Introduction to Chemistry Matter and Change Chapters 1









































- Slides: 57

Introduction to Chemistry Matter and Change Chapters 1 & 2

What is Chemistry? • The study of matter, its properties and the changes it underdoes What is Matter? • Anything that has mass and takes up space 2

What is Mass? • The amount of matter an object contains What is Volume? • The amount of space occupied by matter 3

Why Study Chemistry? • Helps us better understand nature • Helps us understand how things work (cleaning products, pharmaceuticals, shampoos, biology) • Can improve quality of life (plastics, permanent press, recycling processes, medical treatments) • Develops critical thinking skills, analytical skills, skills to organize and manage information 4

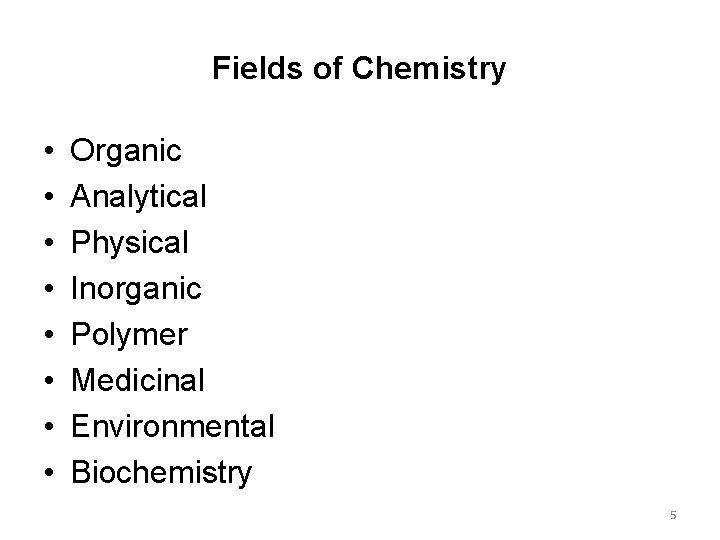
Fields of Chemistry • • Organic Analytical Physical Inorganic Polymer Medicinal Environmental Biochemistry 5

How We Study Chemistry • • Collect data (observations, measurements) Analyze data (tables, graphs, calculations) Interpret & Explain data Draw conclusions Evaluate data Design new experiments Understand evaluate what others have done • Develop models and theories • Identify laws 6

Scientific Method • • • Questions/Observations Hypothesis Design Experiment Collect Data Analyze Data Revise Hypothesis 7

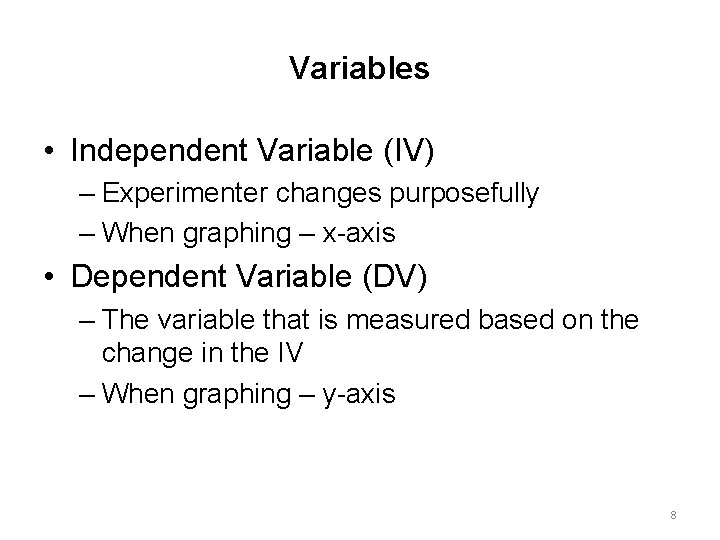
Variables • Independent Variable (IV) – Experimenter changes purposefully – When graphing – x-axis • Dependent Variable (DV) – The variable that is measured based on the change in the IV – When graphing – y-axis 8

Identify the IV and the DV 1. Cut paper towel into strips 2. Fill a container with water. 3. Place the strip into the water for a specified time interval (10, 20, 30, 40 sec) 4. At the end of the time interval mark the water level with a pencil 9

