Adsorption ion exchange and chromatography 1 Adsorption processes
































![Fixed bed considerations: Design Fixed bed: G’ [kg/m 2 s], Y=0 G’ [kg/m 2 Fixed bed considerations: Design Fixed bed: G’ [kg/m 2 s], Y=0 G’ [kg/m 2](https://slidetodoc.com/presentation_image_h2/57d1e7f878c1c6d2d8673251b18f0fc3/image-97.jpg)



- Slides: 113

Adsorption, ion exchange, and chromatography 1

Adsorption processes Recommended reading: R. Treybal, Mass-Transfer operations, Chapter 11 Diran Basmadjian, Little adsorption book

Unit Operations: - Unit Operations is a method of analysis and design of chemical engineering processes in terms of individual tasks/operations - It is a way of organizing chemical engineering knowledge into groups of individual tasks/operations - A unit operation: basic step in a chemical engineering process

Introduction are sorption operations, in which certain components of a fluid phase, called solutes, are selectively transferred to insoluble, rigid particles suspended in a vessel or packed in a column. Sorption includes selective transfer to the surface and/or into the bulk of a solid or liquid. q. The substance that is transferred to the surafce is the adsorbate. q. The material on which the adsorbate deposits is the adsorbent. 4

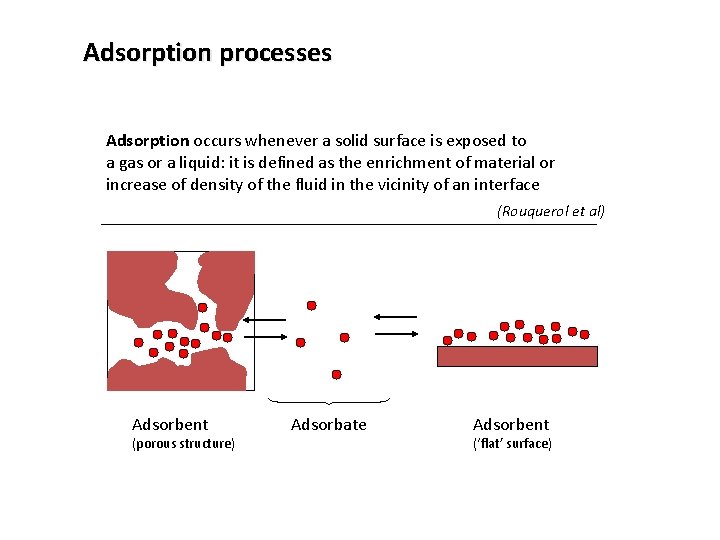
Adsorption processes Adsorption occurs whenever a solid surface is exposed to a gas or a liquid: it is defined as the enrichment of material or increase of density of the fluid in the vicinity of an interface (Rouquerol et al) Adsorbent (porous structure) Adsorbate Adsorbent (‘flat’ surface)

Industrial Example Pressure-swing adsorption for the dehydration of air 6

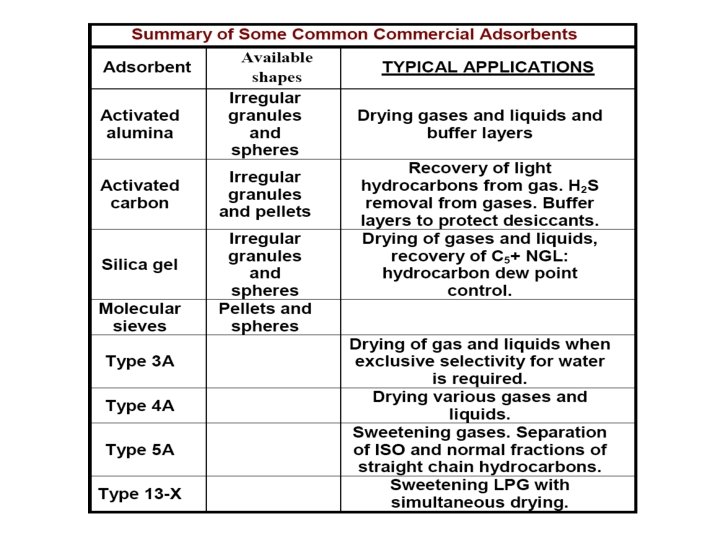
SORBENTS (I) high selectivity to enable sharp separations (II) (2) high capacity to minimize the amount of sorbent needed (III) (3) favorable kinetic and transport properties for rapid sorption (4) chemical and thermal stability (5) hardness and mechanical strength (6) a free-flowing tendency for ease of filling or emptying vessels (7) high resistance to fouling for long life (8) no tendency to promote undesirable chemical reactions (9) the capability of being regenerated (10) relatively low cost. 7

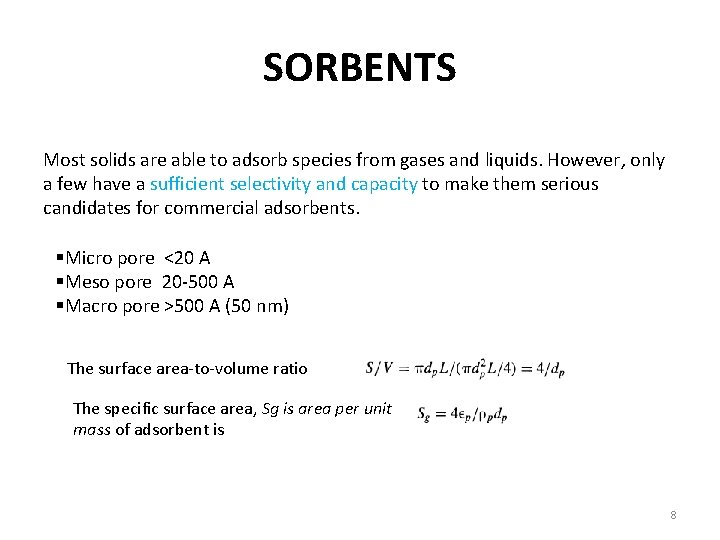
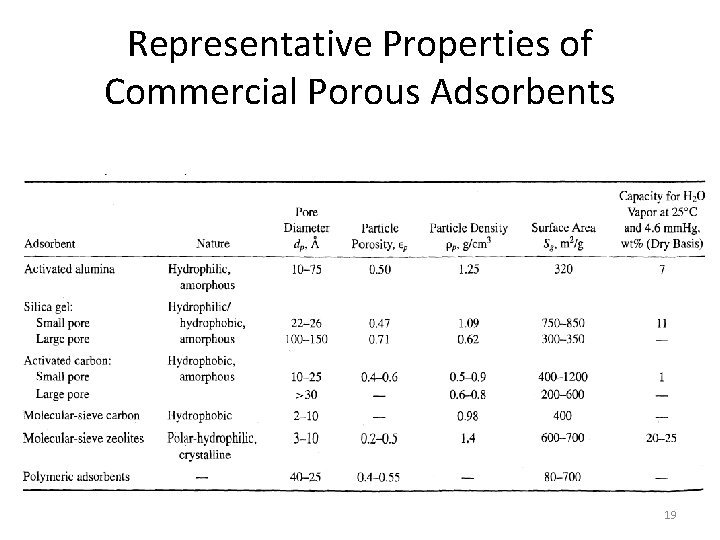
SORBENTS Most solids are able to adsorb species from gases and liquids. However, only a few have a sufficient selectivity and capacity to make them serious candidates for commercial adsorbents. §Micro pore <20 A §Meso pore 20 -500 A §Macro pore >500 A (50 nm) The surface area-to-volume ratio The specific surface area, Sg is area per unit mass of adsorbent is 8

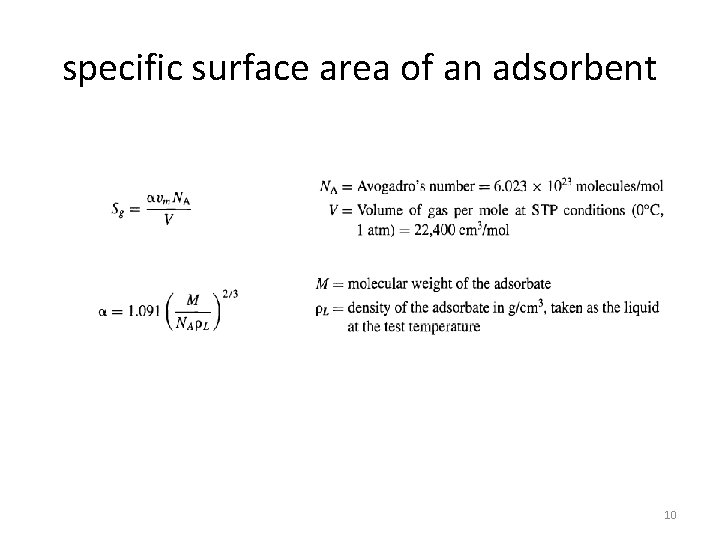
specific surface area of an adsorbent Sg is measured by adsorbing gaseous nitrogen. Typically, the BET apparalus operates at the normal boiling point of N 2 (-195. 8°C) by measuring the equilibrium volume of pure N 2 physically adsorbed on several grams of the adsorbent at a number of different values of the total pressure in the vacuum range of 5 to at least 250 mm. Hg. Brunauer, Emmett, and assumed that the heat of adsorption during monolayer formation is constant and that the heat effect associated with subsequent layers is equal to the heat of condensation. The BET equation is 9

specific surface area of an adsorbent 10









Representative Properties of Commercial Porous Adsorbents 19

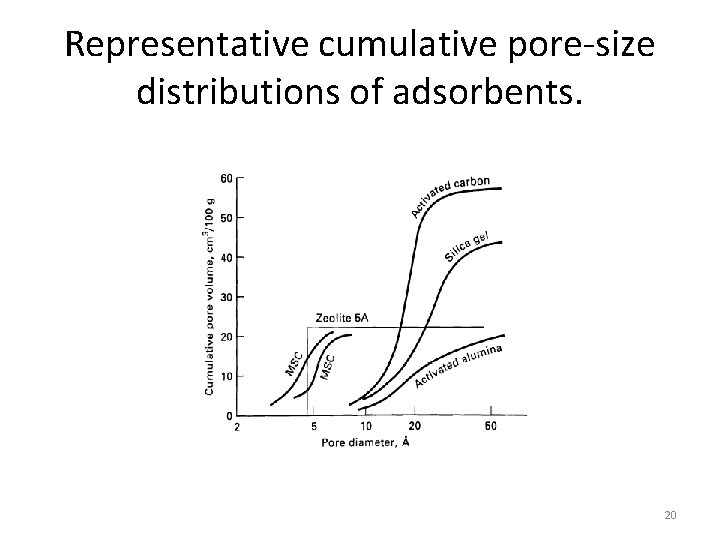
Representative cumulative pore-size distributions of adsorbents. 20

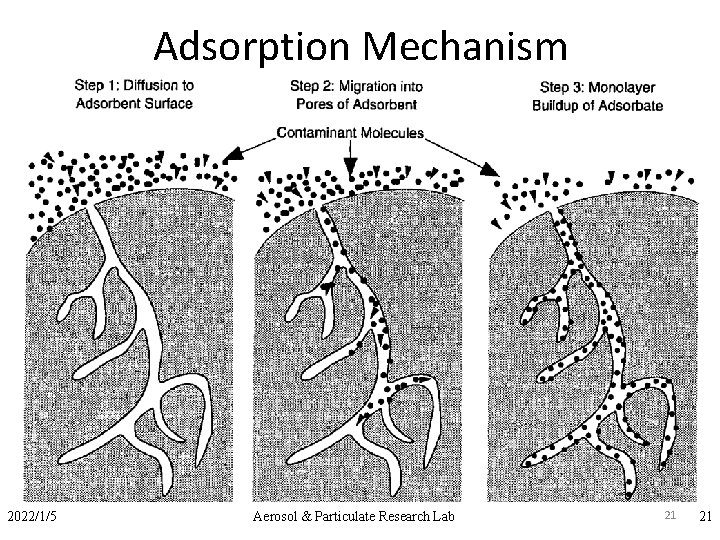
Adsorption Mechanism – 2) Chemical adsorption • Results from a chemical interaction between the adsorbate and adsorbent. Therefore formed bond is much stronger than that for physical adsorption • Heat liberated during chemisorption is in the range of 20 -400 kj/g mole 2022/1/5 Aerosol & Particulate Research Lab 21 21

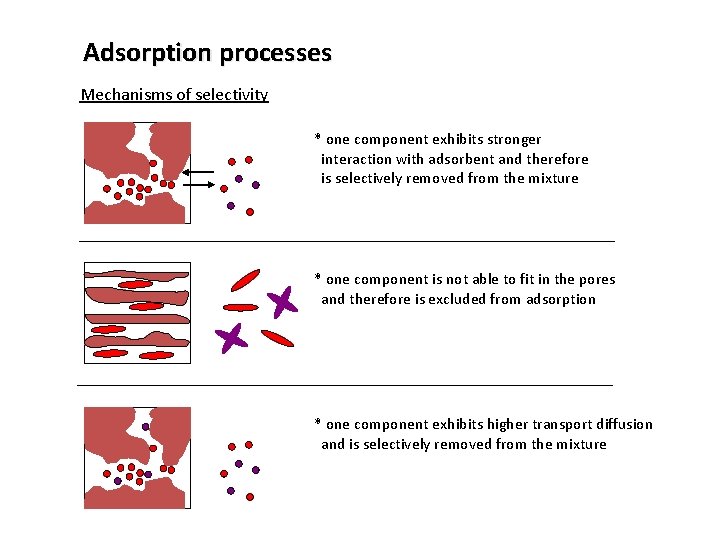
Adsorption processes Mechanisms of selectivity * one component exhibits stronger interaction with adsorbent and therefore is selectively removed from the mixture * one component is not able to fit in the pores and therefore is excluded from adsorption * one component exhibits higher transport diffusion and is selectively removed from the mixture

Adsorption Process Classified as Physical and Chemical Adsorption 1) Physical adsorption 2) Chemical adsorption 23

