Kinetic Particle Theory Recap Physical Properties of Ionic








- Slides: 39

Kinetic Particle Theory

Recap • Physical Properties of Ionic and Covalent compounds -Melting and Boiling Point -Electrical Conductivity -Solubility in water Mp and bp Electrical conductivity Solubility in water Ionic Compound High Yes. Only in molten and aqueous state. Yes. Covalent Compound Low Not for all states. No.

Recap • Questions: • What is/are the states that ionic compounds usually exist as at r. t. p (room temperature and pressure)? What about covalent molecules? • Can ionic compound exist as other states? What do you need to do to change state? How does it happen?

Lesson Objectives At the end of this lesson, you should be able to: ü Describe the solid, liquid and gaseous states of matter

WHAT IS A MATTER? (a)Matter is anything that has mass and occupies space. (b)All matter is made up of tiny particles (atoms, molecules or ions). Use of the general term 'particle' means the precise nature of the particles does not have to be specified. (c)Matters can exist in 3 states: Solid, Liquid and gas.

• These three forms of matter are called the states of matter. • As shown here, water (liquid) can exist as ice (solid) or water vapour (gas).

MAKE OBSERVATIONS Look at the purple spot and orange spot…. . • What do you see? Why do you think it behave that way?

Food for thought…. . • Why do you smell a hamburger from a distance away? What about perfumes? How are the bees attracted to the flowers? • When you place a small piece of food into boiling water, what do you observe to it?

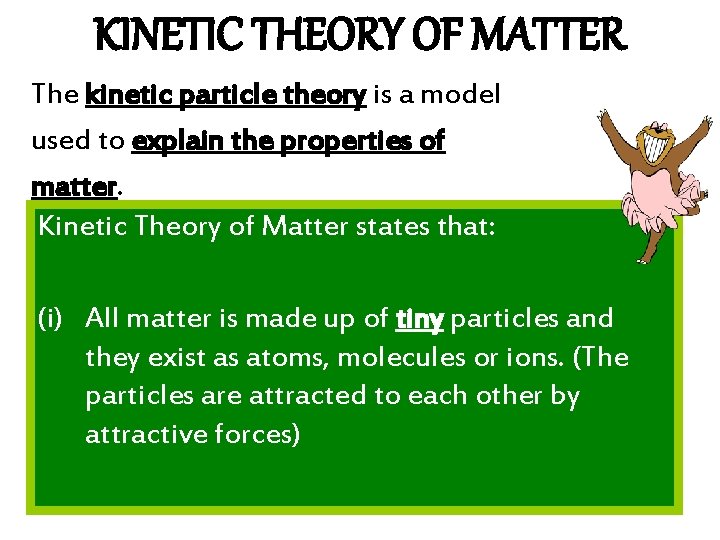
KINETIC THEORY OF MATTER The kinetic particle theory is a model used to explain the properties of matter. Kinetic Theory of Matter states that: (i) All matter is made up of tiny particles and they exist as atoms, molecules or ions. (The particles are attracted to each other by attractive forces)

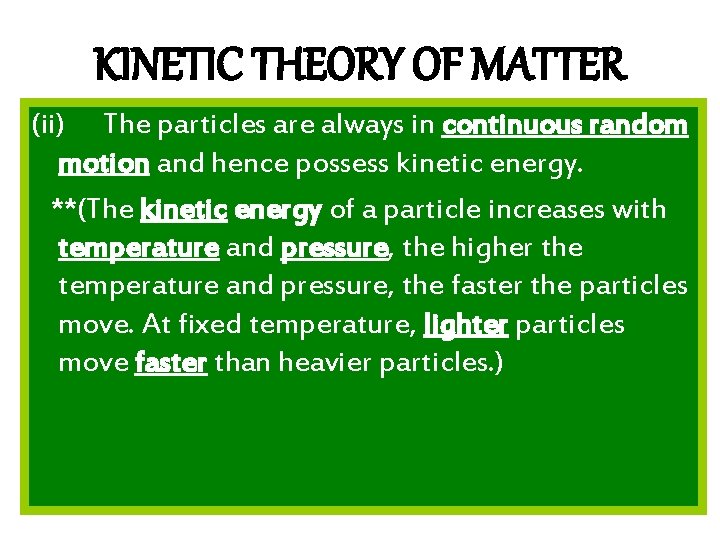
KINETIC THEORY OF MATTER (ii) The particles are always in continuous random motion and hence possess kinetic energy. **(The kinetic energy of a particle increases with temperature and pressure, the higher the temperature and pressure, the faster the particles move. At fixed temperature, lighter particles move faster than heavier particles. )

