The Particle Theory of Matter The Particle Theory





- Slides: 16

The Particle Theory of Matter

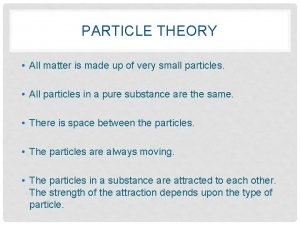
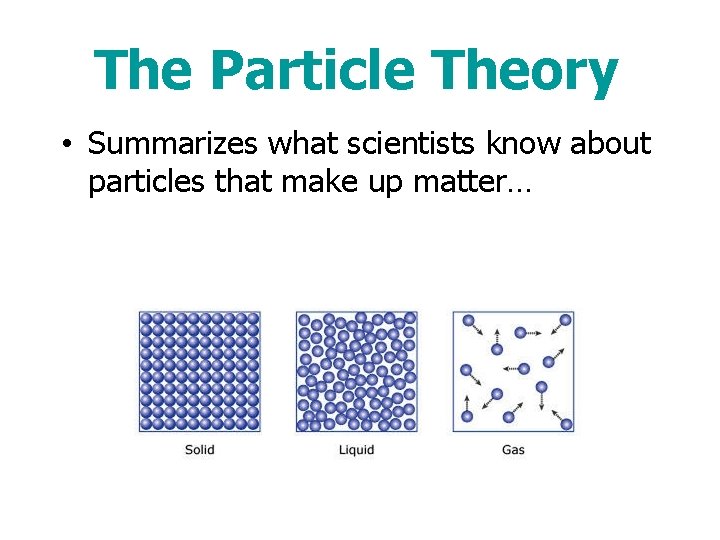
The Particle Theory • Summarizes what scientists know about particles that make up matter…

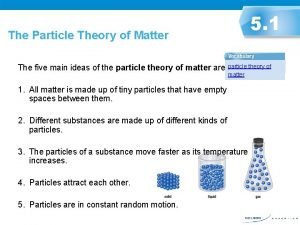
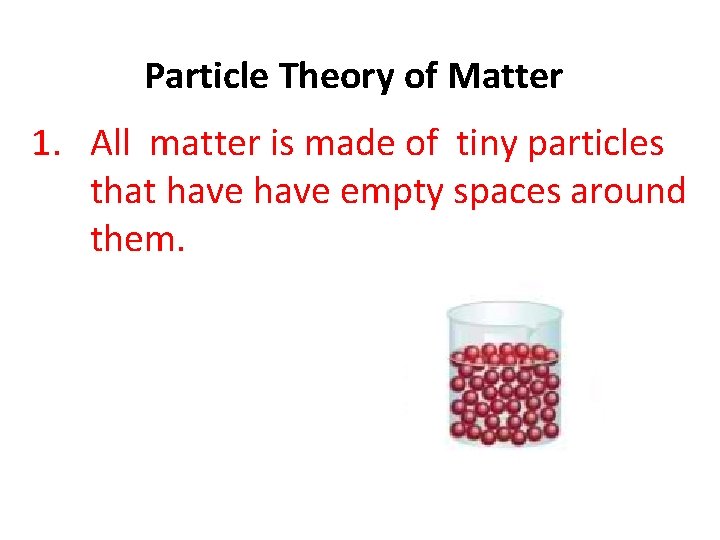
Particle Theory of Matter 1. All matter is made of tiny particles that have empty spaces around them.