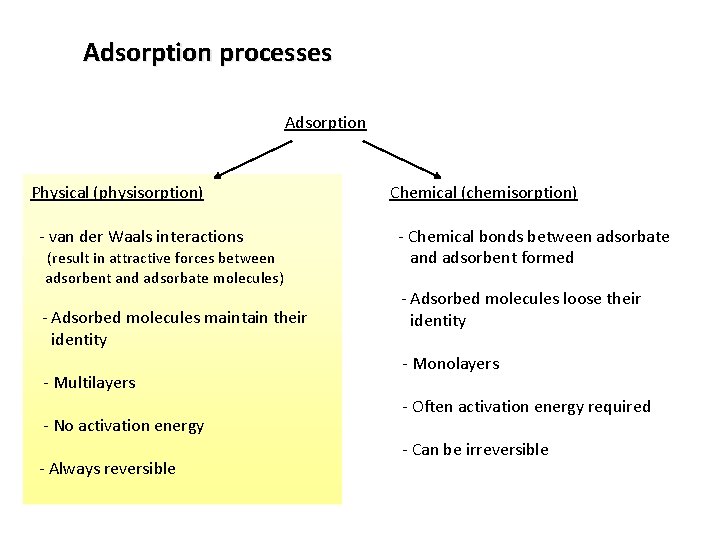
Adsorption processes Adsorption Physical (physisorption) - van der Waals interactions (result in attractive forces between adsorbent and adsorbate molecules) - Adsorbed molecules maintain their identity - Multilayers - No activation energy - Always reversible Chemical (chemisorption) - Chemical bonds between adsorbate and adsorbent formed - Adsorbed molecules loose their identity - Monolayers - Often activation energy required - Can be irreversible

Adsorption Process 1) Physical adsorption § § § § The gas molecules adhere to the surface of the solid adsorbent as a result of intermolecular attractive forces (van der Waals forces) between them The process is exothermic The process is reversible (recovery of adsorbent material or adsorbed gas is possible) by increasing the temperature or lowering the adsorbate conc. Physical adsorption usually directly proportional to the amount of solid surface area Adsorbate can be adsorbed on a monolayer or a number of layers The adsorption rate is generally quite rapid The amount of heat released during this process is equal to the heat of condensation 25

Adsorption Process 2) Chemical adsorption § occurs when there is sharing of electrons between adsorbent and adsorbate § The amount of heat released during this process is equal to the heat of reaction. § Heat liberated during chemisorption is much larger than the heat of vaporization. § It is frequently irreversible. On desorption the chemical nature of the original adsorbate will have undergone a change. § Only a monomolecular layer of adsorbate appears on the adsorbing medium. § Chemisorption from a gas generally takes place only at temperatures greater than 200°C may be slow and irreversible. § Commercial adsorbents rely on physical adsorption; catalysis relies on chemisorption. 26

27

Factors affecting adsorption § physical and chemical properties of the adsorbate § properties of the adsorbent § Adsorption isotherm § Solubility § p. H often affects the surface charge on the adsorbent as well as the charge on the solute. Generally, for organic material as p. H goes down adsorption goes up. § Temperature Adsorption reactions are typically exothermic as T increases extent of adsorption decreases. § Presence of other solutes 28

Adsorption Equilibrium If the adsorbent and adsorbate are contacted long enough an equilibrium will be established between the amount of adsorbate adsorbed and the amount of adsorbate in solution. The equilibrium relationship is described by isotherms. • This equilibrium is usually expressed in terms of (1) concentration (if the fluid is a liquid) or partial pressure (if the fluid is a gas) of the adsorbate in the fluid (2) solute loading on the adsorbent, expressed as mass, moles, or volume of adsorbate per unit mass or per unit BET surface area of the adsorbent. • This equilibrium isotherm places a limit on the extent to which a solute is adsorbed from a given fluid mixture on an adsorbent of given chemical composition and geometry for a given set of conditions. 29

1. Pure Gas Adsorption 30

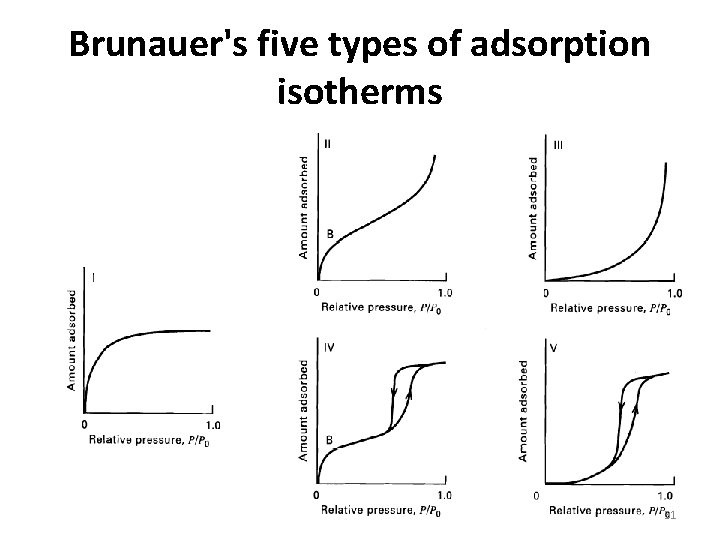
Brunauer's five types of adsorption isotherms 31

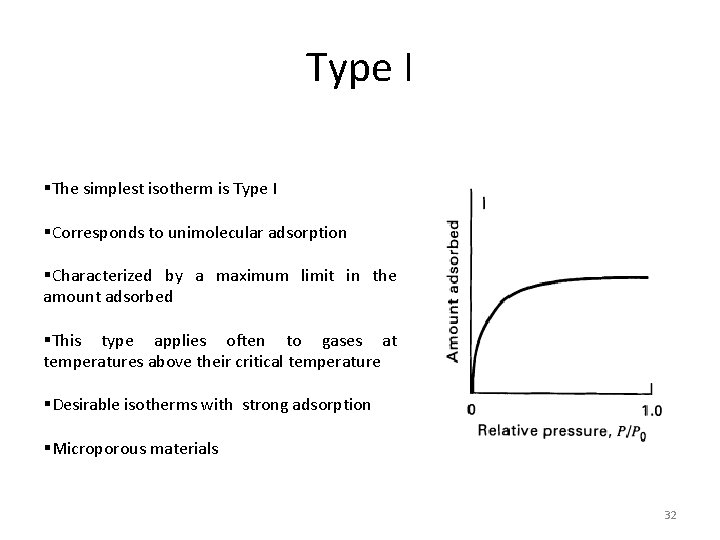
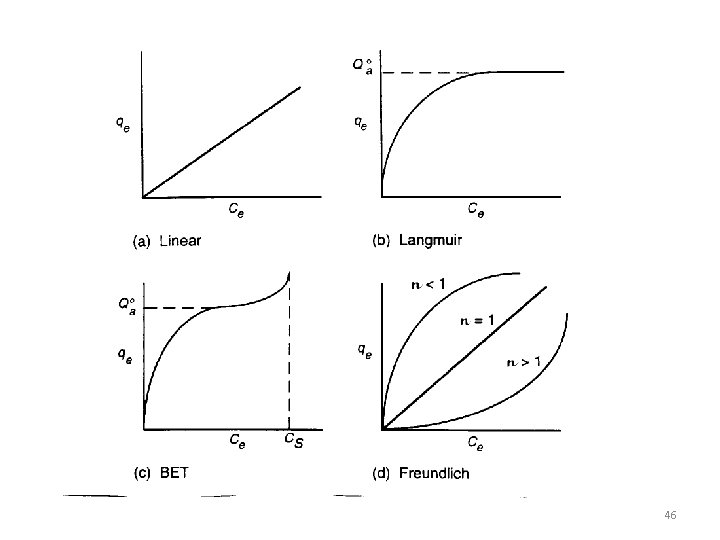
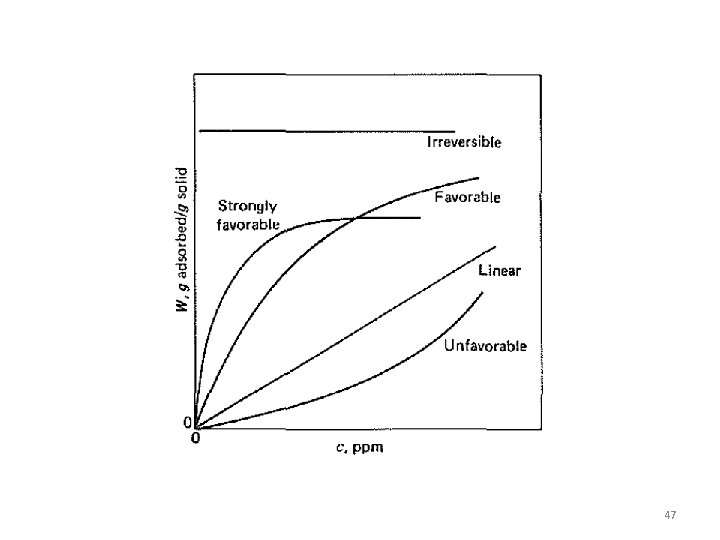
Type I §The simplest isotherm is Type I §Corresponds to unimolecular adsorption §Characterized by a maximum limit in the amount adsorbed §This type applies often to gases at temperatures above their critical temperature §Desirable isotherms with strong adsorption §Microporous materials 32

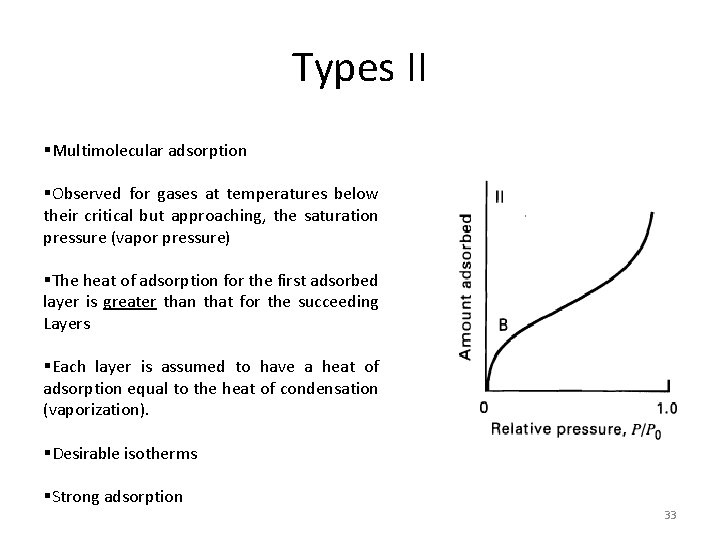
Types II §Multimolecular adsorption §Observed for gases at temperatures below their critical but approaching, the saturation pressure (vapor pressure) §The heat of adsorption for the first adsorbed layer is greater than that for the succeeding Layers §Each layer is assumed to have a heat of adsorption equal to the heat of condensation (vaporization). §Desirable isotherms §Strong adsorption 33

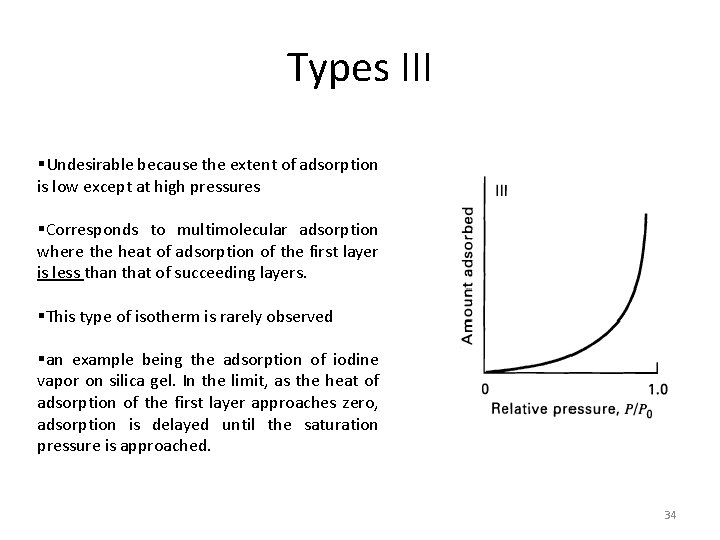
Types III §Undesirable because the extent of adsorption is low except at high pressures §Corresponds to multimolecular adsorption where the heat of adsorption of the first layer is less than that of succeeding layers. §This type of isotherm is rarely observed §an example being the adsorption of iodine vapor on silica gel. In the limit, as the heat of adsorption of the first layer approaches zero, adsorption is delayed until the saturation pressure is approached. 34

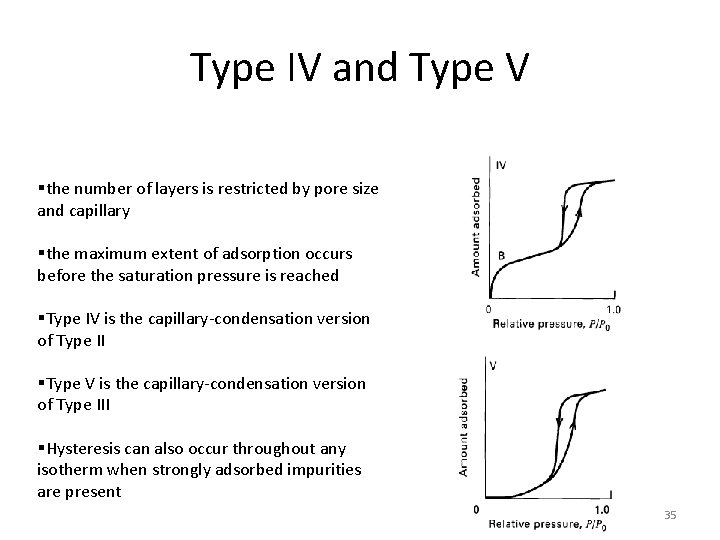
Type IV and Type V §the number of layers is restricted by pore size and capillary §the maximum extent of adsorption occurs before the saturation pressure is reached §Type IV is the capillary-condensation version of Type II §Type V is the capillary-condensation version of Type III §Hysteresis can also occur throughout any isotherm when strongly adsorbed impurities are present 35