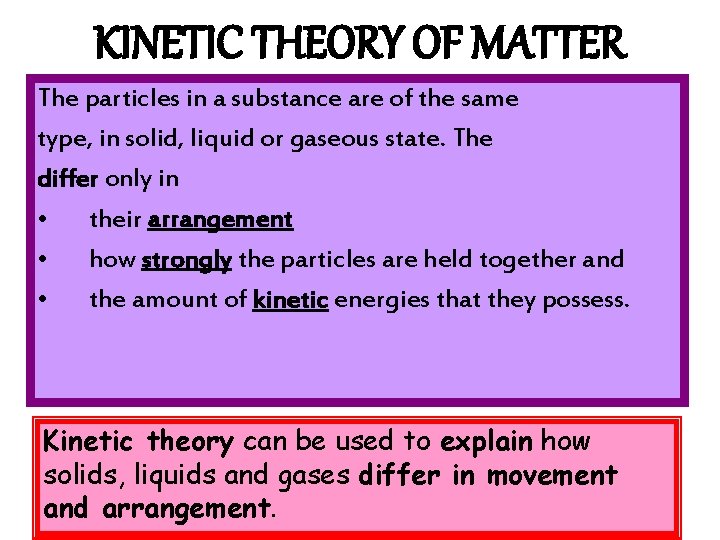
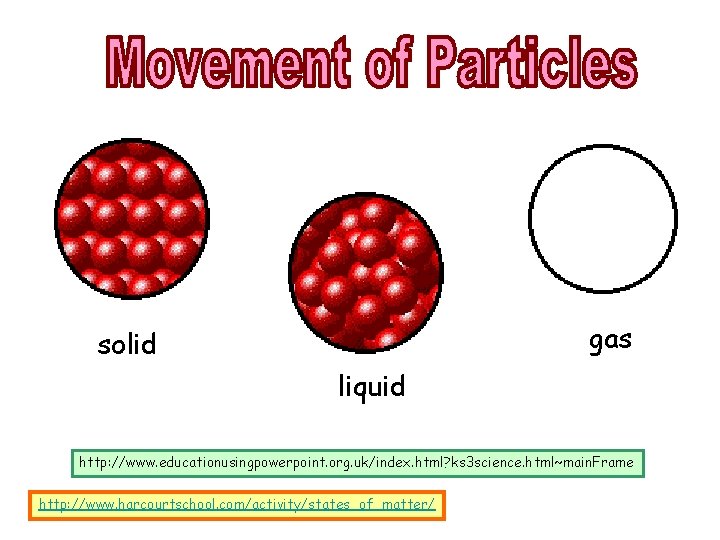
KINETIC THEORY OF MATTER The particles in a substance are of the same type, in solid, liquid or gaseous state. The differ only in • their arrangement • how strongly the particles are held together and • the amount of kinetic energies that they possess. Kinetic theory can be used to explain how solids, liquids and gases differ in movement and arrangement.

Group Work (5 mins for discussion) Get into groups of 4. Discuss about • How do molecules of matter behave? • How do the behaviour of particles account for the property of solid, liquid and gas? (Each group’ll be allocated 1 state) • Selected groups will role play on the movement of the particles in the particular state allocated.

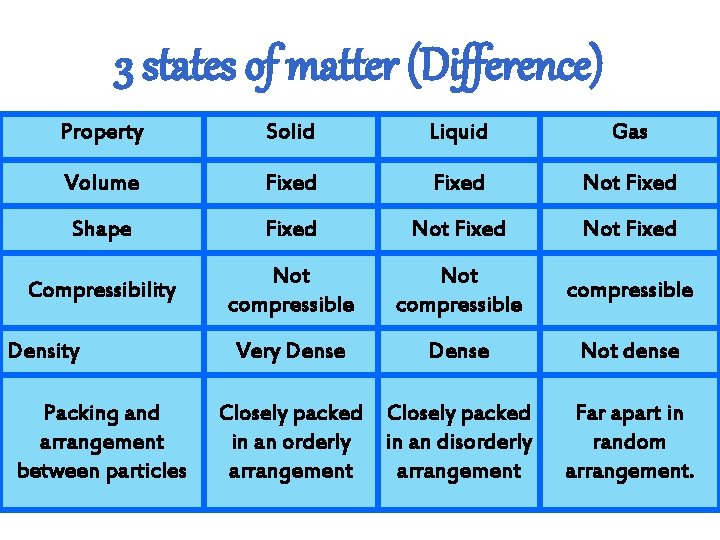
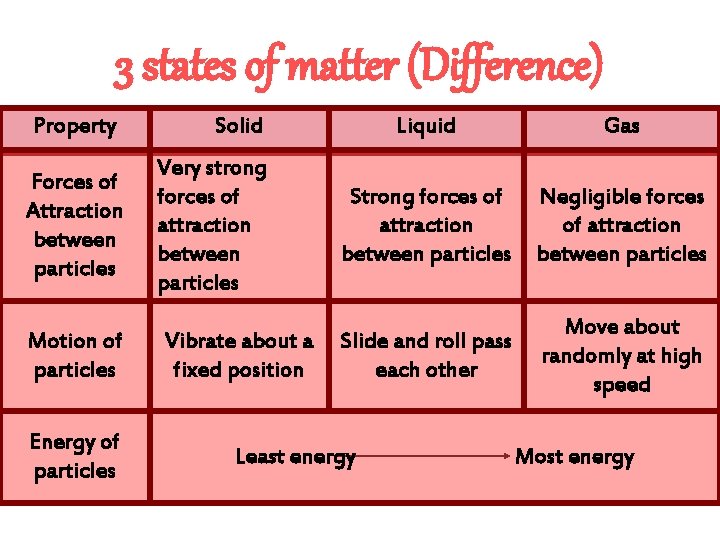
3 states of matter (Difference) Property Solid Liquid Gas Volume Fixed Not Fixed Shape Fixed Not Fixed Compressibility Not compressible Very Dense Not dense Density Packing and arrangement between particles Closely packed in an orderly in an disorderly arrangement Far apart in random arrangement.

