States of Matter Poster SOLID LIQUID GAS PLASMA











- Slides: 30

States of Matter Poster SOLID LIQUID GAS PLASMA Pic Pic pic

Heating/Cooling of Molecules 1. Describe what the colors looked like and how they moved and mixed in the cold water. 2. Describe what the colors looked like and how they moved and mixed in the hot water. 3. What does the speed of the mixing colors tell you about the speed of the molecules in hot and cold water?

States of Matter There are five states of matter: 1 – Solid 2– Liquid 3 – Gas 4 – Plasma 5 – Bose - Einstein

States of Matter -- Solid Charateristics: § Definite shape § Definite volume § Do not easily compress or expand § Very strong energy binding particles

States of Matter -- Solid Distances between atoms or molecules: Particles are tightly packed together

States of Matter -- Solid Movement of Atoms or molecules Particles vibrate back and forth

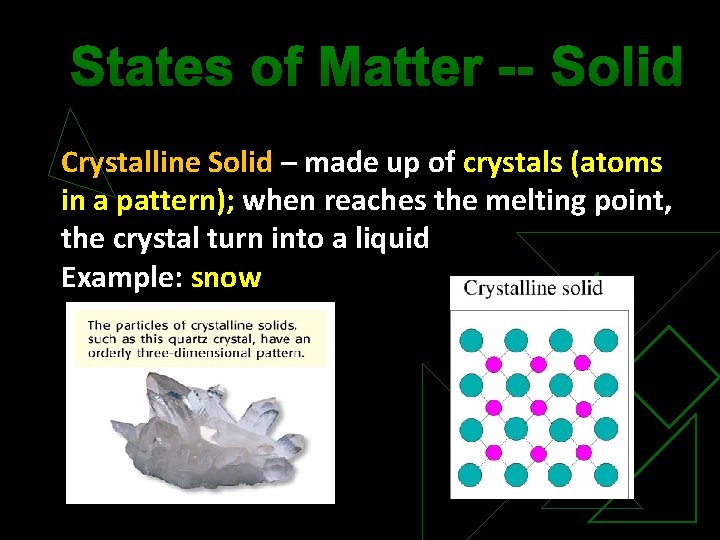
States of Matter -- Solid Crystalline Solid – made up of crystals (atoms in a pattern); when reaches the melting point, the crystal turn into a liquid Example: snow

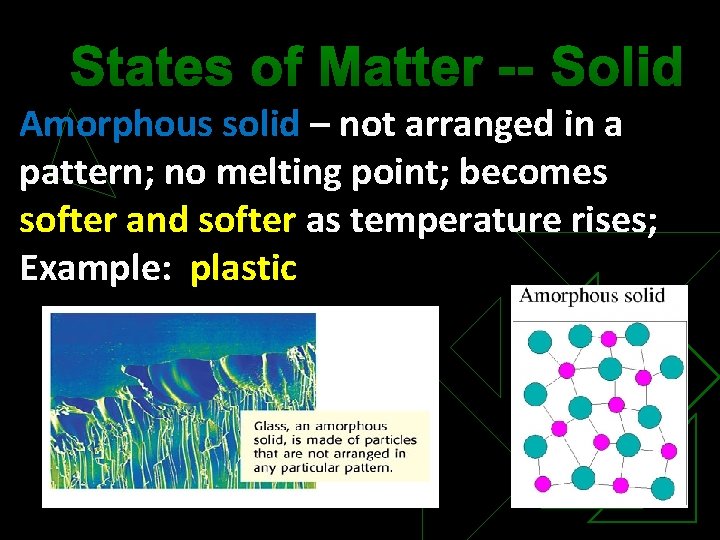
States of Matter -- Solid Amorphous solid – not arranged in a pattern; no melting point; becomes softer and softer as temperature rises; Example: plastic

http: //www. harcourtschool. com/activity/states_of_matter/ Phet States of Matter Annimation

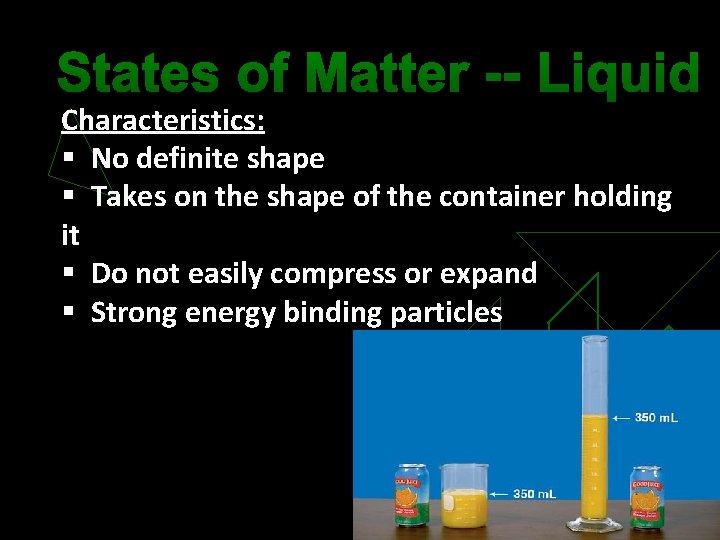
States of Matter -- Liquid Characteristics: § No definite shape § Takes on the shape of the container holding it § Do not easily compress or expand § Strong energy binding particles