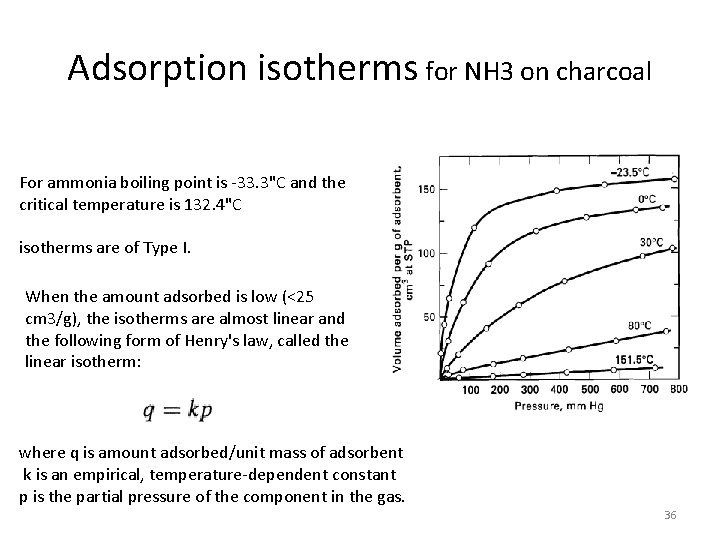
Adsorption isotherms for NH 3 on charcoal For ammonia boiling point is -33. 3"C and the critical temperature is 132. 4"C isotherms are of Type I. When the amount adsorbed is low (<25 cm 3/g), the isotherms are almost linear and the following form of Henry's law, called the linear isotherm: where q is amount adsorbed/unit mass of adsorbent k is an empirical, temperature-dependent constant p is the partial pressure of the component in the gas. 36

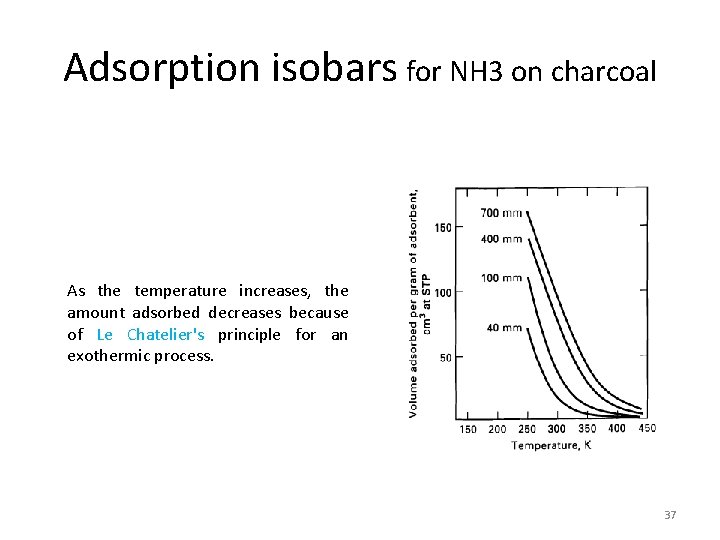
Adsorption isobars for NH 3 on charcoal As the temperature increases, the amount adsorbed decreases because of Le Chatelier's principle for an exothermic process. 37

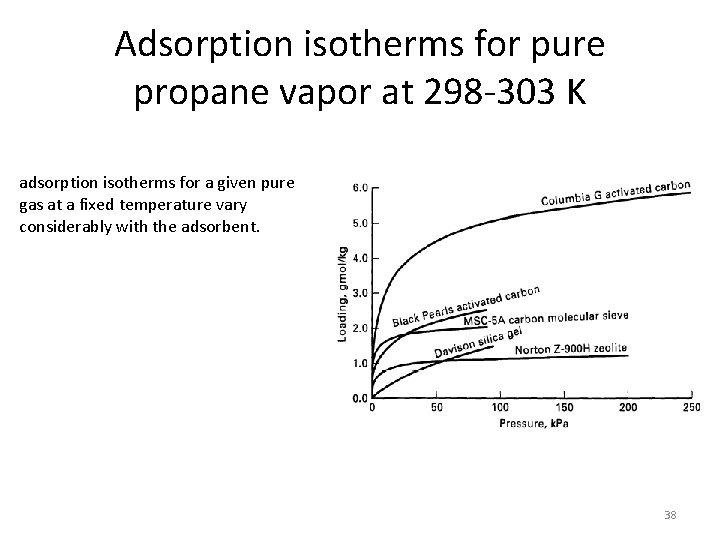
Adsorption isotherms for pure propane vapor at 298 -303 K adsorption isotherms for a given pure gas at a fixed temperature vary considerably with the adsorbent. 38

Adsorption isotherms for water in air at 20 to 50'C. 39

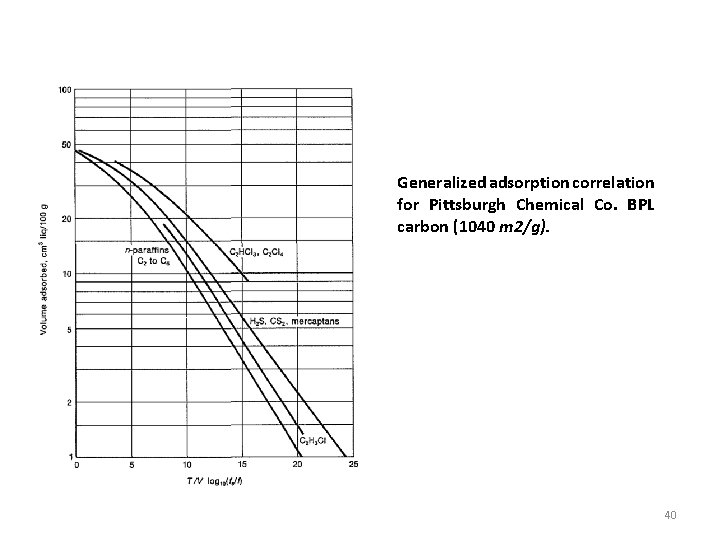
Generalized adsorption correlation for Pittsburgh Chemical Co. BPL carbon (1040 m 2/g). 40

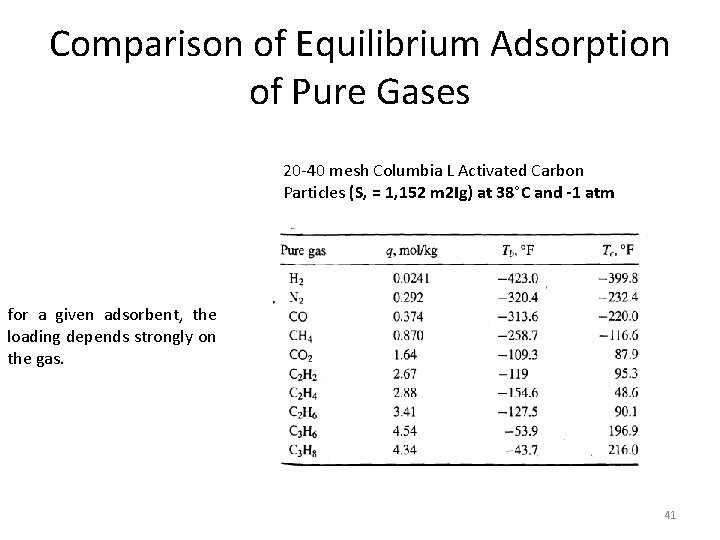
Comparison of Equilibrium Adsorption of Pure Gases 20 -40 mesh Columbia L Activated Carbon Particles (S, = 1, 152 m 2 Ig) at 38°C and -1 atm for a given adsorbent, the loading depends strongly on the gas. 41

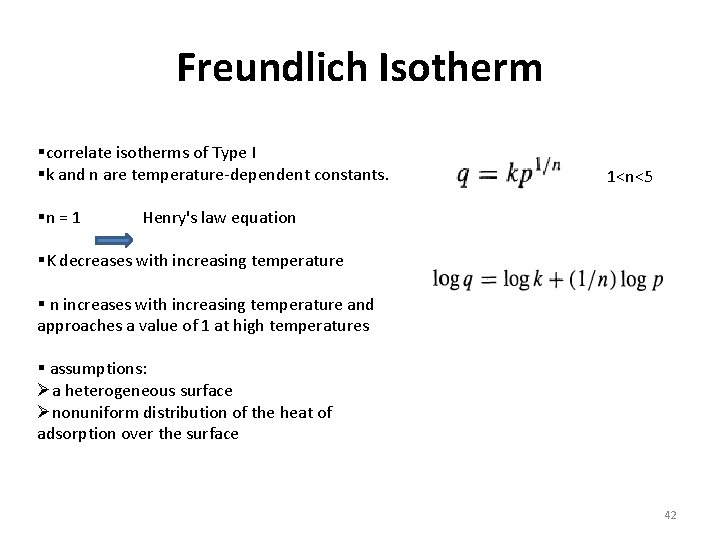
Freundlich Isotherm §correlate isotherms of Type I §k and n are temperature-dependent constants. §n = 1 1<n<5 Henry's law equation §K decreases with increasing temperature § n increases with increasing temperature and approaches a value of 1 at high temperatures § assumptions: Øa heterogeneous surface Ønonuniform distribution of the heat of adsorption over the surface 42

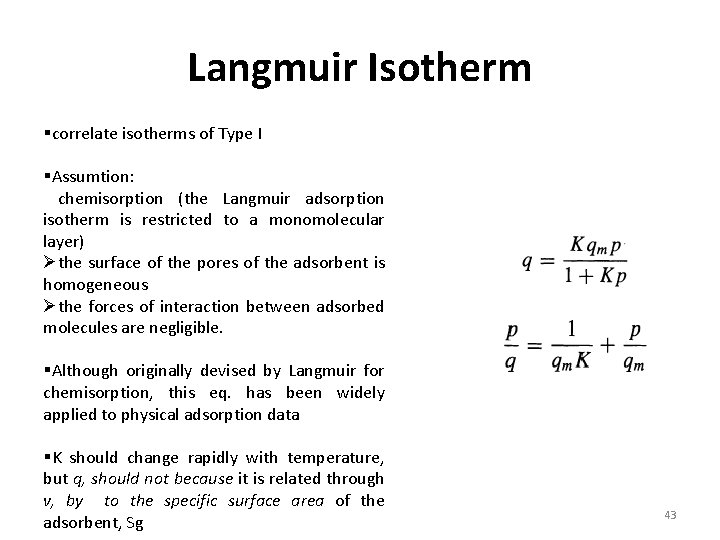
Langmuir Isotherm §correlate isotherms of Type I §Assumtion: chemisorption (the Langmuir adsorption isotherm is restricted to a monomolecular layer) Øthe surface of the pores of the adsorbent is homogeneous Øthe forces of interaction between adsorbed molecules are negligible. §Although originally devised by Langmuir for chemisorption, this eq. has been widely applied to physical adsorption data §K should change rapidly with temperature, but q, should not because it is related through v, by to the specific surface area of the adsorbent, Sg 43

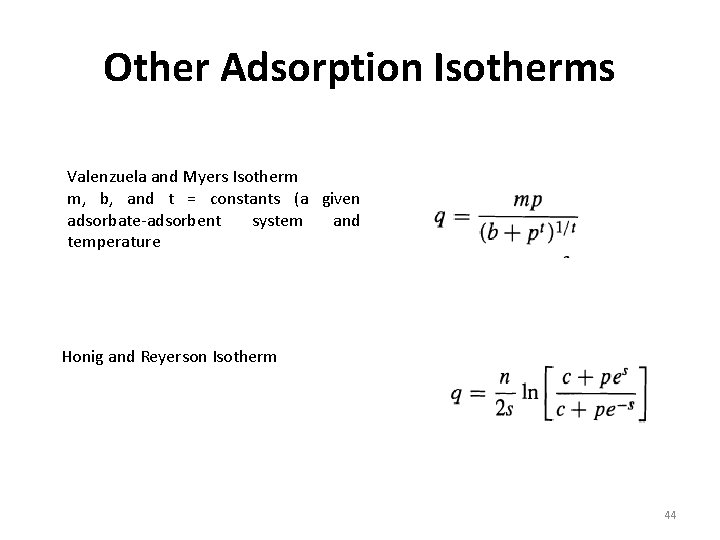
Other Adsorption Isotherms Valenzuela and Myers Isotherm m, b, and t = constants (a given adsorbate-adsorbent system and temperature Honig and Reyerson Isotherm 44

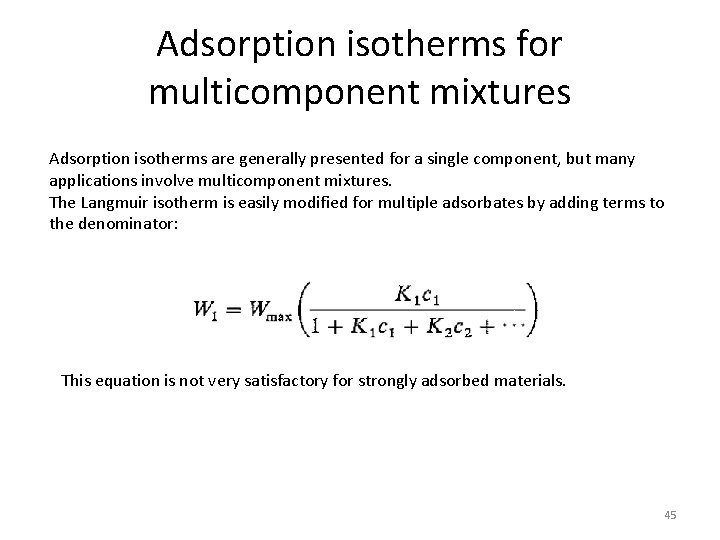
Adsorption isotherms for multicomponent mixtures Adsorption isotherms are generally presented for a single component, but many applications involve multicomponent mixtures. The Langmuir isotherm is easily modified for multiple adsorbates by adding terms to the denominator: This equation is not very satisfactory for strongly adsorbed materials. 45