3 states of matter (Difference) Property Forces of Attraction between particles Motion of particles Energy of particles Solid Very strong forces of attraction between particles Vibrate about a fixed position Liquid Gas Strong forces of attraction between particles Negligible forces of attraction between particles Slide and roll pass each other Move about randomly at high speed Least energy Most energy

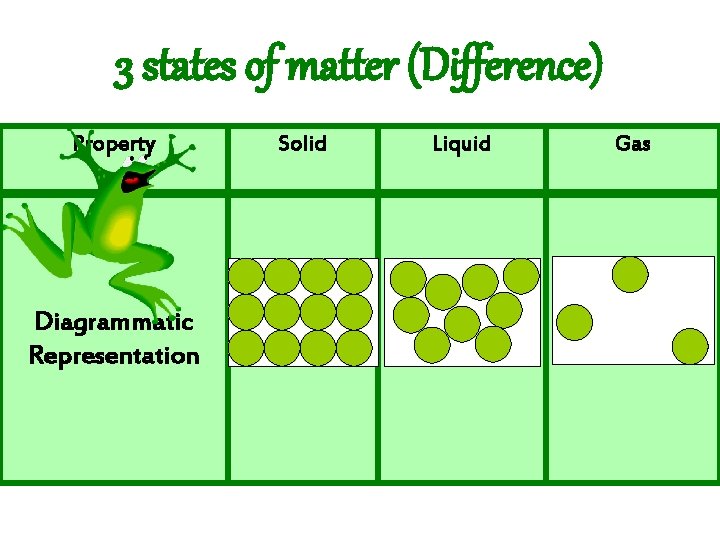
3 states of matter (Difference) Property Diagrammatic Representation Solid Liquid Gas

gas solid liquid http: //www. educationusingpowerpoint. org. uk/index. html? ks 3 science. html~main. Frame http: //www. harcourtschool. com/activity/states_of_matter/

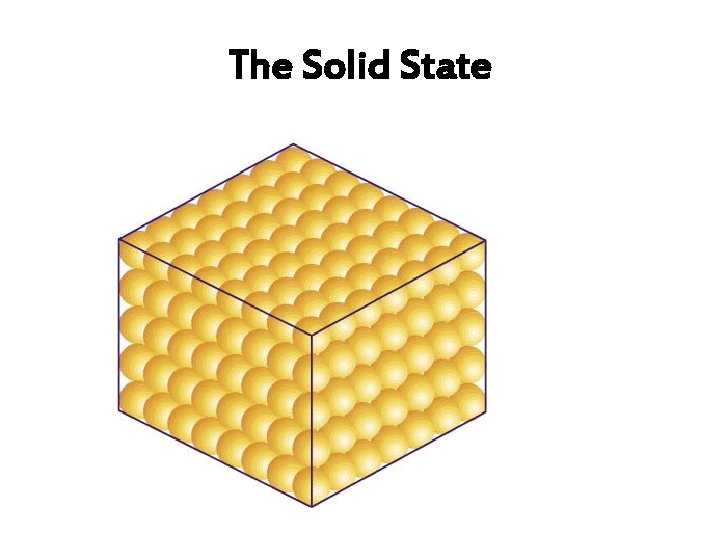
The Solid State

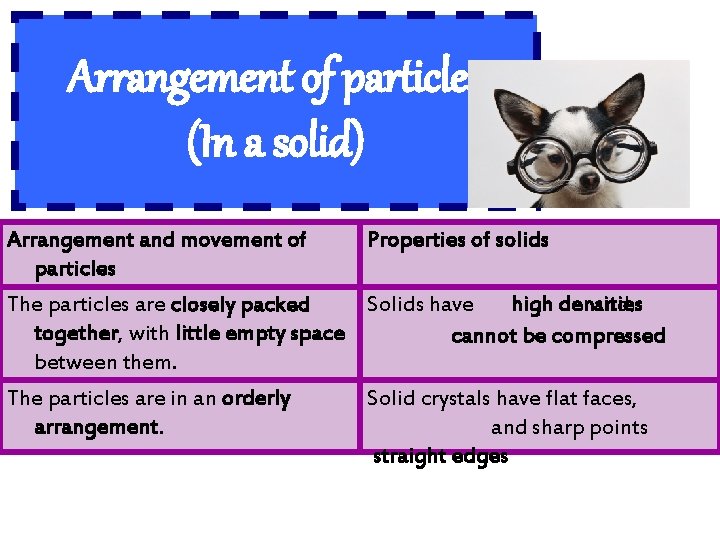
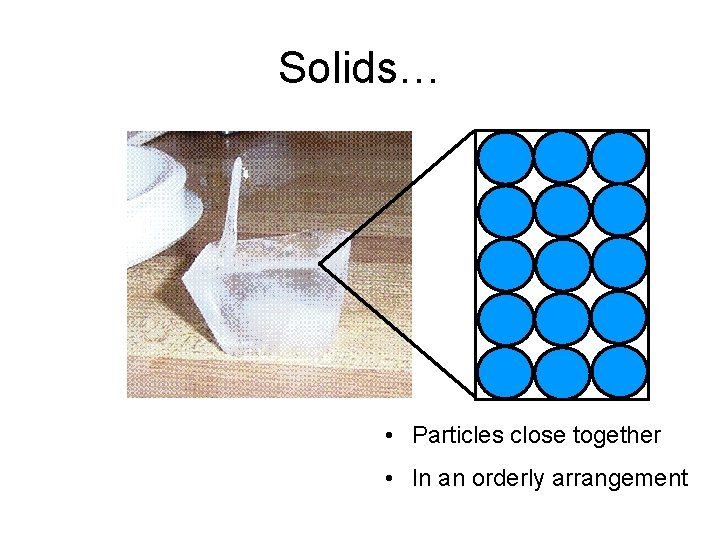
Arrangement of particles (In a solid) Arrangement and movement of particles Properties of solids high densities The particles are closely packed Solids have and. together, with little empty space cannot be compressed between them. The particles are in an orderly arrangement. Solid crystals have flat faces, and sharp points straight edges