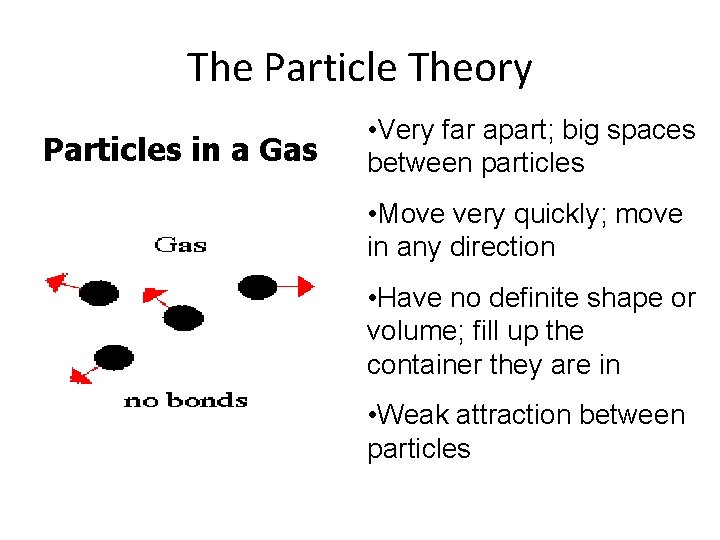
The Particle Theory Particles in a Gas • Very far apart; big spaces between particles • Move very quickly; move in any direction • Have no definite shape or volume; fill up the container they are in • Weak attraction between particles

The Particle Theory Particles in a Liquid • Particles are closer together; move more slowly • Particles in a liquid are loosely bonded so they slide over each other • Liquids have a definite volume, but not shape

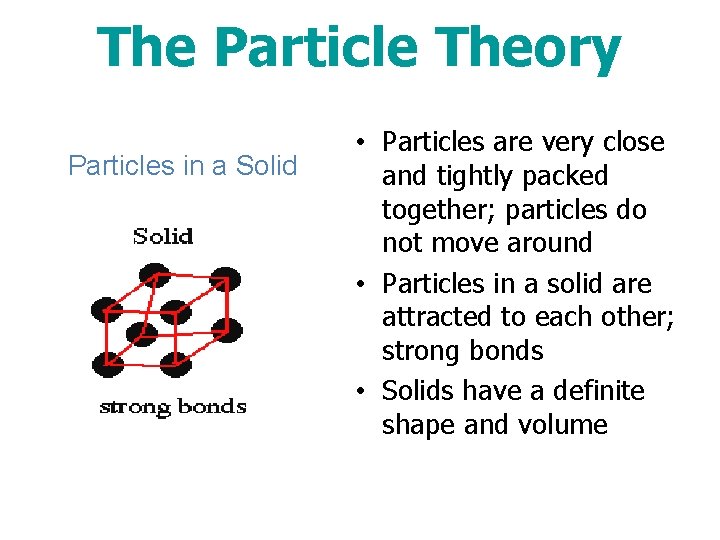
The Particle Theory Particles in a Solid • Particles are very close and tightly packed together; particles do not move around • Particles in a solid are attracted to each other; strong bonds • Solids have a definite shape and volume

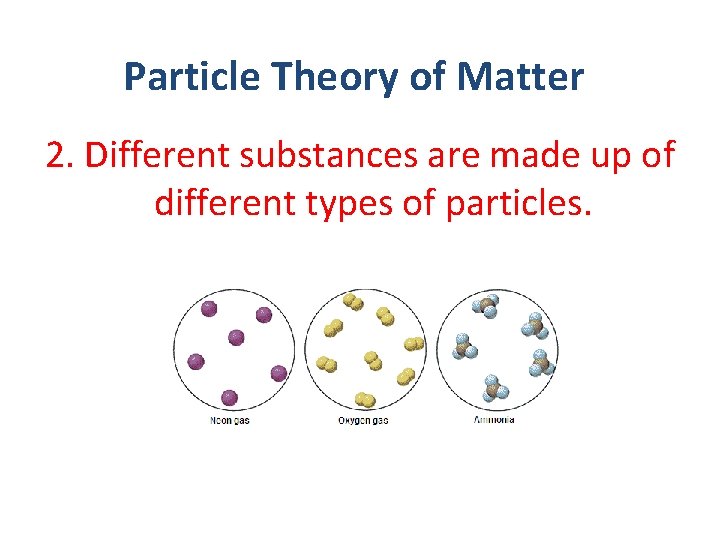
Particle Theory of Matter 2. Different substances are made up of different types of particles.

Particle Theory of Matter 3. Particles are always in constant random motion.

