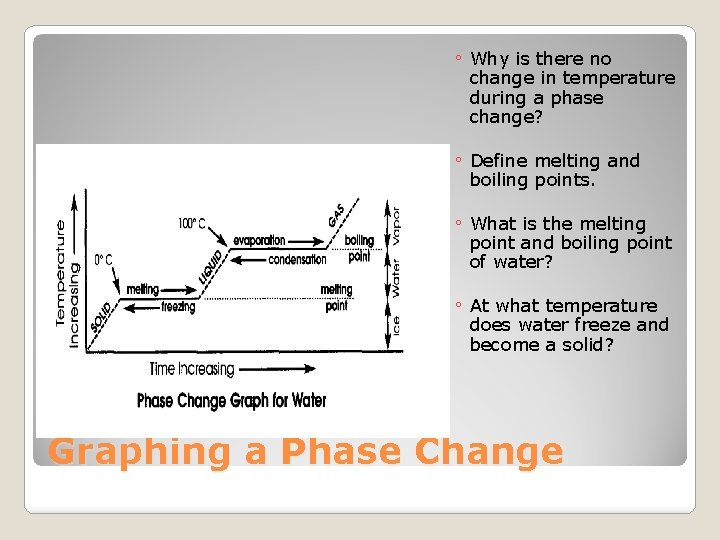
◦ Why is there no change in temperature during a phase change? ◦ Define melting and boiling points. ◦ What is the melting point and boiling point of water? ◦ At what temperature does water freeze and become a solid? Graphing a Phase Change