46

47

2. Liquid Adsorption 48

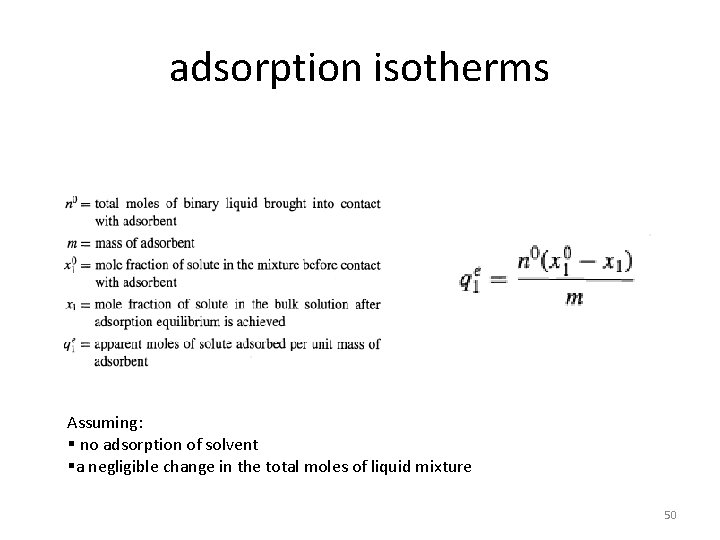
§When porous adsorbent particles are immersed in a pure gas, the amount of adsorbed gas is determined by the decrease in total pressure. §With a liquid, the pressure does not change, and no simple experimental procedure has been devised for determining the extent of adsorption of a pure liquid. § If the liquid is a homogeneous binary mixture, it is customary to designate one component the solute (1) and the other the solvent (2). §adsorption of the solvent is tacitly assumed not to occur. 49

adsorption isotherms Assuming: § no adsorption of solvent §a negligible change in the total moles of liquid mixture 50

adsorption isotherms When the solvent is not a Composite curve without negative Concentration Composite isotherms or isothems of concentration change > 51

§When data for the binary mixture are only available in the dilute region, the amount of adsorption of the solvent may be constant and all changes in the total amount adsorbed are due to just the solute. § In that case, the adsorption mass of adsorbent isotherms are of the form of Figure a, which resembles the form obtained with pure gases. §It is then common to fit with adsorbent the data with concentration forms of the Freundlich equation 52

SORPTION SYSTEMS 53

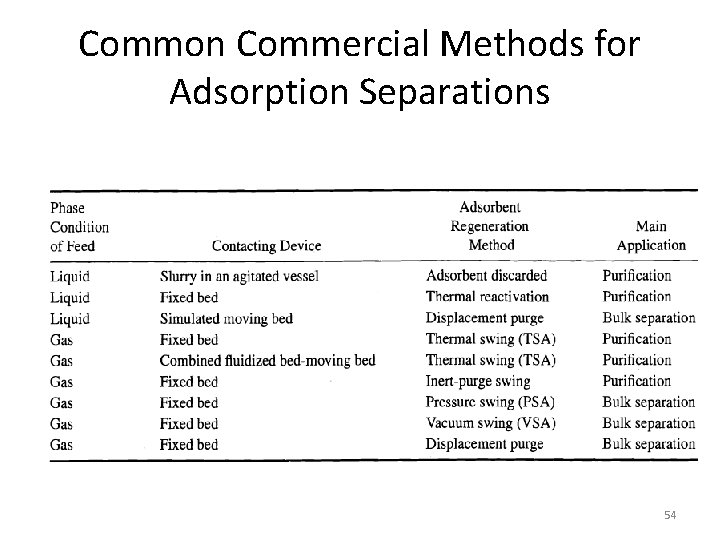
Common Commercial Methods for Adsorption Separations 54

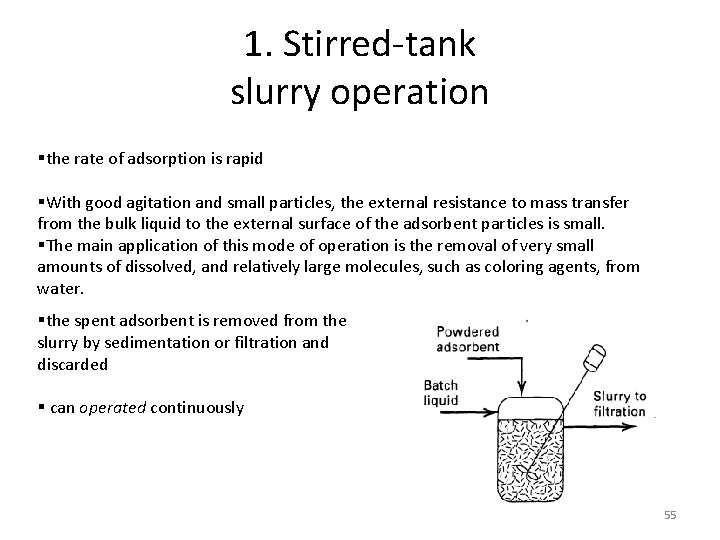
1. Stirred-tank slurry operation §the rate of adsorption is rapid §With good agitation and small particles, the external resistance to mass transfer from the bulk liquid to the external surface of the adsorbent particles is small. §The main application of this mode of operation is the removal of very small amounts of dissolved, and relatively large molecules, such as coloring agents, from water. §the spent adsorbent is removed from the slurry by sedimentation or filtration and discarded § can operated continuously 55

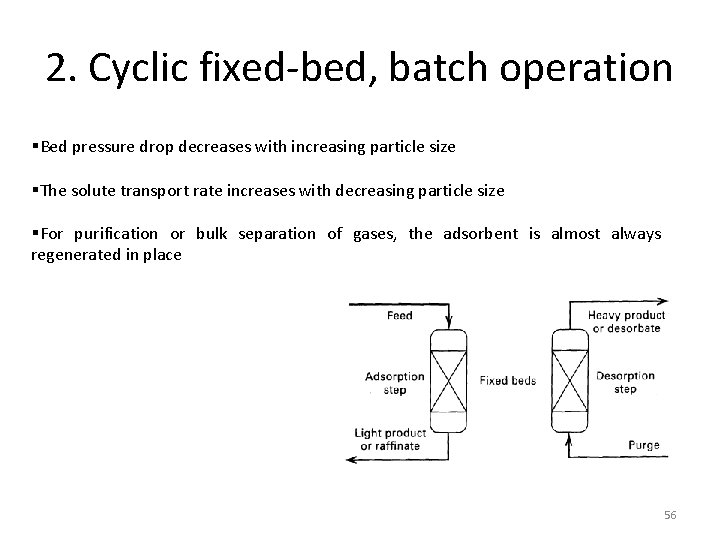
2. Cyclic fixed-bed, batch operation §Bed pressure drop decreases with increasing particle size §The solute transport rate increases with decreasing particle size §For purification or bulk separation of gases, the adsorbent is almost always regenerated in place 56

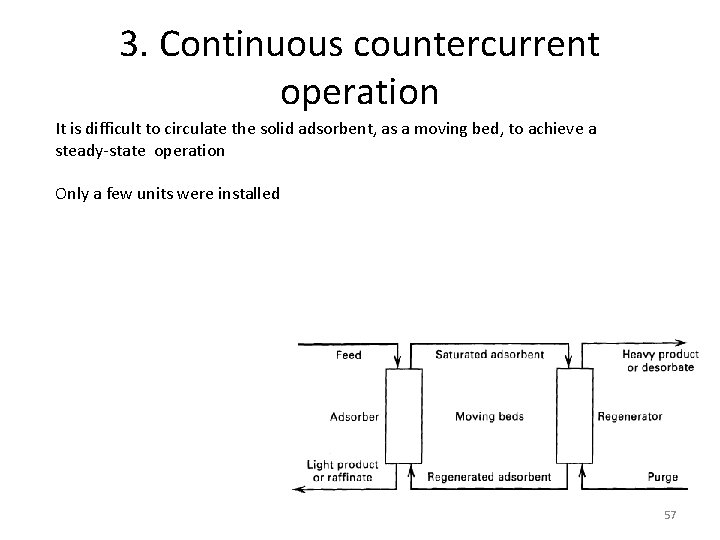
3. Continuous countercurrent operation It is difficult to circulate the solid adsorbent, as a moving bed, to achieve a steady-state operation Only a few units were installed 57

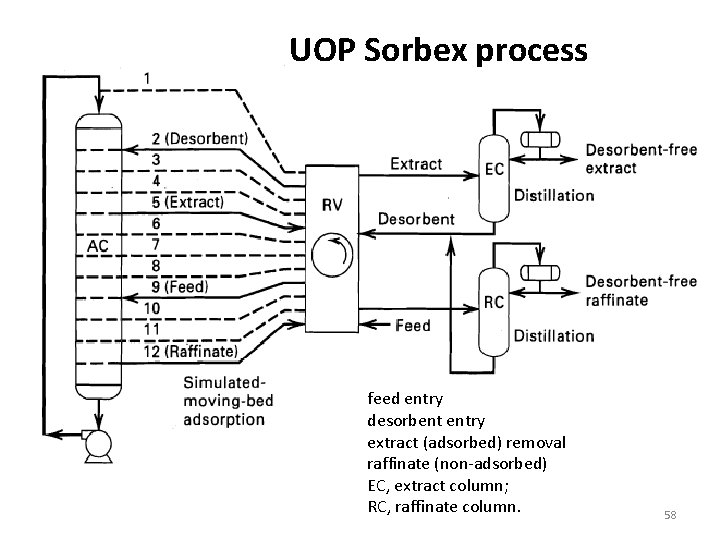
UOP Sorbex process feed entry desorbent entry extract (adsorbed) removal raffinate (non-adsorbed) EC, extract column; RC, raffinate column. 58

Regeneration methods 1. Thermal (temperature)-swing-adsorption (TSA) method 2. Inert-purge-swing method 3. Pressure-swing-adsorption (PSA) cycle 4. Vacuum-swing-adsorption 5. Displacement-purge (displacement desorption) cycle 59

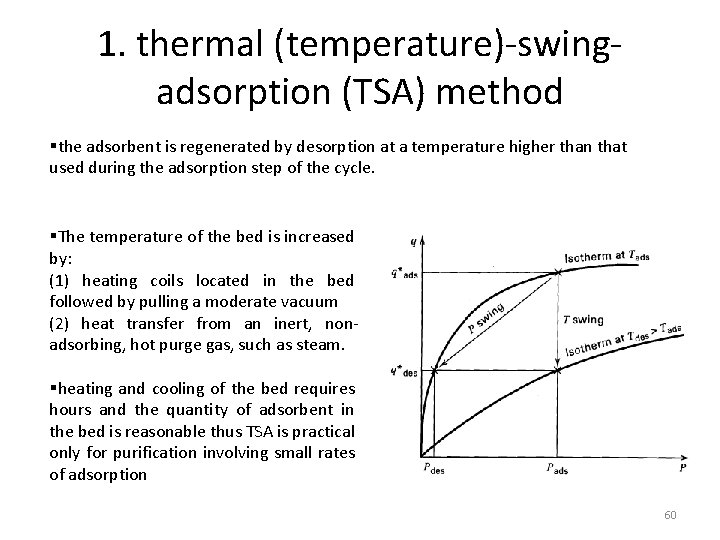
1. thermal (temperature)-swingadsorption (TSA) method §the adsorbent is regenerated by desorption at a temperature higher than that used during the adsorption step of the cycle. §The temperature of the bed is increased by: (1) heating coils located in the bed followed by pulling a moderate vacuum (2) heat transfer from an inert, nonadsorbing, hot purge gas, such as steam. §heating and cooling of the bed requires hours and the quantity of adsorbent in the bed is reasonable thus TSA is practical only for purification involving small rates of adsorption 60

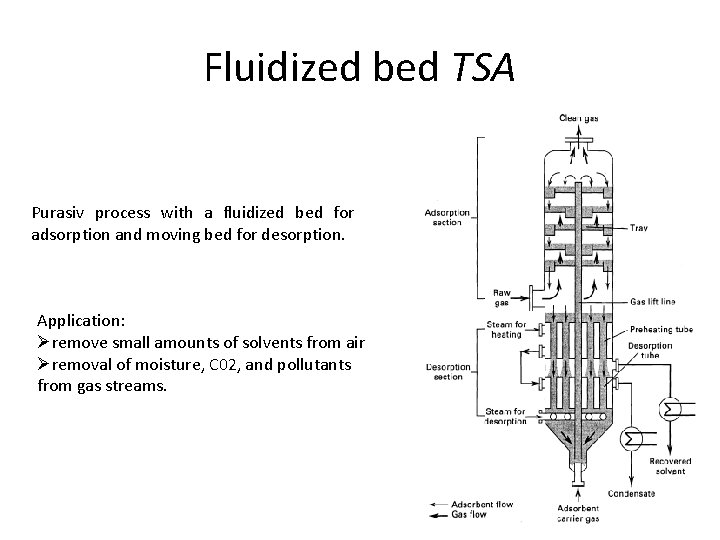
Fluidized bed TSA Purasiv process with a fluidized bed for adsorption and moving bed for desorption. Application: Øremove small amounts of solvents from air Øremoval of moisture, C 02, and pollutants from gas streams. 61