Experimental Design - Trials • Trial – repetition of the experiment for the same level of the IV • Improvement in experiment: – Increase the number of trials for each time limit from one to three. – Take average of the DV for each value of the IV 10

Theories and Laws Theory • Well-tested explanation for a broad set of observations Law • A statement that summarizes observations but does not explain them 11

What is a Model? • A representation • May or may not be to scale • May represent something very large or something very small • Models take many forms… a diagram, a graph, a physical model • Used to help explain a concept 12

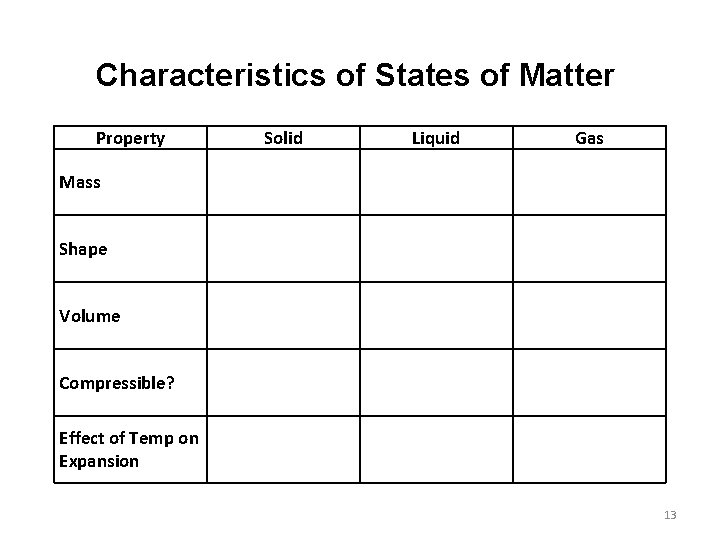
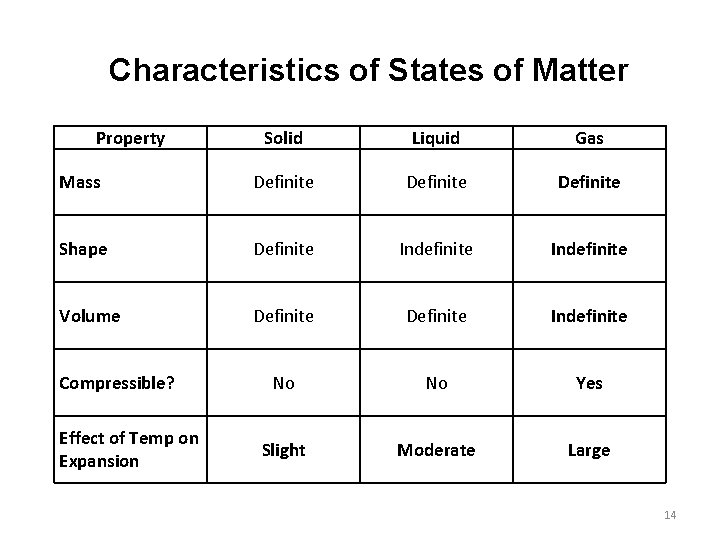
Characteristics of States of Matter Property Solid Liquid Gas Mass Shape Volume Compressible? Effect of Temp on Expansion 13

Characteristics of States of Matter Property Solid Liquid Gas Mass Definite Shape Definite Indefinite Volume Definite Indefinite No No Yes Slight Moderate Large Compressible? Effect of Temp on Expansion 14

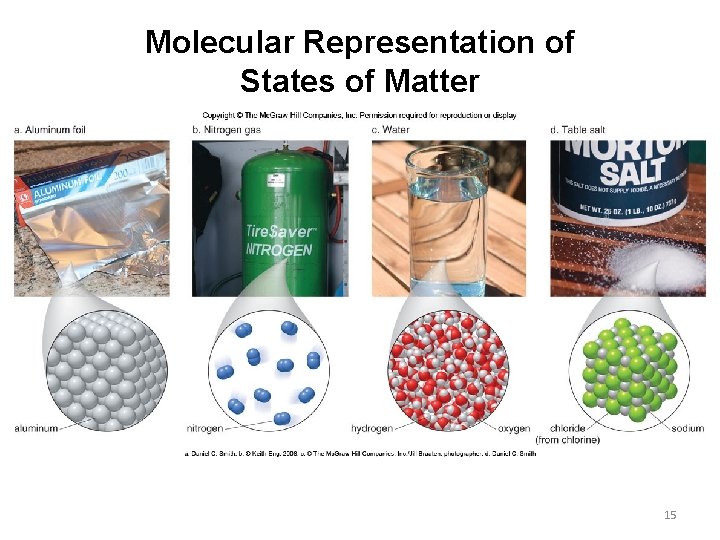
Molecular Representation of States of Matter 15