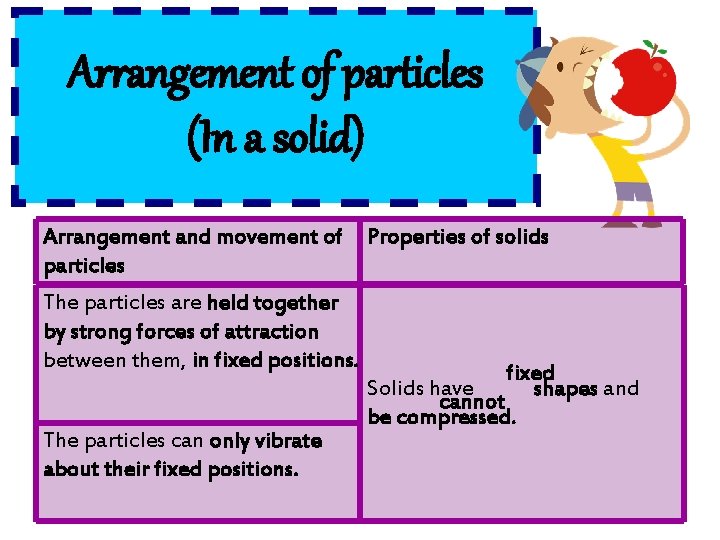
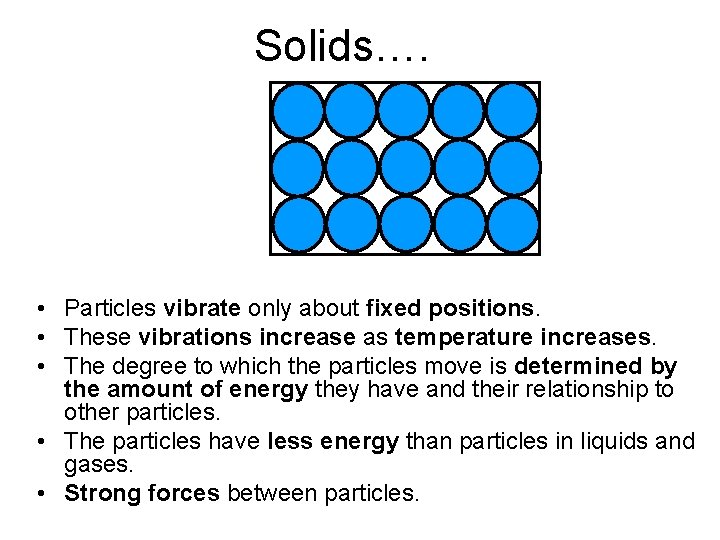
Arrangement of particles (In a solid) Arrangement and movement of Properties of solids particles The particles are held together by strong forces of attraction between them, in fixed positions. The particles can only vibrate about their fixed positions. fixed Solids have shapes and cannot be compressed.