States of Matter -- Liquid Distances between atoms or molecules: Even though particles move freely, they stay close to each other (almost as close as in a solid)

States of Matter -- Liquid Movement of Atoms or molecules Particles are free to move

http: //www. harcourtschool. com/activity/states_of_matter/ Phet States of Matter Annimation

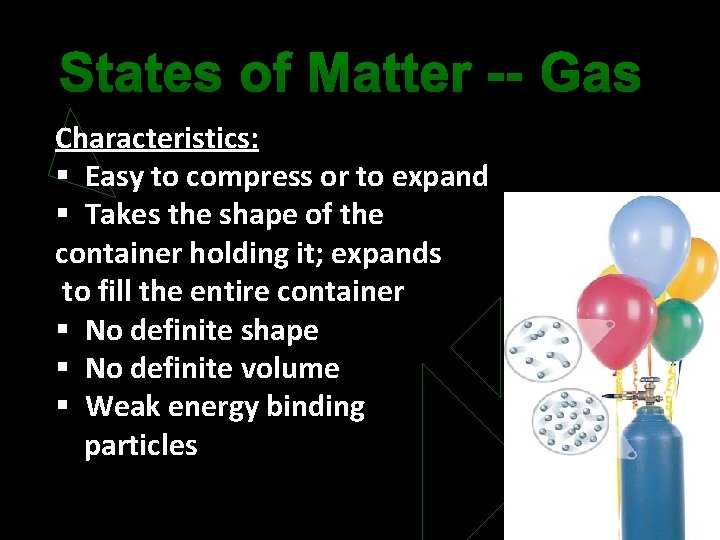
States of Matter -- Gas Characteristics: § Easy to compress or to expand § Takes the shape of the container holding it; expands to fill the entire container § No definite shape § No definite volume § Weak energy binding particles

States of Matter -- Gas Distances between atoms or molecules: particles move more freely than liquids

States of Matter -- Gas Movement of Atoms or molecules Particles are free to move at high speeds.

http: //www. harcourtschool. com/activity/states_of_matter/ Phet States of Matter Annimation

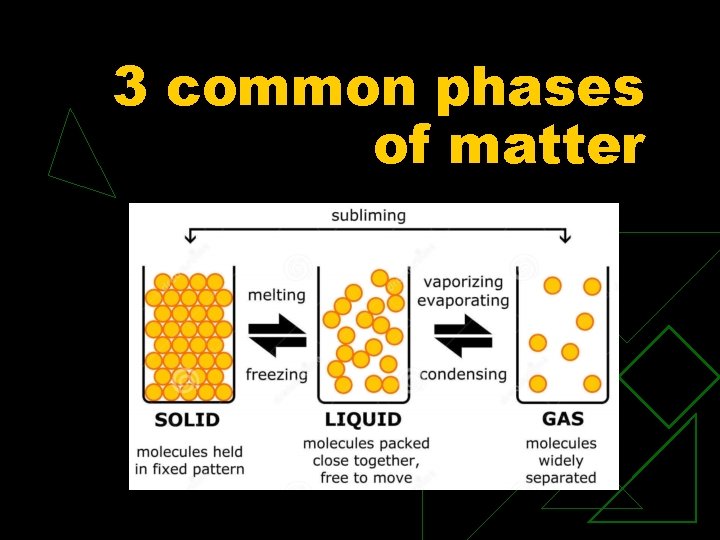
3 common phases of matter

States of Matter -- Plasma Characteristics: § Atoms are stripped of their electrons § Nuclei are packed close together § Occurs at extremely high temperatures (like inside a star)

States of Matter -- Plasma Distances between atoms or molecules: Nuclei are close together and moving fast enough to collide with one another; fusion can take place (two nuclei combine to form a new larger nuclei

States of Matter -- Plasma Movement of Atoms or molecules Nuclei and electrons are moving at incredibly fast speeds but they are held close together


States of Matter – Bose-Einstein http: //www. chem 4 kids. com/files/matter_beconden sate. html

BOSE-EINSTEIN CONDENSATE (B. E. C) u Bose-Einstein condensate (BEC) are super-unexcited and super-cold atoms.