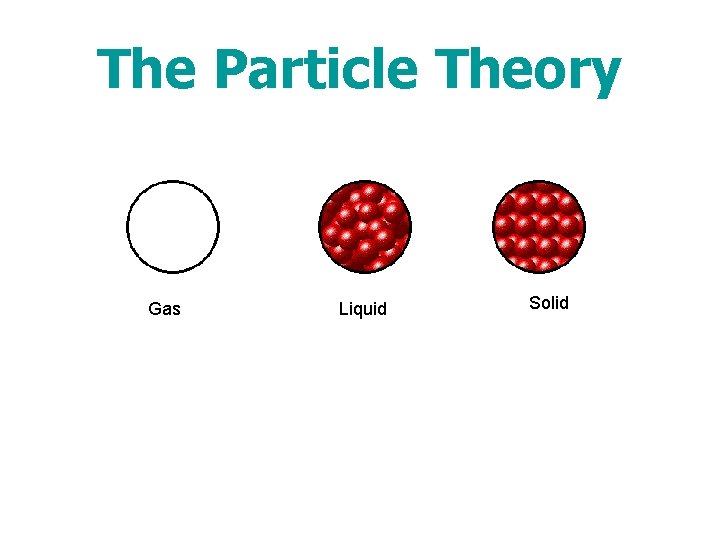
The Particle Theory Gas Liquid Solid

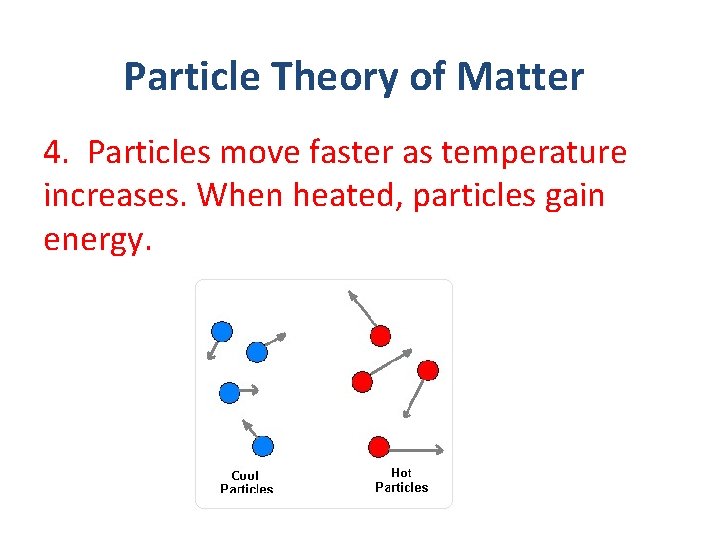
Particle Theory of Matter 4. Particles move faster as temperature increases. When heated, particles gain energy.

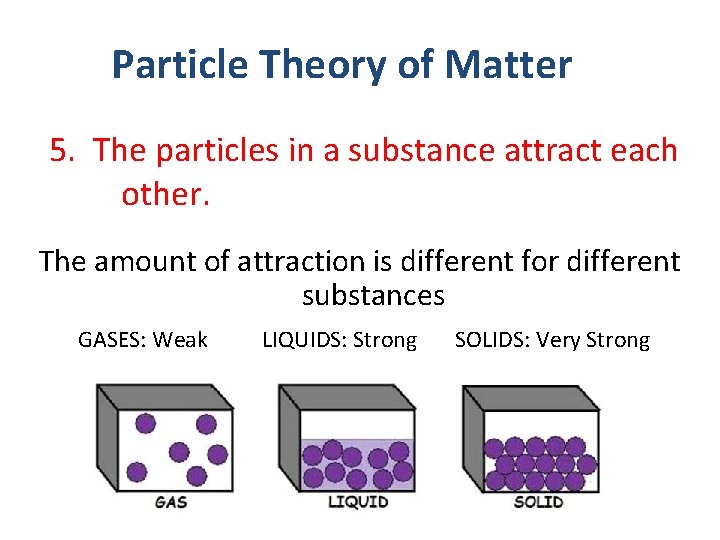
Particle Theory of Matter 5. The particles in a substance attract each other. The amount of attraction is different for different substances GASES: Weak LIQUIDS: Strong SOLIDS: Very Strong