How we describe matter Substance • A sample of matter having a constant (uniform or definite) composition. It can be either an element or a compound. 16

How we describe matter Element • A substance that cannot be decomposed into simpler substances by chemical or physical means. It consists of atoms having the same atomic number. 17

How we describe matter Compound • A substance with constant composition that can be broken down into elements by chemical processes. 18

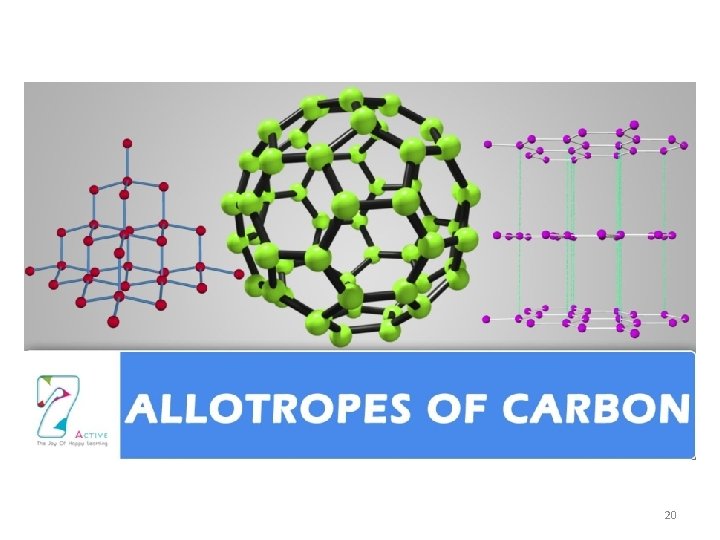
How we describe matter Allotrope • Different forms of the same element Example: Carbon: diamond, graphite, buckyballs Phosphorus: red, white, black 19

20

Allotropes of Phosphorus White phosphorus can be transformed to red, black or violet by in creasing temperature or pressure By Weißer_Phosphor. JPG: BXXXD at de. wikipedia Phosphor_rot. jpg: Tomihahndorf Phosphor-rot-violett. jpg: Maksim derivative work: Materialscientist [GFDL (http: //www. gnu. org/copyleft/fdl. html) or CC-BY-SA-3. 0 (http: //creativecommons. org/licenses/by-sa/3. 0/)], via Wikimedia Commons https: //commons. wikimedia. org/wiki/File%3 APhosph. Comby. jpg 21

How we describe matter - Particles Atom • Smallest particle of an element that retains the properties of that element; the fundamental unit of which elements are composed 22

How we describe matter - Particles Molecule • A bonded collection of two or more atoms of the same element or different elements. A representative particle of a molecular compound. Examples: Br 2, CH 4, H 2 O 23

Properties of Matter Extensive property • Depends upon the amount of matter in a sample • Examples – mass, volume Intensive property • Depends upon the type of matter, not the amount • Example – density, absorbancy 24

Properties of Matter Physical Property • A quality or condition of a substance that can be observed or measured without changing the substance’s composition 25

Examples of Physical Properties Density Boiling Point Melting Point Color Odor Luster State Solubility Ductility Malleability Conductivity Hardness 26

Properties of Matter Chemical Property • Ability to form new substances 27

Examples of Chemical Properties Reactivity – reacts, neutralizes Flammability – burns Note: The fact that a substance does NOT react is a chemical property 28

Physical or Chemical Property? • • Water melts at 0 o. C Propane burns in air Leaves turn color in the fall Lead is a very dense metal 29

Physical and Chemical Changes Physical Change • A change in the physical form of a substance but not its chemical composition; • Chemical bonds are not broken 30

Physical and Chemical Changes Chemical Change • The change of one substance into another substance through a reorganization of the atoms by breaking or making of chemical bonds; • A chemical reaction 31

Indicators of Chemical Change • • Unexpected color change Formation of a precipitate Evolution of a gas Change in energy 32