The Liquid State

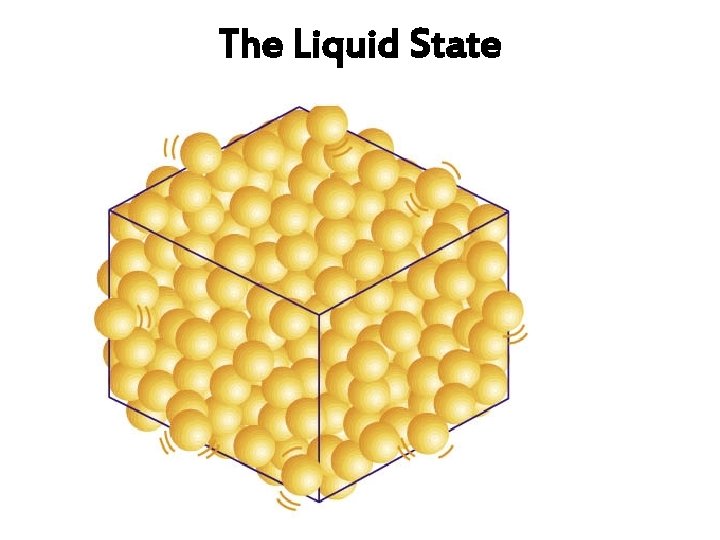
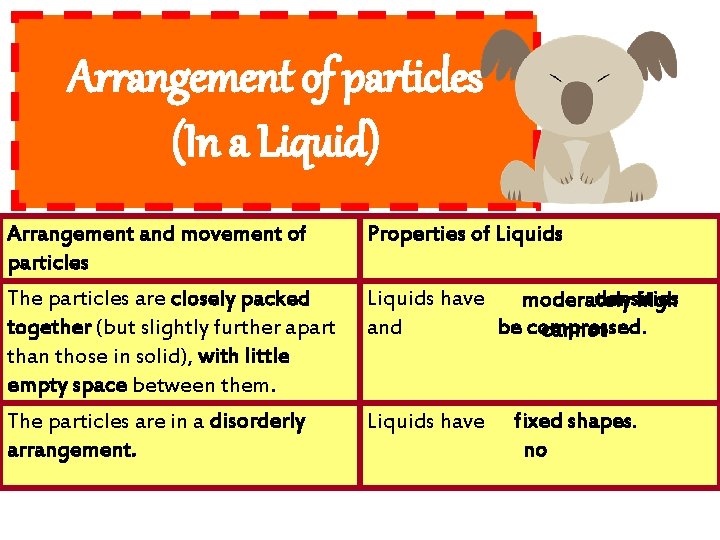
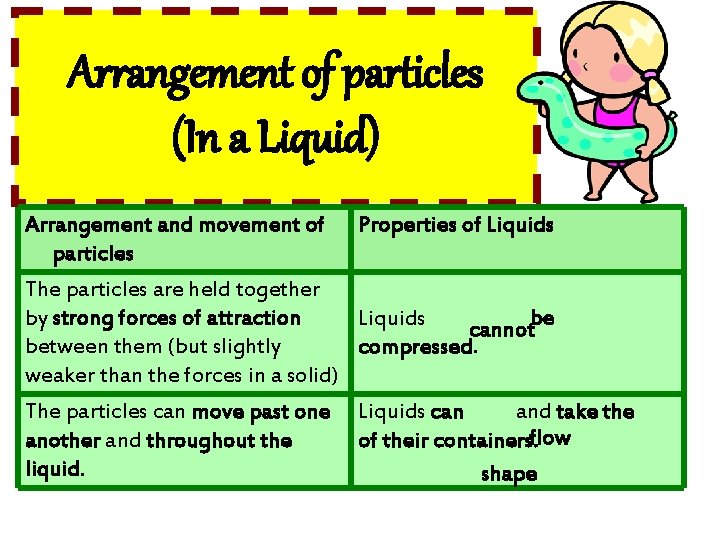
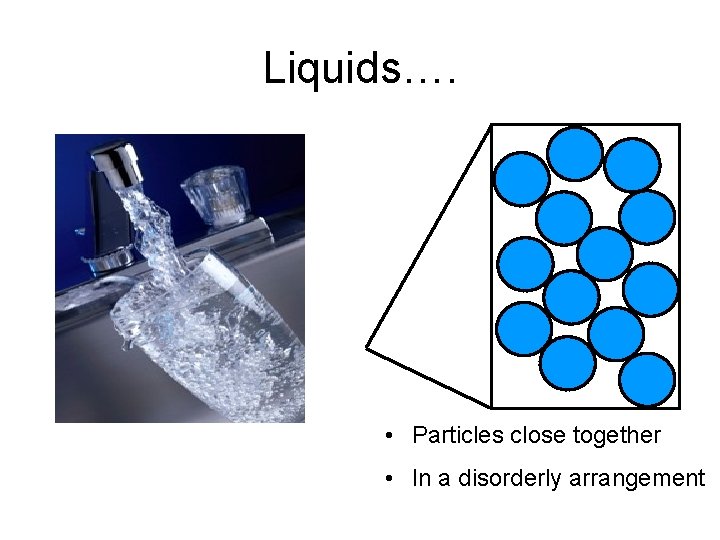
Arrangement of particles (In a Liquid) Arrangement and movement of particles Properties of Liquids The particles are closely packed together (but slightly further apart than those in solid), with little empty space between them. Liquids have densities moderately high and be compressed. cannot The particles are in a disorderly arrangement. Liquids have fixed shapes. no

Arrangement of particles (In a Liquid) Arrangement and movement of Properties of Liquids particles The particles are held together by strong forces of attraction Liquids cannotbe between them (but slightly compressed. weaker than the forces in a solid) The particles can move past one another and throughout the liquid. Liquids can and take the of their containers. flow shape