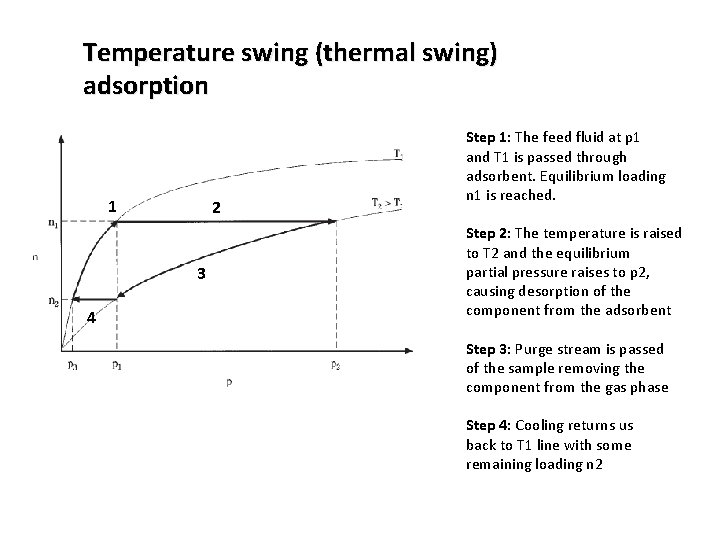
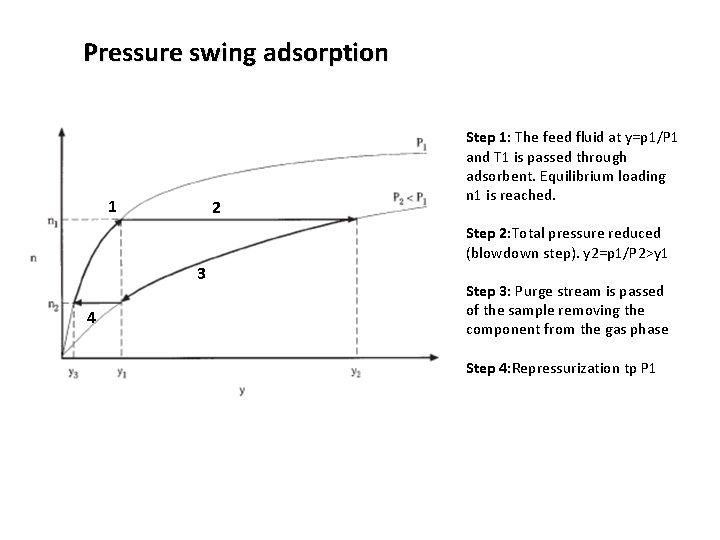
Temperature swing (thermal swing) adsorption 1 2 3 4 Step 1: The feed fluid at p 1 and T 1 is passed through adsorbent. Equilibrium loading n 1 is reached. Step 2: The temperature is raised to T 2 and the equilibrium partial pressure raises to p 2, causing desorption of the component from the adsorbent Step 3: Purge stream is passed of the sample removing the component from the gas phase Step 4: Cooling returns us back to T 1 line with some remaining loading n 2

Temperature swing (thermal swing) adsorption Applications: 1) Drying 2) Sweetening (H 2 S, SO 2 removal from natural gas, H 2) 3) NOx removal 4) HCl removal from Cl 2


2. inert-purge-swing method §Desorption is at the same temperature and pressure §the gas used for purging is non-adsorbing (inert) or only weakly adsorbing § This method is used only when the solute is weakly adsorbed, easily desorbed, and of little or no value §The purge gas must be inexpensive so that it does not have to be purified before recycle 65

3. Pressure-swing-adsorption (PSA) cycle §Adsorption takes place at an elevated pressure, whereas desorption occurs at near-ambient pressure §because the bed can be depressurized and repressurized rapidly, making it possible to operate at cycle times of seconds to minutes §Because of these short times, the beds need not be large §Small plants often use PSA because that cycle is simpler. §If adsorption takes place at near-ambient pressure and desorption under vacuum, the cycle is referred to as vacuum-swing-adsorption (VSA) 66

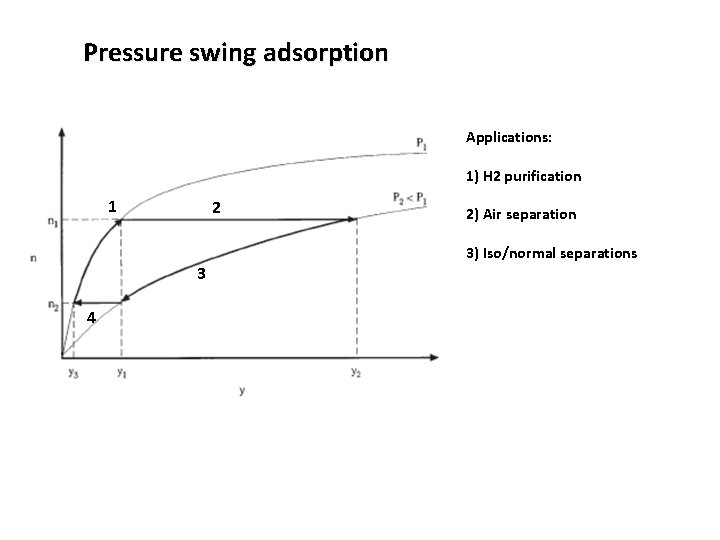
Pressure swing adsorption 1 2 Step 1: The feed fluid at y=p 1/P 1 and T 1 is passed through adsorbent. Equilibrium loading n 1 is reached. Step 2: Total pressure reduced (blowdown step). y 2=p 1/P 2>y 1 3 4 Step 3: Purge stream is passed of the sample removing the component from the gas phase Step 4: Repressurization tp P 1

Pressure swing adsorption Applications: 1) H 2 purification 1 2 2) Air separation 3) Iso/normal separations 3 4

4. Displacement-purge cycle §a strongly adsorbed purge gas is used in the desorption step to displace the adsorbed species. §Another step is required to recover the purge gas. §This method is considered only where TSA, PSA, and VSA cannot be used because of pressure or temperature limitations. 69

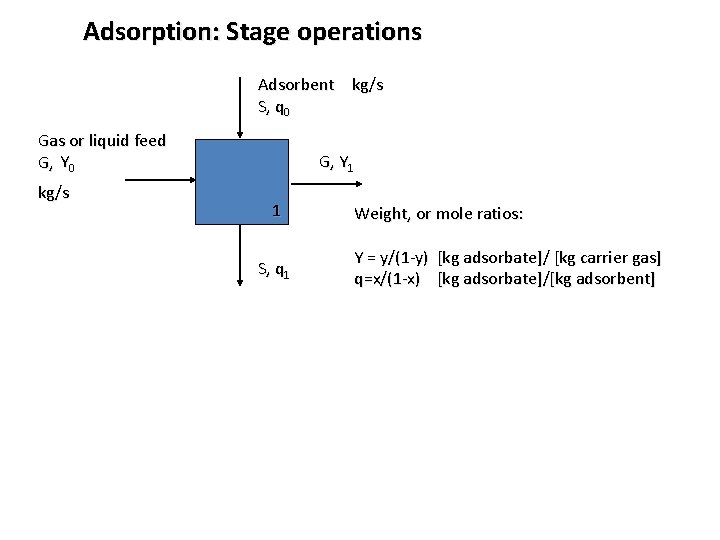
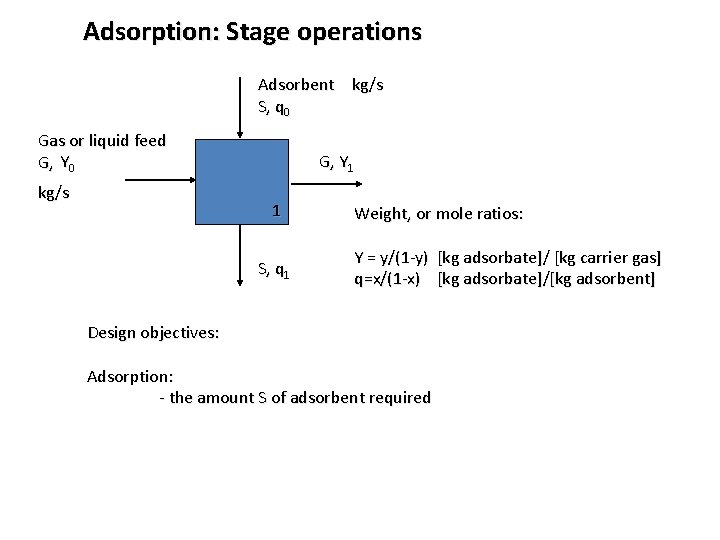
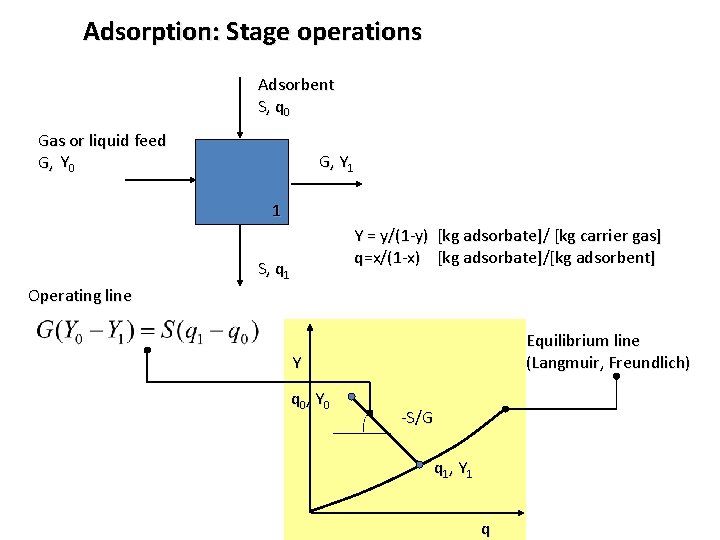
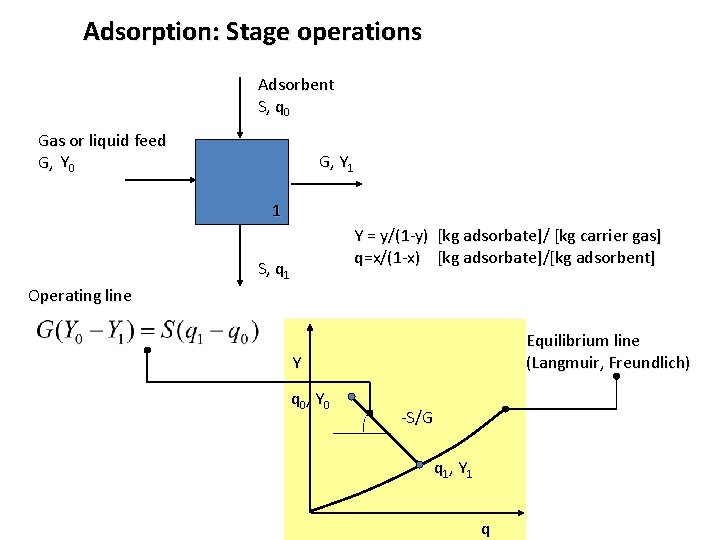
Adsorption: Stage operations Adsorbent kg/s S, q 0 Gas or liquid feed G, Y 0 kg/s G, Y 1 1 S, q 1 Weight, or mole ratios: Y = y/(1 -y) [kg adsorbate]/ [kg carrier gas] q=x/(1 -x) [kg adsorbate]/[kg adsorbent]

Adsorption: Stage operations Adsorbent kg/s S, q 0 Gas or liquid feed G, Y 0 kg/s G, Y 1 1 S, q 1 Weight, or mole ratios: Y = y/(1 -y) [kg adsorbate]/ [kg carrier gas] q=x/(1 -x) [kg adsorbate]/[kg adsorbent] Design objectives: Adsorption: - the amount S of adsorbent required

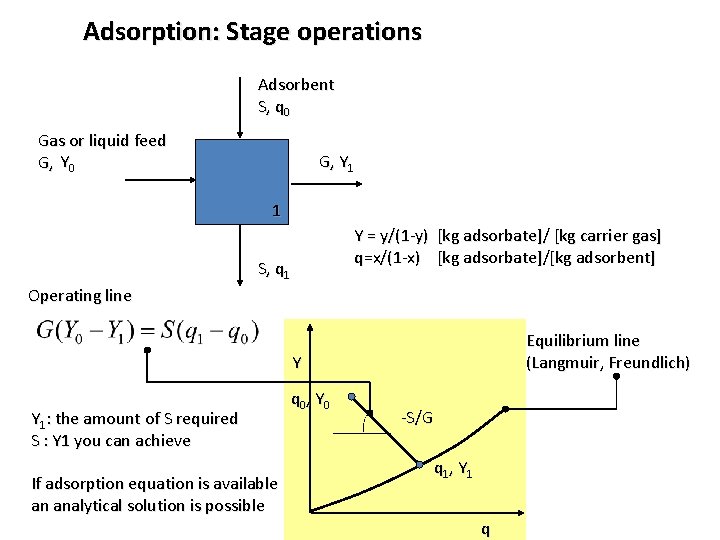
Adsorption: Stage operations Adsorbent S, q 0 Gas or liquid feed G, Y 0 G, Y 1 1 Y = y/(1 -y) [kg adsorbate]/ [kg carrier gas] q=x/(1 -x) [kg adsorbate]/[kg adsorbent] S, q 1 Operating line Equilibrium line (Langmuir, Freundlich) Y q 0, Y 0 -S/G q 1, Y 1 q

Adsorption: Stage operations Adsorbent S, q 0 Gas or liquid feed G, Y 0 G, Y 1 1 Y = y/(1 -y) [kg adsorbate]/ [kg carrier gas] q=x/(1 -x) [kg adsorbate]/[kg adsorbent] S, q 1 Operating line Equilibrium line (Langmuir, Freundlich) Y q 0, Y 0 -S/G q 1, Y 1 q

Adsorption: Stage operations Adsorbent S, q 0 Gas or liquid feed G, Y 0 G, Y 1 1 Y = y/(1 -y) [kg adsorbate]/ [kg carrier gas] q=x/(1 -x) [kg adsorbate]/[kg adsorbent] S, q 1 Operating line Equilibrium line (Langmuir, Freundlich) Y Y 1: the amount of S required S : Y 1 you can achieve If adsorption equation is available an analytical solution is possible q 0, Y 0 -S/G q 1, Y 1 q