Differentiating Between Physical or Chemical Change • Physical change does not alter the composition of a substance though it may alter the appearance of the substance • Key words – Bend – Break – Crush – Dissolve Boil Melt Solidify Evaporate 33

Differentiating Between Physical or Chemical Change Examples Salt dissolves in water. Solubility is a physical property so the change is physical. A liquid boils and is converted from a liquid to a gas. Boiling point is a physical property. A change in state is a physical change. 34

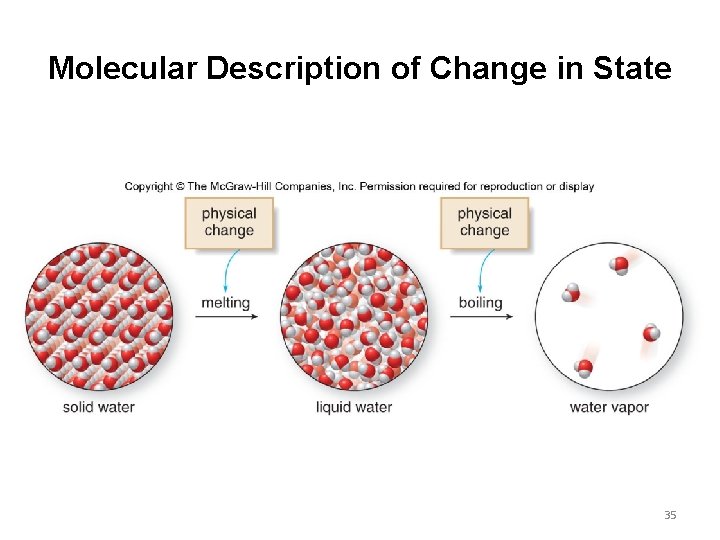
Molecular Description of Change in State 35

Differentiating Between Physical or Chemical Change Chemical change produces a different substance; composition and properties are different. Requires a chemical reaction to reclaim the starting material. Not always easily done! Reactants Products 36

Differentiating Between Physical or Chemical Change Key words that indicate a chemical change: Reacts Burn s Bubbles Fizzes Gives off Precipitates Explodes Emits A flame 37

Differentiating Between Physical or Chemical Change Examples • A match lights when struck • Substances are combined and light is emitted • An antacid tablet fizzes when added to water 38

How to differentiate between physical and chemical change • Physical change does not alter the composition of a substance although it may alter its appearance • Chemical change produces a different substance 39

Mixture A combination of two or more substances that are not chemically combined. The components of the mixture is variable (can be made up differently at different times). 40

Types of Mixtures Homogeneous Mixture: • Completely uniform in composition • Components are not distinguishable • A solution 41

Homogeneous Mixtures Examples: • Salt water • Stainless steel • Sugar in water • Gasoline • Brass • Clean air • Jello 42

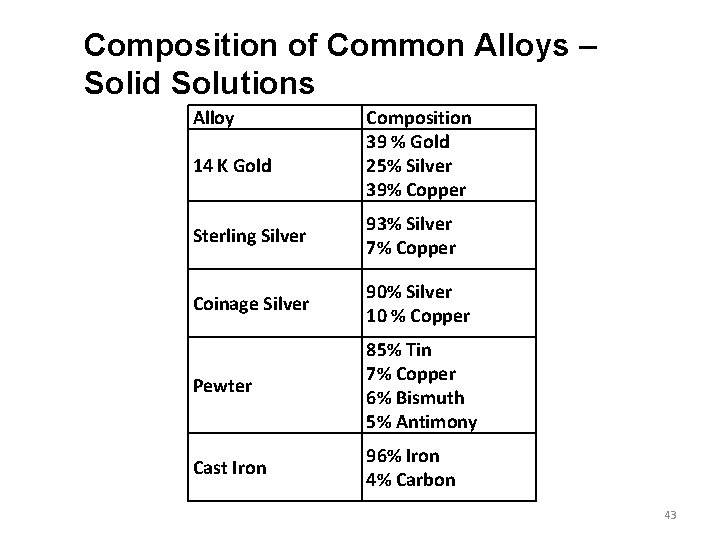
Composition of Common Alloys – Solid Solutions Alloy 14 K Gold Composition 39 % Gold 25% Silver 39% Copper Sterling Silver 93% Silver 7% Copper Coinage Silver 90% Silver 10 % Copper Pewter 85% Tin 7% Copper 6% Bismuth 5% Antimony Cast Iron 96% Iron 4% Carbon 43