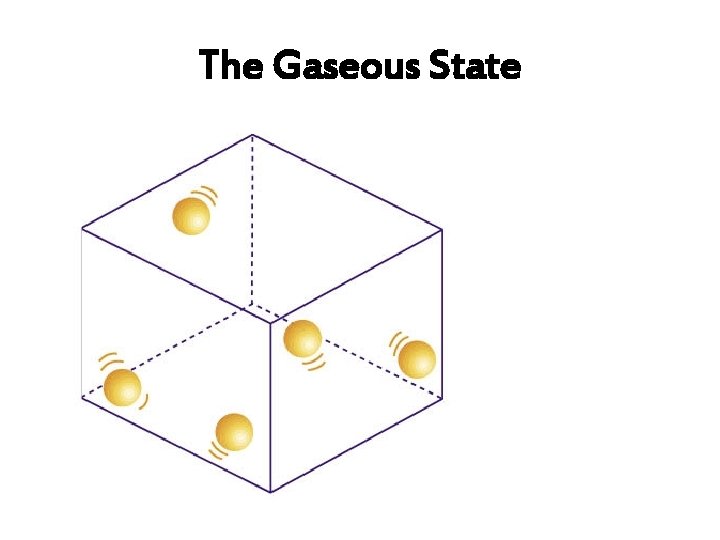
The Gaseous State

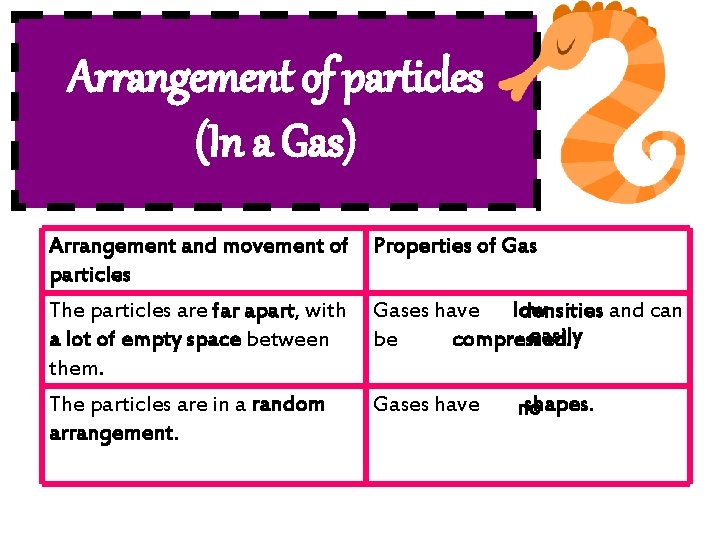
Arrangement of particles (In a Gas) Arrangement and movement of Properties of Gas particles The particles are far apart, with Gases have low densities and can easily a lot of empty space between be compressed. them. The particles are in a random arrangement. Gases have shapes. no

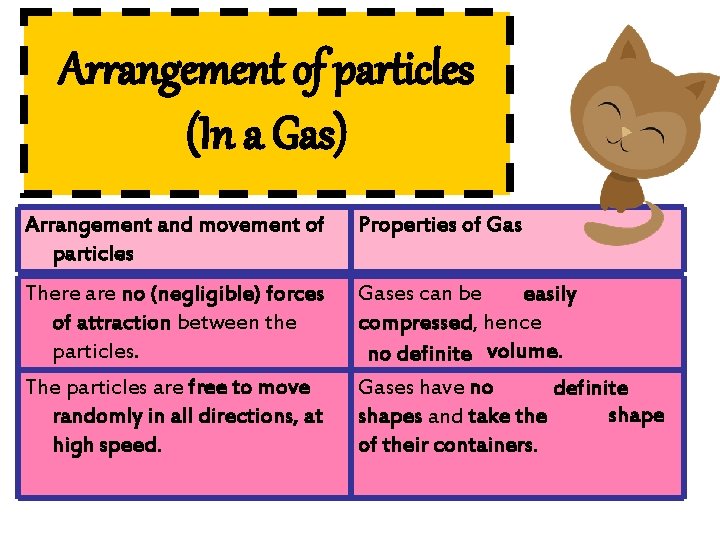
Arrangement of particles (In a Gas) Arrangement and movement of particles Properties of Gas There are no (negligible) forces of attraction between the particles. Gases can be easily compressed, hence no definite volume. Gases have no definite shapes and take the of their containers. The particles are free to move randomly in all directions, at high speed.


What we covered for today: Kinetic Particle Theory • Says that all matter consists of many, very small particles. • The particles are constantly moving or in a continual state of motion. • The particles might be atoms, molecules or ions.

Solids… • Particles close together • In an orderly arrangement

Solids…. • Particles vibrate only about fixed positions. • These vibrations increase as temperature increases. • The degree to which the particles move is determined by the amount of energy they have and their relationship to other particles. • The particles have less energy than particles in liquids and gases. • Strong forces between particles.