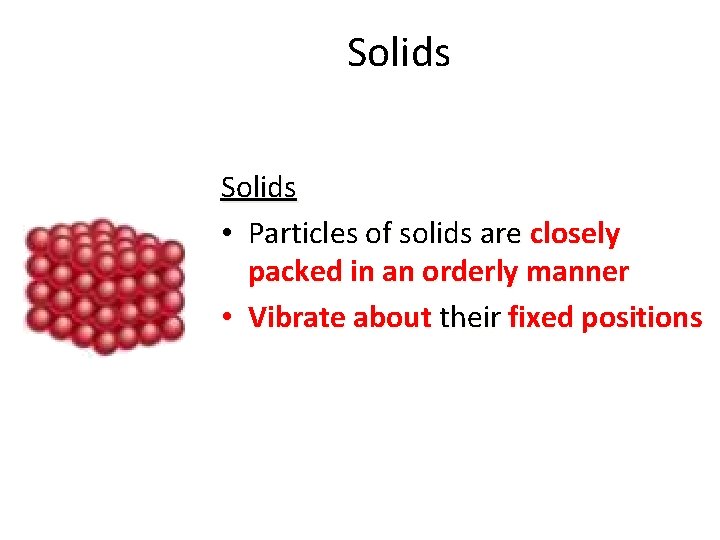
Solids • Particles of solids are closely packed in an orderly manner • Vibrate about their fixed positions

Liquids • Particles of a liquid are closely packed in a disorderly pattern • The particles move past one another

Gases • Particles of a gas are spread far apart from one another • The attraction between the particles is weak

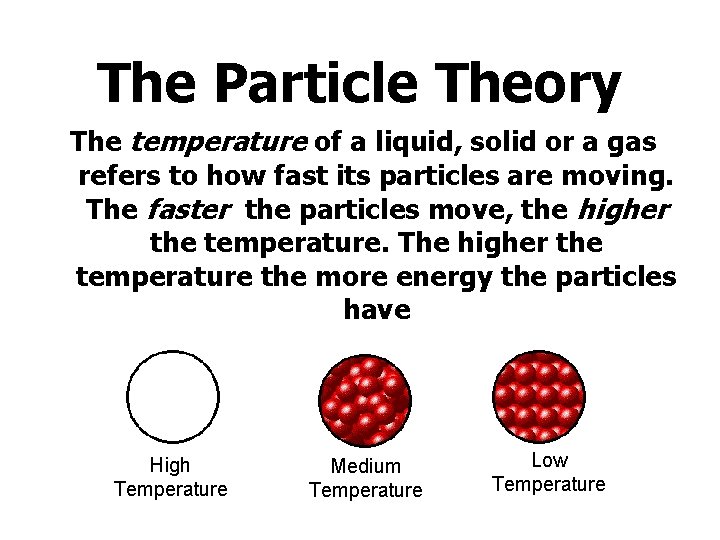
The Particle Theory The temperature of a liquid, solid or a gas refers to how fast its particles are moving. The faster the particles move, the higher the temperature. The higher the temperature the more energy the particles have High Temperature Medium Temperature Low Temperature

Chemistry…a volatile history • http: //vimeo. com/23948902
 Tightly packed in a disorderly manner
Tightly packed in a disorderly manner State of matter in chemical equations
State of matter in chemical equations Define particle theory of matter
Define particle theory of matter Particle theory of matter
Particle theory of matter Particle theory
Particle theory What is matter?
What is matter? Particle theory grade 9
Particle theory grade 9 Particle theory dissolving
Particle theory dissolving Kinetic particle theory o level questions
Kinetic particle theory o level questions Particle model of matter exam questions
Particle model of matter exam questions Whats the smallest particle of matter
Whats the smallest particle of matter Whats the smallest particle of matter
Whats the smallest particle of matter Particle diagram of a solid
Particle diagram of a solid Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Koosha golmohammadi
Koosha golmohammadi Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Chapter 2 matter section 1 classifying matter answer key
Chapter 2 matter section 1 classifying matter answer key