Types of Mixtures Heterogeneous Mixture: • Not uniform in composition • Components are readily distinguishable • Different composition in different parts of the mixture 44

Heterogeneous Mixtures Examples: • Tossed salad • Soil • Granite 45

Mixture lingo Phase Any part of a system with uniform composition and properties. Homogeneous mixtures consist of one phase Heterogeneous mixtures consist of two or more phases (e. g. , vinegar and oil) 46

Classification of Matter • Differentiate whether a sample is a substance (element or compound) or a mixture (homogeneous or heterogeneous) 47

Separation of Mixtures • Mixtures of substances can be separated by physical means. The mixtures may be homogeneous or heterogeneous. One or more processes may be required to separate mixtures. • Make use of type of mixture and physical properties of its components to select the best means of separation. 48

Techniques for Separation of Mixtures • • • Filtration Evaporation Extraction Chromatography Distillation Decantation Sieve Magnet Centrifuge Forceps 49

Examples • • Water and salt Water and ethanol Salt and sand Sand wood chips 50

How are physical & chemical properties used? • • Identification Reactivity Separation of mixtures State of substance under certain conditions 51

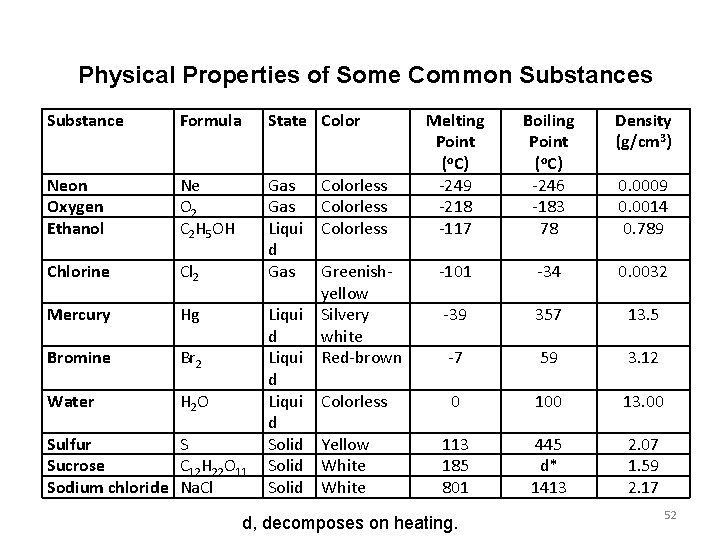
Physical Properties of Some Common Substances Substance Formula State Color Neon Oxygen Ethanol Ne O 2 C 2 H 5 OH Chlorine Cl 2 Gas Liqui d Gas Mercury Hg Bromine Br 2 Water H 2 O Sulfur S Sucrose C 12 H 22 O 11 Sodium chloride Na. Cl Liqui d Solid Colorless Greenishyellow Silvery white Red-brown Colorless Yellow White Melting Point (o. C) -249 -218 -117 Boiling Point (o. C) -246 -183 78 Density (g/cm 3) -101 -34 0. 0032 -39 357 13. 5 -7 59 3. 12 0 100 13. 00 113 185 801 445 d* 1413 2. 07 1. 59 2. 17 d, decomposes on heating. 0. 0009 0. 0014 0. 789 52

Changes in State 53

Given physical properties and current temperature, what is the state of the substance? BP MP SOLID LIQUID GAS Increasing Temperature 54

Water: mp = 0 o. C, bp = 100 o. C If temperature is 45 o. C, what is state of water? (liquid) If temperature is -29 o. C, what is state of water? (solid) If temperature is 150 o. C, what is state of water? (gas) 55