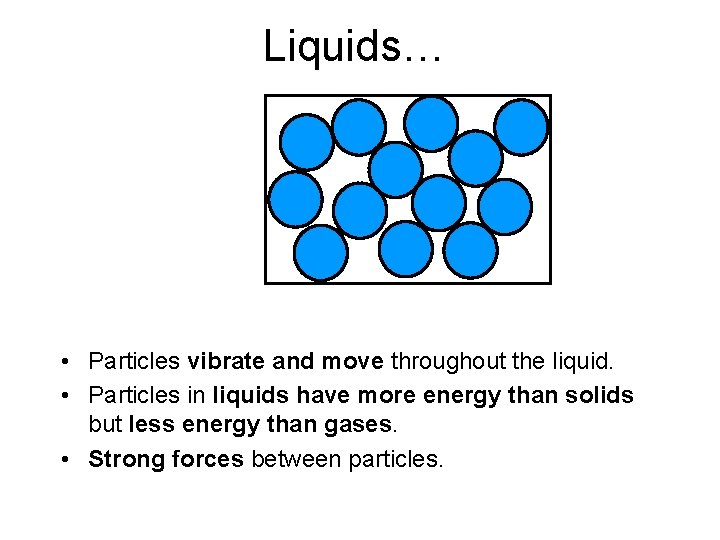
Liquids…. • Particles close together • In a disorderly arrangement

Liquids… • Particles vibrate and move throughout the liquid. • Particles in liquids have more energy than solids but less energy than gases. • Strong forces between particles.

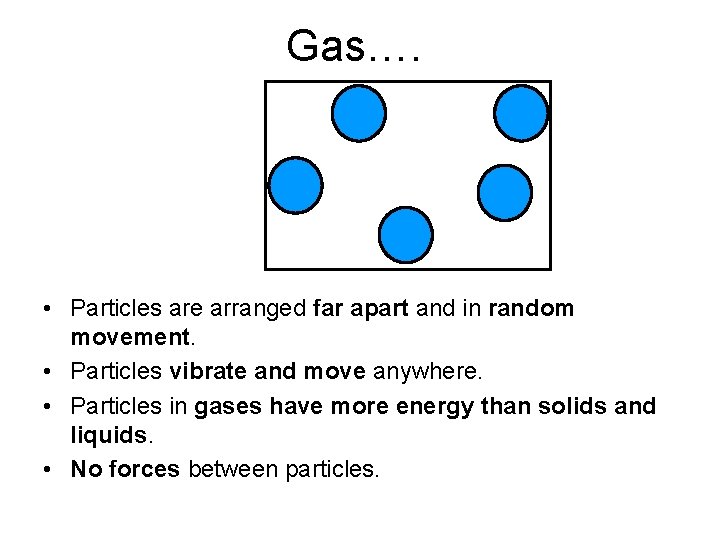
Gas…. • Particles are arranged far apart and in random movement. • Particles vibrate and move anywhere. • Particles in gases have more energy than solids and liquids. • No forces between particles.

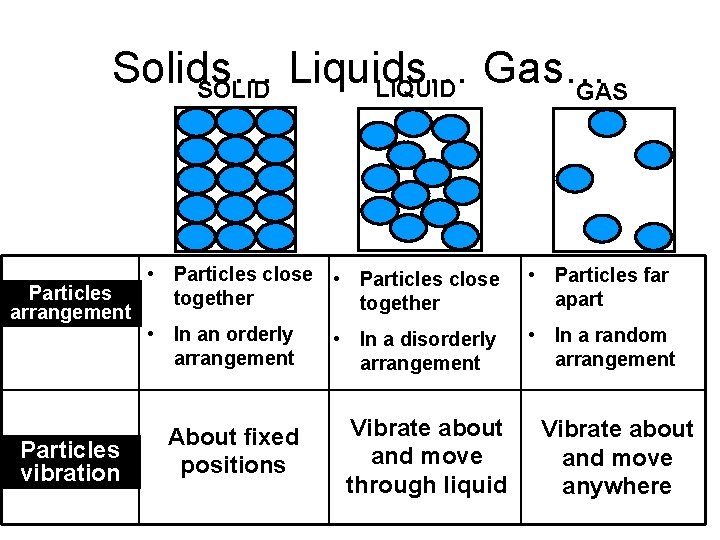
Solids… Liquids… Gas… LIQUID SOLID GAS • Particles close Particles together arrangement • In an orderly • In a disorderly arrangement Particles vibration About fixed positions Vibrate about and move through liquid • Particles far apart • In a random arrangement Vibrate about and move anywhere

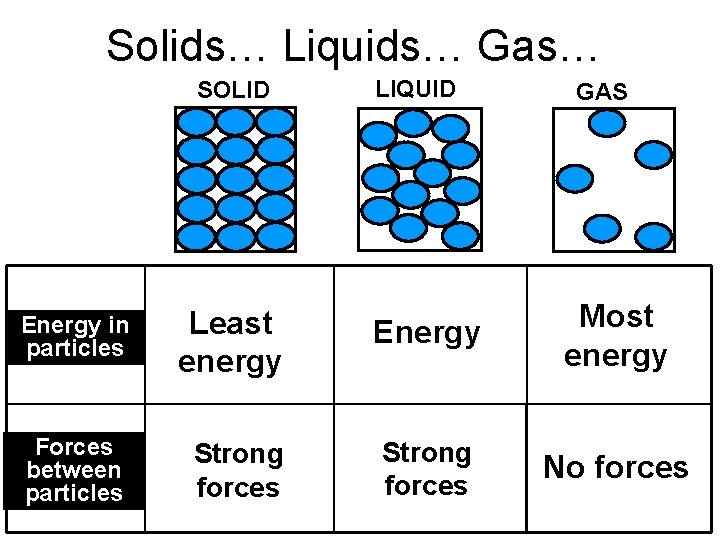
Solids… Liquids… Gas… SOLID LIQUID GAS Energy in particles Least energy Energy Most energy Forces between particles Strong forces No forces