Note: The word “point” is added to each change type to refer to the temperature at which the change takes place

CHANGE BETWEEN SOLIDS AND LIQUIDS Melting -- change from a solid to a liquid Freezing– change from liquid to solid

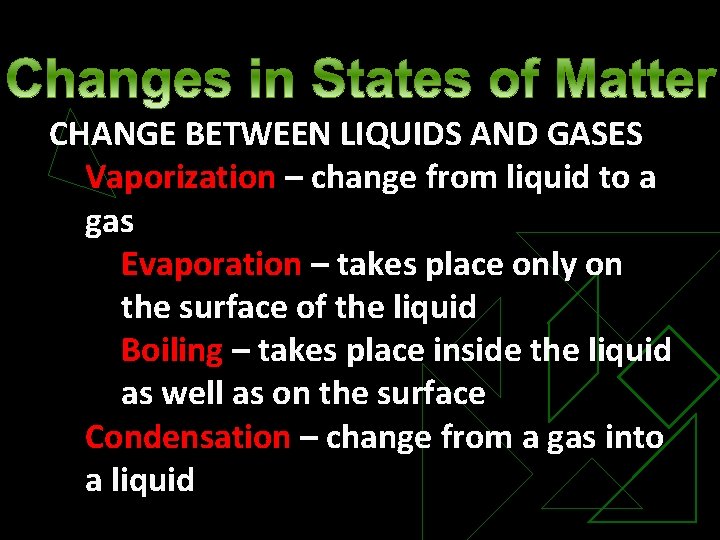
CHANGE BETWEEN LIQUIDS AND GASES Vaporization – change from liquid to a gas Evaporation – takes place only on the surface of the liquid Boiling – takes place inside the liquid as well as on the surface Condensation – change from a gas into a liquid

CHANGES BETWEEN SOLIDS AND GASES Sublimation – solid particles “jump” directly to the gas state without first becoming a liquid

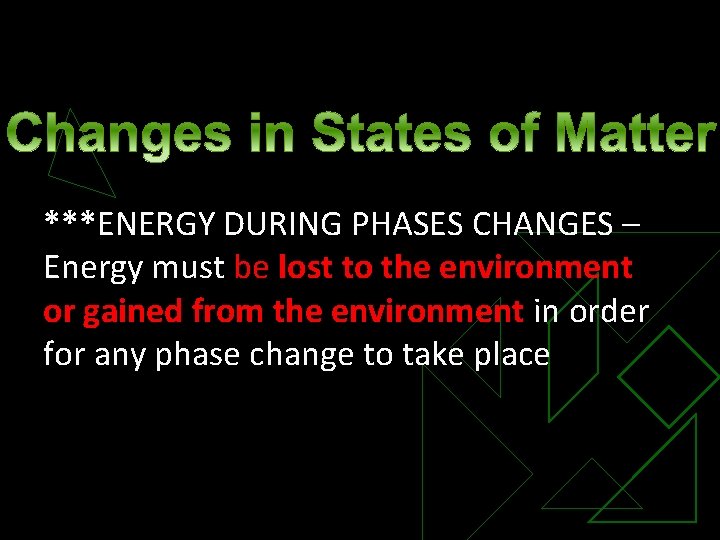
***ENERGY DURING PHASES CHANGES – Energy must be lost to the environment or gained from the environment in order for any phase change to take place

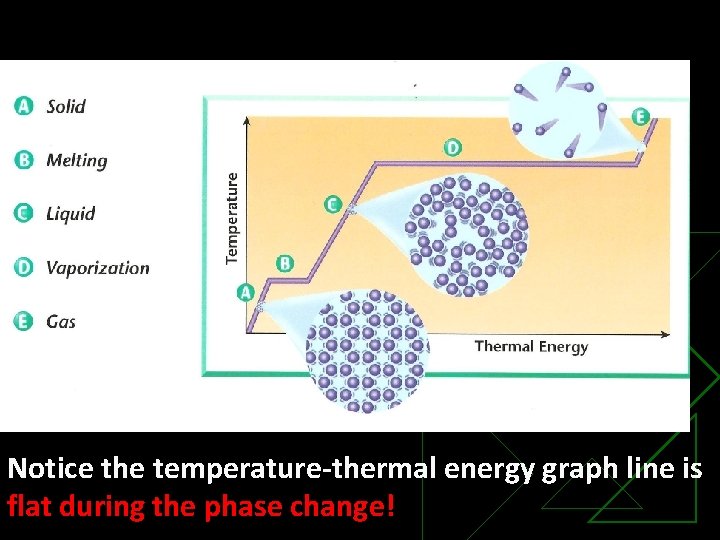
Notice the temperature-thermal energy graph line is flat during the phase change!