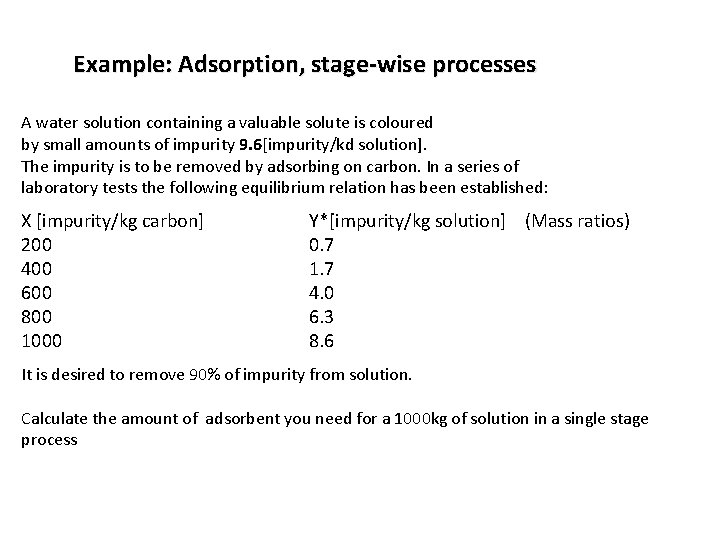
Example: Adsorption, stage-wise processes A water solution containing a valuable solute is coloured by small amounts of impurity 9. 6[impurity/kd solution]. The impurity is to be removed by adsorbing on carbon. In a series of laboratory tests the following equilibrium relation has been established: X [impurity/kg carbon] 200 400 600 800 1000 Y*[impurity/kg solution] (Mass ratios) 0. 7 1. 7 4. 0 6. 3 8. 6 It is desired to remove 90% of impurity from solution. Calculate the amount of adsorbent you need for a 1000 kg of solution in a single stage process

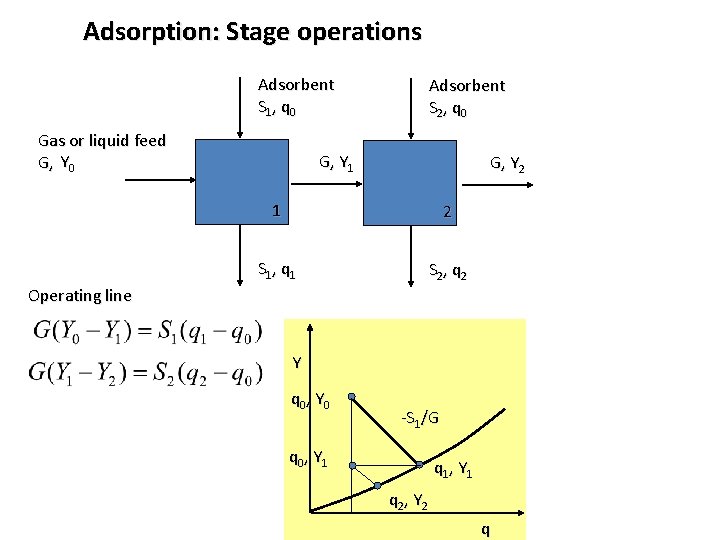
Adsorption: Stage operations Adsorbent S 1, q 0 Gas or liquid feed G, Y 0 Adsorbent S 2, q 0 G, Y 1 G, Y 2 1 2 S 1, q 1 S 2, q 2 Operating line Y q 0, Y 0 -S 1/G q 0, Y 1 q 1, Y 1 q 2, Y 2 q

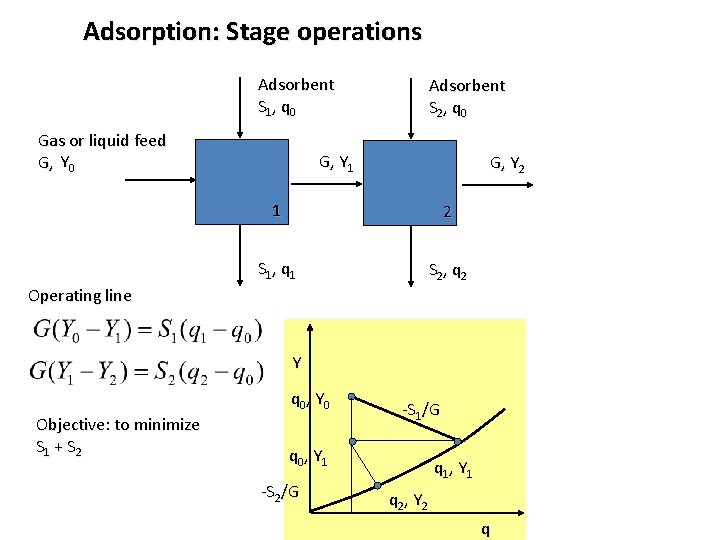
Adsorption: Stage operations Adsorbent S 1, q 0 Gas or liquid feed G, Y 0 Adsorbent S 2, q 0 G, Y 1 G, Y 2 1 2 S 1, q 1 S 2, q 2 Operating line Y q 0, Y 0 Objective: to minimize S 1 + S 2 -S 1/G q 0, Y 1 -S 2/G q 1, Y 1 q 2, Y 2 q

Adsorption: Stage operations Gas or liquid feed G, Y 0 S, q 1 G, Y 1 1 S, q 2 G, Y 2 2 Design objectives: Adsorption: - the amount S of adsorbent required - number of stages G, YN S, q. N+1

Adsorption: Stage operations Gas or liquid feed G, Y 0 S, q 1 G, Y 1 1 G, Y 2 2 S, q. N+1 Operating line Y q 1, Y 0 S/G G, YN q. N+1, YN q

Adsorption: Stage operations Gas or liquid feed G, Y 0 S, q 1 G, Y 1 1 G, Y 2 2 S, q. N+1 Operating line Minimum adsorbent requirement: Design: analogy with stage-wise absorption Y Smin/G G, YN q 1, Y 0 q. N+1, YN q

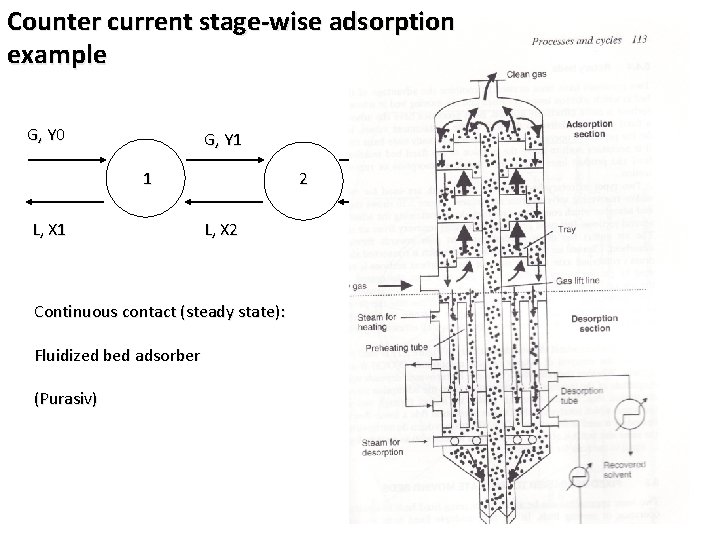
Counter current stage-wise adsorption example G, Y 0 1 L, X 2 Fluidized bed adsorber G, YN G, Y(N-1) N 2 Continuous contact (steady state): (Purasiv) G, Y 2 G, Y 1 L 3, X 3 LN, XN L, X(N+1)

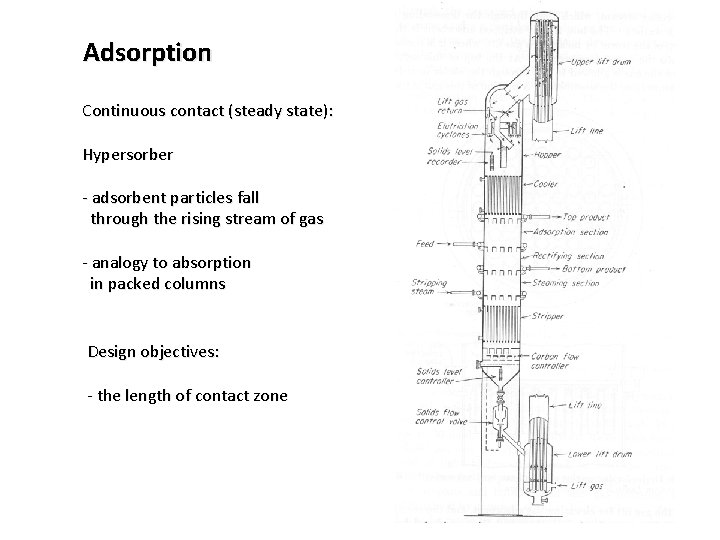
Adsorption Continuous contact (steady state): Hypersorber - adsorbent particles fall through the rising stream of gas - analogy to absorption in packed columns Design objectives: - the length of contact zone

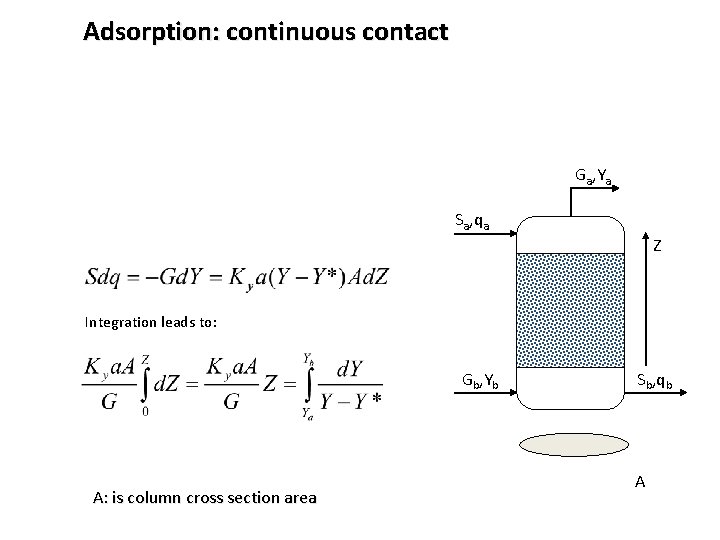
Adsorption: continuous contact Ga, Ya Sa, qa Z Integration leads to: Gb, Yb A: is column cross section area Sb, qb A

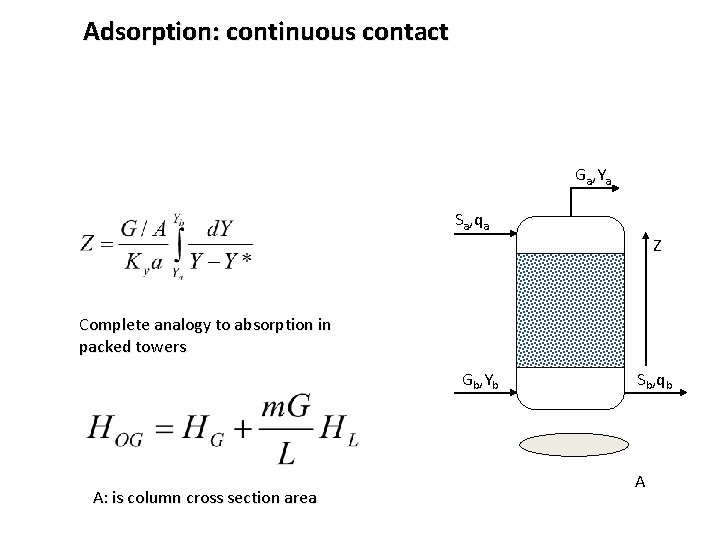
Adsorption: continuous contact Ga, Ya Sa, qa Z Complete analogy to absorption in packed towers Gb, Yb A: is column cross section area Sb, qb A

Adsorption: Design considerations - Continuous contact: Unsteady state Fixed bed: Gas (liquid) is send through a system containing fixed (not moving) adsorbent. A component is removed from passing mixture until saturation of the bed is reached. The process is then stopped and the system regenerated

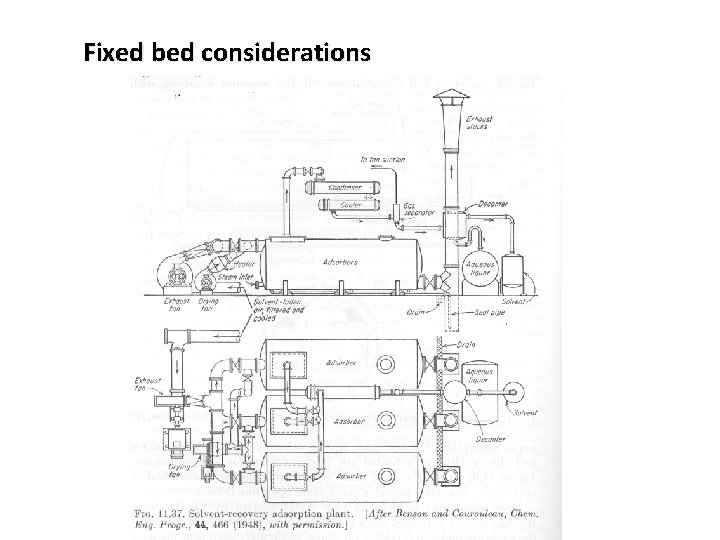
Fixed bed considerations

Fixed bed considerations

Fixed bed considerations saturated section of the bed: no more adsorption

Fixed bed considerations saturated section of the bed: no more adsorption

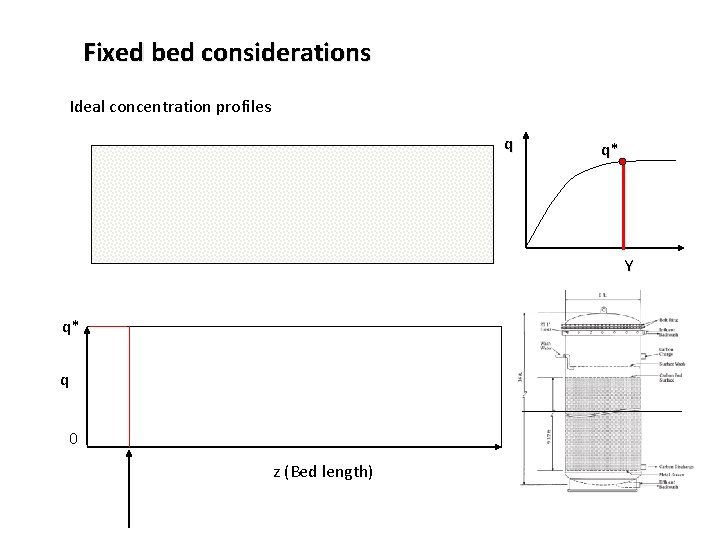
Fixed bed considerations Ideal concentration profiles q q* Y q* q 0 z (Bed length)