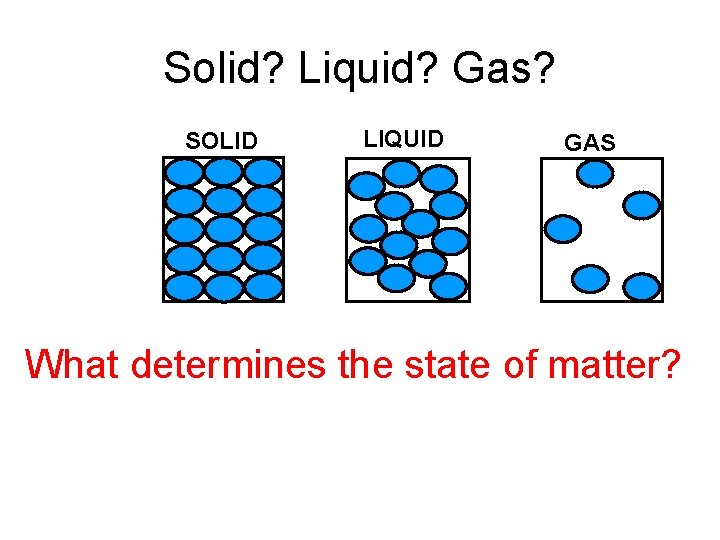
Solid? Liquid? Gas? SOLID LIQUID GAS What determines the state of matter?

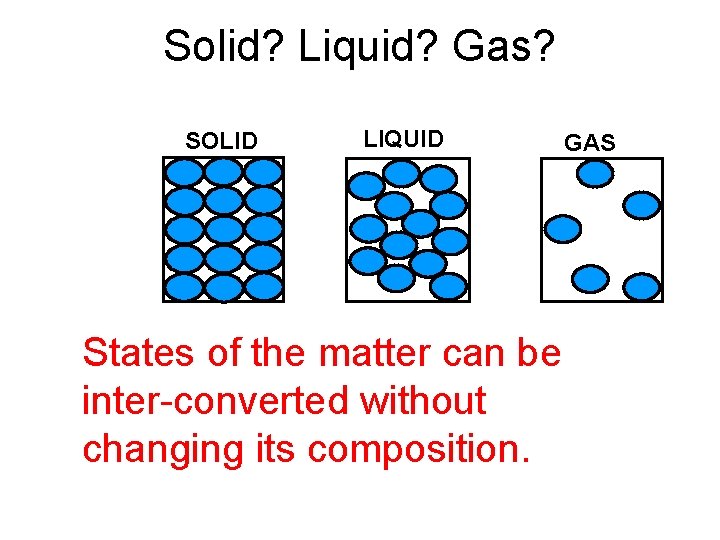
Solid? Liquid? Gas? SOLID LIQUID States of the matter can be inter-converted without changing its composition. GAS

Next Lesson In the next lesson, you will be learning on: ü Explain their inter-conversion of states in terms of the kinetic particle theory. ü Explain the inter-conversion of states in terms of the energy changes involved.

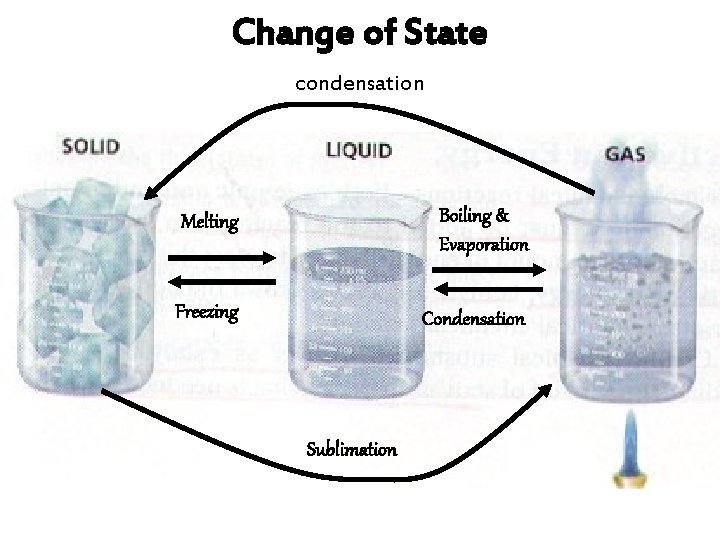
Change of State condensation Melting Boiling & Evaporation Freezing Condensation Sublimation

Changes of State and the Kinetic Particle Theory Melting, freezing, boiling and condensation are examples of changes of state.