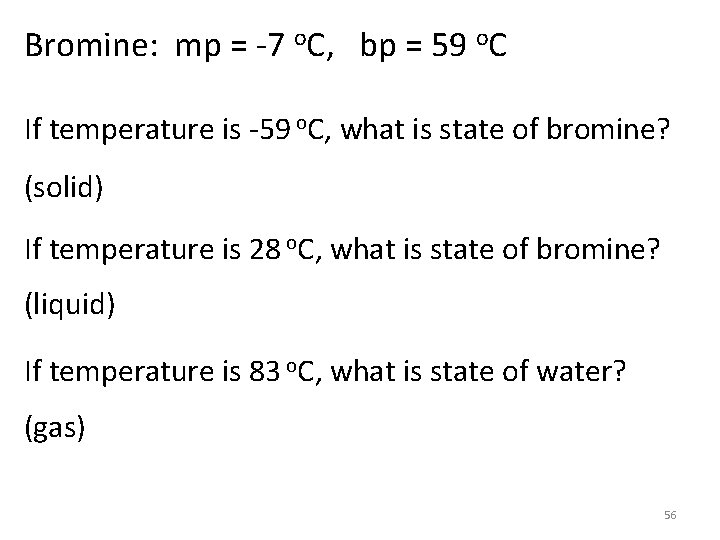
Bromine: mp = -7 o. C, bp = 59 o. C If temperature is -59 o. C, what is state of bromine? (solid) If temperature is 28 o. C, what is state of bromine? (liquid) If temperature is 83 o. C, what is state of water? (gas) 56

Symbols and Formulas • Element or chemical symbols Al = Aluminum C = Carbon Pb = Lead Many symbols based on Latin or German names 57
 Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chapter 10 the mole study guide
Chapter 10 the mole study guide 1s 22 s22 p63 s23 p64 s2
1s 22 s22 p63 s23 p64 s2 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chapter 10 study guide the mole
Chapter 10 study guide the mole 2 matter and change answer key
2 matter and change answer key Organic chemistry chapter 1 problem 59pp
Organic chemistry chapter 1 problem 59pp Gray matter
Gray matter Brain falx
Brain falx Gray matter and white matter
Gray matter and white matter Matter
Matter Chemistry matter and its changes
Chemistry matter and its changes Section 1 composition of matter
Section 1 composition of matter Composition of matter section 1
Composition of matter section 1 Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Energy naturally flows from warmer matter to cooler matter
Energy naturally flows from warmer matter to cooler matter Chapter 1 review matter and change
Chapter 1 review matter and change Changes in matter grade 5
Changes in matter grade 5 Unit 2 matter and change
Unit 2 matter and change Circle the solute and underline the solvent
Circle the solute and underline the solvent Examples of matter in chemistry
Examples of matter in chemistry Matter flowchart
Matter flowchart Flowchart undissolved solids
Flowchart undissolved solids Mixture graphic organizer
Mixture graphic organizer Non examples of homogeneous mixture
Non examples of homogeneous mixture Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Basic food chemistry the nature of matter
Basic food chemistry the nature of matter Functional groups ib chemistry
Functional groups ib chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Change in state of matter
Change in state of matter Two types of changes in matter
Two types of changes in matter What is physical change
What is physical change Absolute change and relative change formula
Absolute change and relative change formula Difference between chemical and physical change
Difference between chemical and physical change Supply and demand curve shifts
Supply and demand curve shifts Physical vs chemical change examples
Physical vs chemical change examples Rocks change due to temperature and pressure change
Rocks change due to temperature and pressure change Whats chemical change
Whats chemical change Second order change
Second order change Fusion chemistry phase change
Fusion chemistry phase change Examples for physical change
Examples for physical change What is integer
What is integer A change in supply vs a change in quantity supplied
A change in supply vs a change in quantity supplied Enagic comp plan
Enagic comp plan Proactive and reactive change
Proactive and reactive change Spare change physical versus chemical change
Spare change physical versus chemical change Physical change
Physical change Chemical change in baking
Chemical change in baking Is a magnet sticking to the refrigerator a chemical change
Is a magnet sticking to the refrigerator a chemical change Climate change 2014 mitigation of climate change
Climate change 2014 mitigation of climate change Introduction to clinical chemistry
Introduction to clinical chemistry Common inorganic pharmaceutical compounds
Common inorganic pharmaceutical compounds Organic vs inorganic compounds
Organic vs inorganic compounds Chemistry goals and objectives
Chemistry goals and objectives Patrick: an introduction to medicinal chemistry 3e
Patrick: an introduction to medicinal chemistry 3e How to determine number of protons
How to determine number of protons Chapter 1 introduction to chemistry
Chapter 1 introduction to chemistry