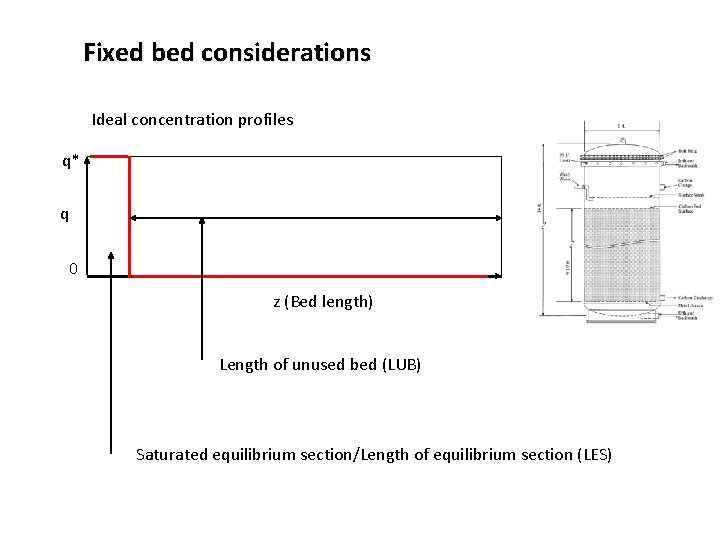
Fixed bed considerations Ideal concentration profiles q* q 0 z (Bed length) Length of unused bed (LUB) Saturated equilibrium section/Length of equilibrium section (LES)

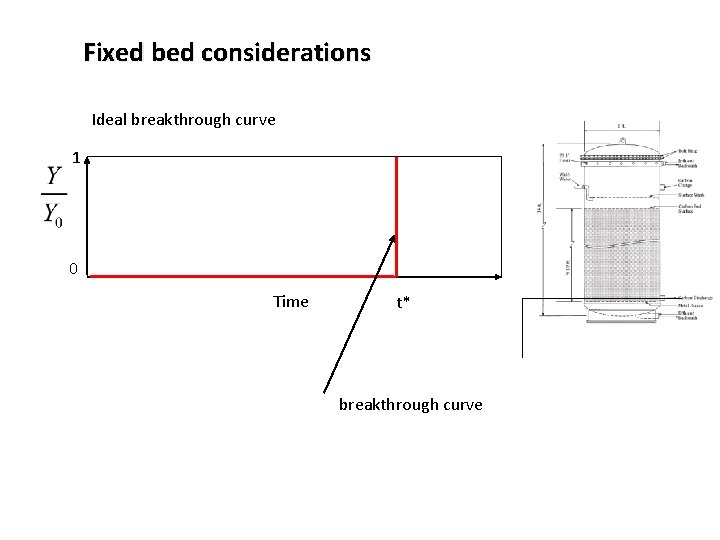
Fixed bed considerations Ideal breakthrough curve 1 0 Time t* breakthrough curve

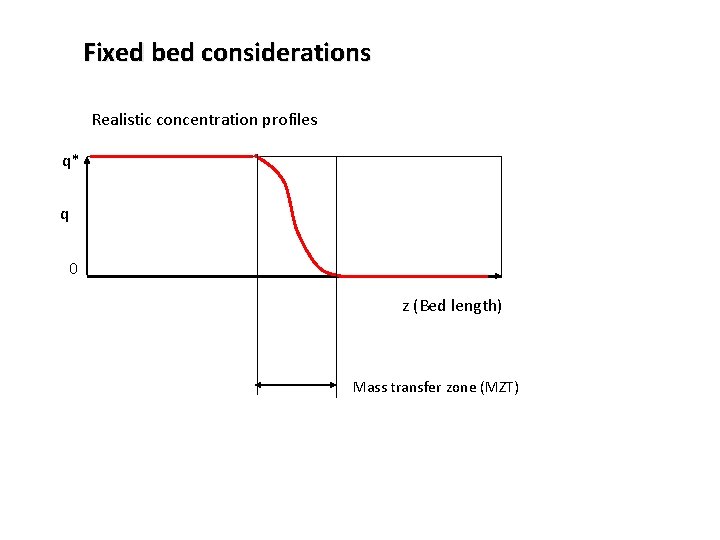
Fixed bed considerations Realistic concentration profiles q* q 0 z (Bed length) Mass transfer zone (MZT)

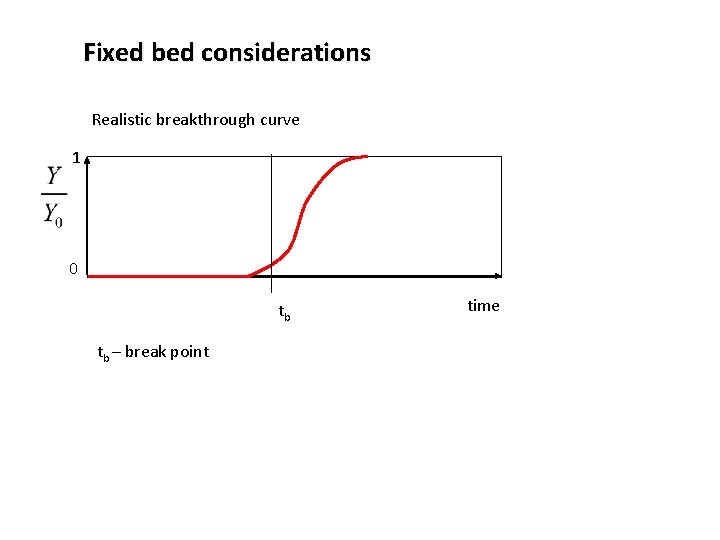
Fixed bed considerations Realistic breakthrough curve 1 0 tb tb – break point time

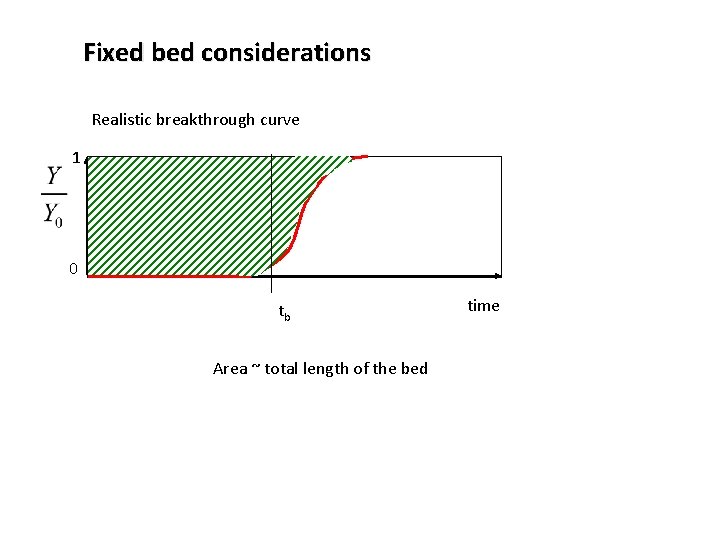
Fixed bed considerations Realistic breakthrough curve 1 0 tb Area ~ total length of the bed time

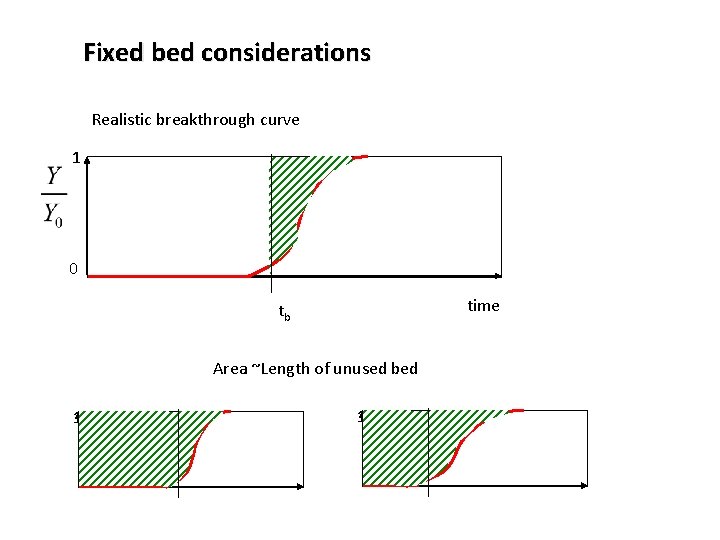
Fixed bed considerations Realistic breakthrough curve 1 0 time tb Area ~Length of unused bed 1 1
![Fixed bed considerations Design Fixed bed G kgm 2 s Y0 G kgm 2 Fixed bed considerations: Design Fixed bed: G’ [kg/m 2 s], Y=0 G’ [kg/m 2](https://slidetodoc.com/presentation_image_h2/57d1e7f878c1c6d2d8673251b18f0fc3/image-97.jpg)
Fixed bed considerations: Design Fixed bed: G’ [kg/m 2 s], Y=0 G’ [kg/m 2 s], Y A [m 2] Density of the bed [kg/m 3] Design objectives: Adsorption: - operation time before bed becomes saturated - amount of adsorbent required (minimum bed) Desorption: - time required for complete regeneration - amount of purge required for regeneration

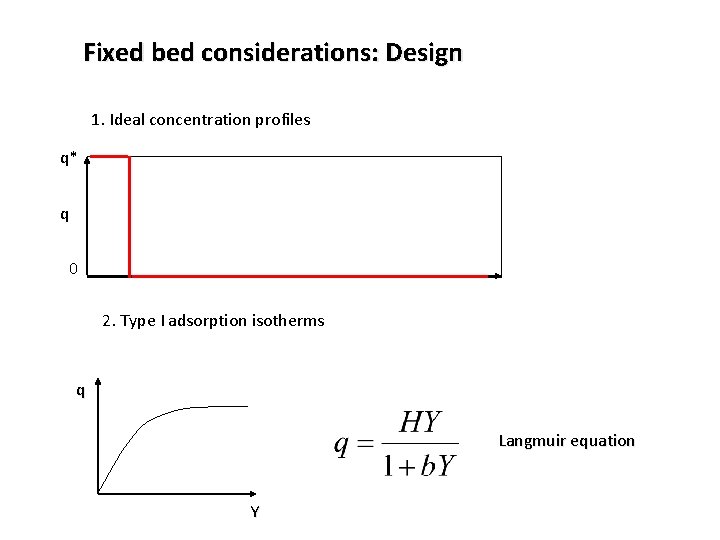
Fixed bed considerations: Design 1. Ideal concentration profiles q* q 0 2. Type I adsorption isotherms q Langmuir equation Y

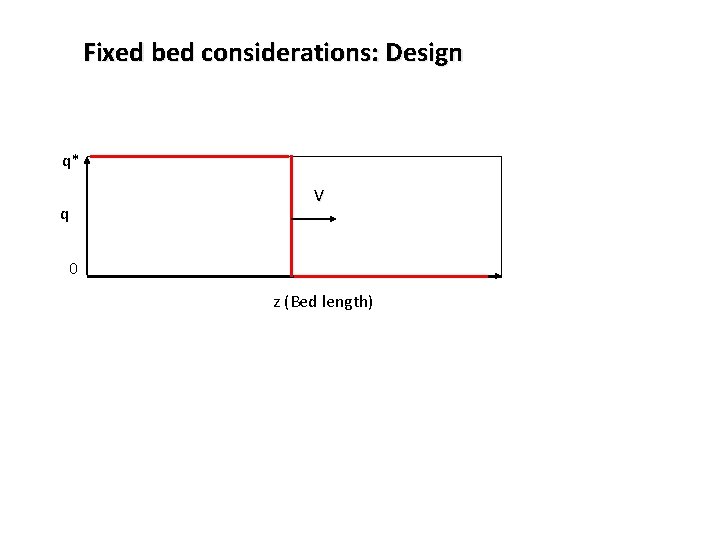
Fixed bed considerations: Design q* V q 0 z (Bed length)

Fixed bed considerations: Design q* V q qin 0 z (Bed length) Amount adsorbed up to position z Or, if qin = 0 = kg

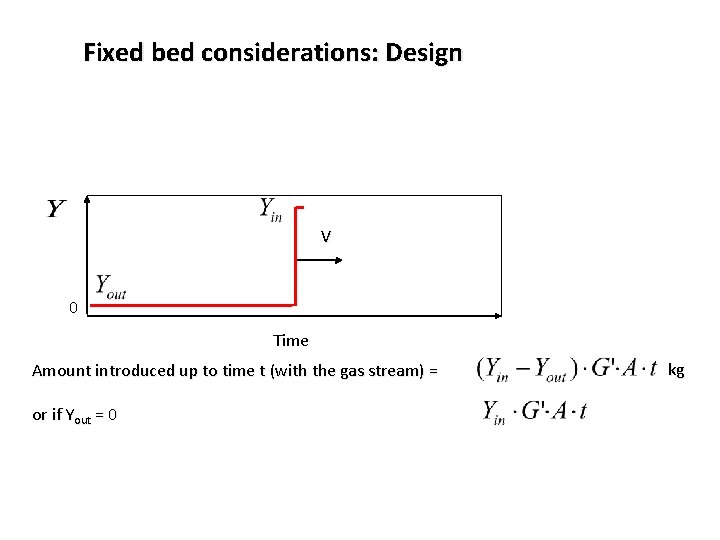
Fixed bed considerations: Design V 0 Time Amount introduced up to time t (with the gas stream) = or if Yout = 0 kg

Fixed bed considerations: Design Amount introduced up to time t (with the gas stream) = kg Amount adsorbed up to position z kg Propagation velocity =

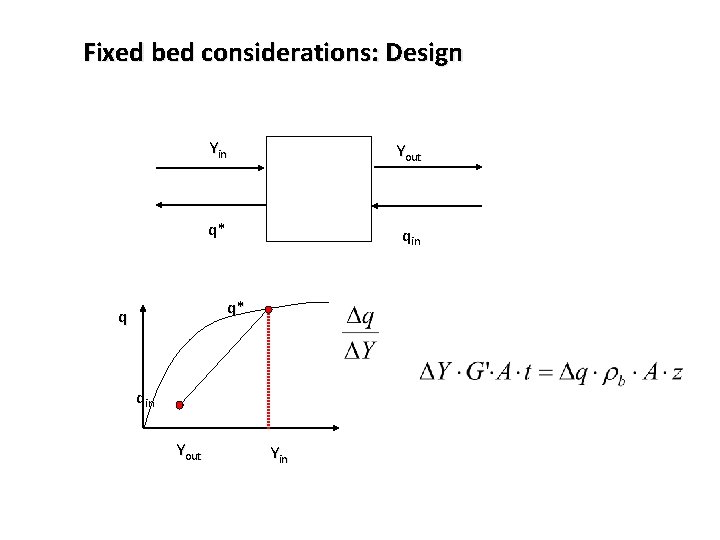
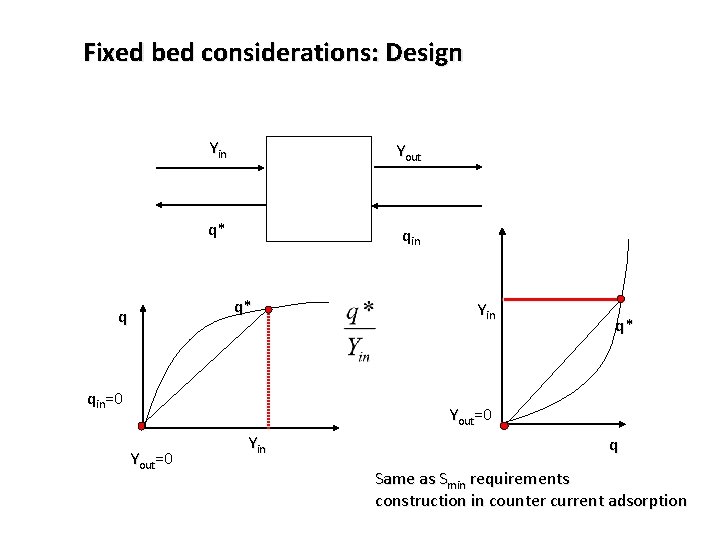
Fixed bed considerations: Design Yin Yout q* qin Yin q Yout qin q*

Fixed bed considerations: Design Yin Yout q* qin q* q qin Yout Yin

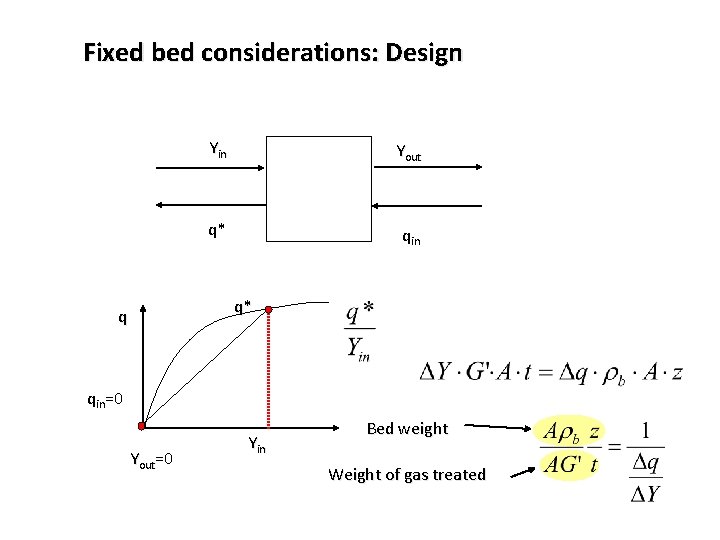
Fixed bed considerations: Design Yin Yout q* qin q* q qin=0 Yin q* Yout=0 Yin q Same as Smin requirements construction in counter current adsorption

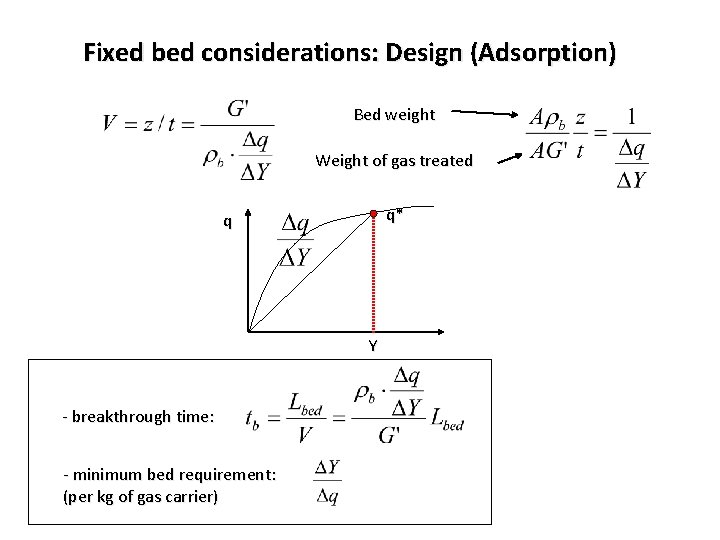
Fixed bed considerations: Design Yin Yout q* qin q* q qin=0 Yout=0 Yin Bed weight Weight of gas treated

Fixed bed considerations: Design (Adsorption) Bed weight Weight of gas treated q* q Y - breakthrough time: - minimum bed requirement: (per kg of gas carrier)

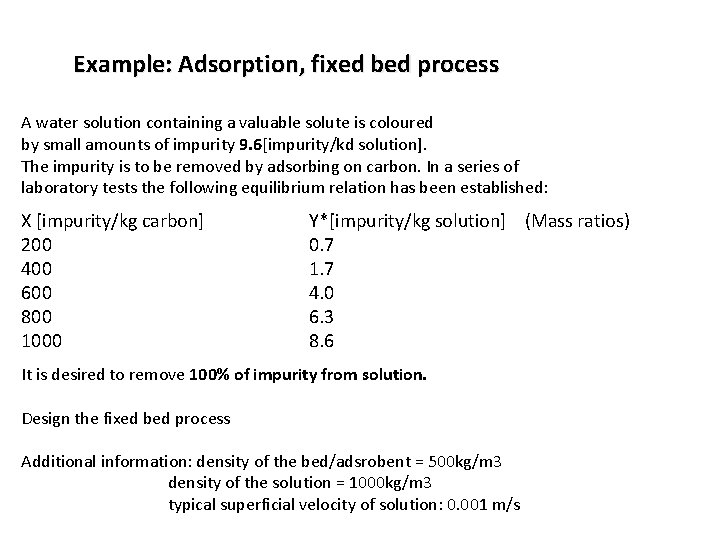
Example: Adsorption, fixed bed process A water solution containing a valuable solute is coloured by small amounts of impurity 9. 6[impurity/kd solution]. The impurity is to be removed by adsorbing on carbon. In a series of laboratory tests the following equilibrium relation has been established: X [impurity/kg carbon] 200 400 600 800 1000 Y*[impurity/kg solution] (Mass ratios) 0. 7 1. 7 4. 0 6. 3 8. 6 It is desired to remove 100% of impurity from solution. Design the fixed bed process Additional information: density of the bed/adsrobent = 500 kg/m 3 density of the solution = 1000 kg/m 3 typical superficial velocity of solution: 0. 001 m/s

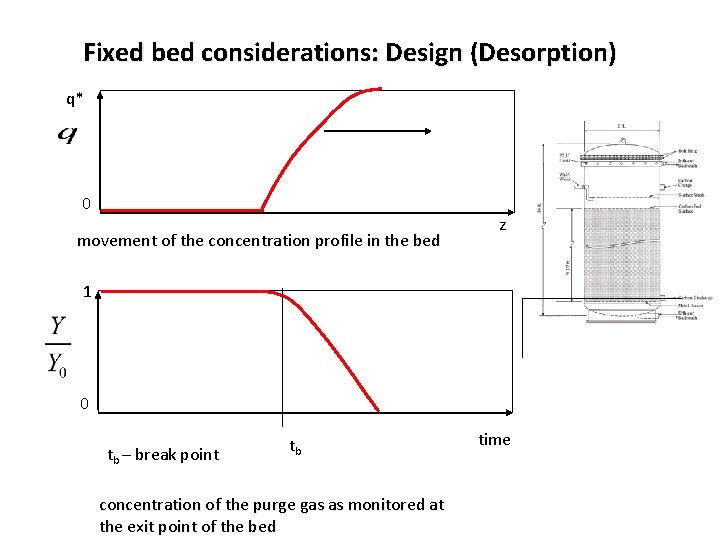
Fixed bed considerations: Design (Desorption) q* 0 movement of the concentration profile in the bed z 1 0 tb – break point tb concentration of the purge gas as monitored at the exit point of the bed time

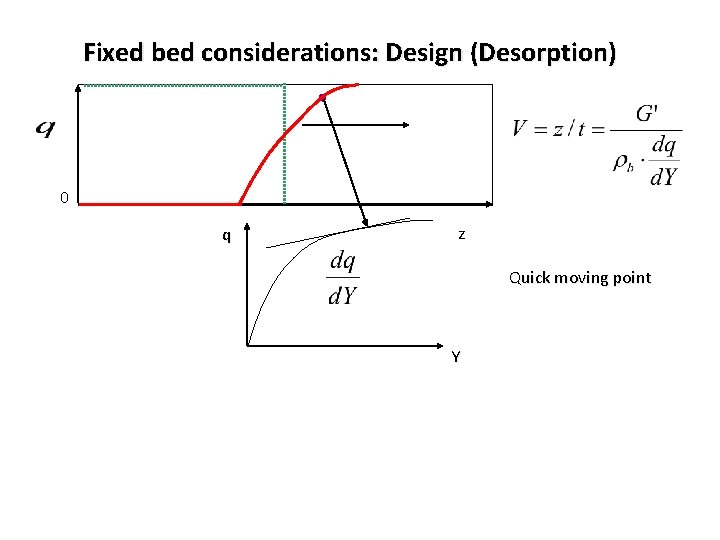
Fixed bed considerations: Design (Desorption) 0 q z Quick moving point Y

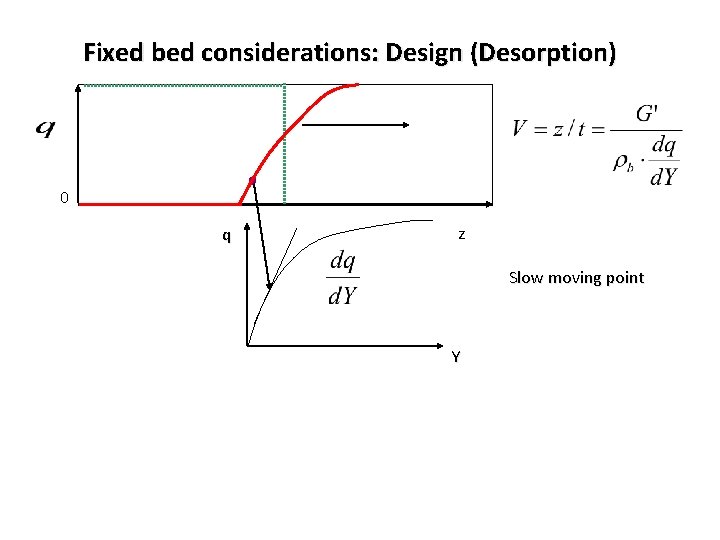
Fixed bed considerations: Design (Desorption) 0 q z Slow moving point Y

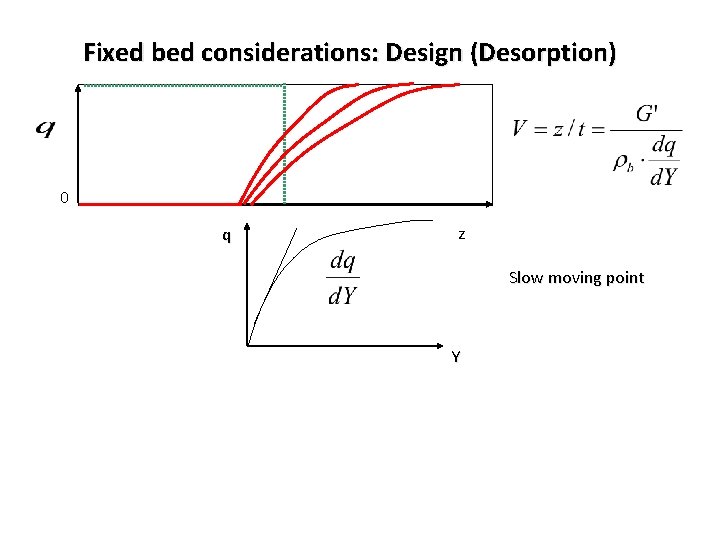
Fixed bed considerations: Design (Desorption) 0 q z Slow moving point Y

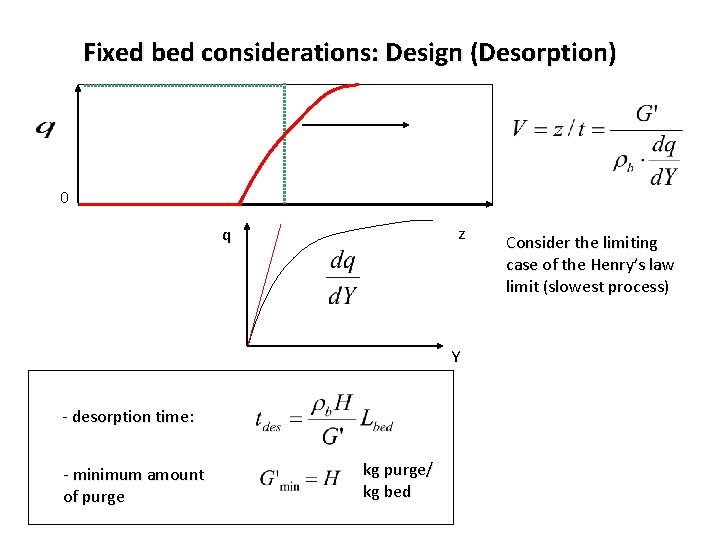
Fixed bed considerations: Design (Desorption) 0 z q Y - desorption time: - minimum amount of purge kg purge/ kg bed Consider the limiting case of the Henry’s law limit (slowest process)