Partition chromatography a liquidliquid chromatography a liquid stationary







- Slides: 15

Partition chromatography a) liquid-liquid chromatography; a liquid stationary phase is retained on the surface of the packing by physical adsorption. b) bonded-phase chromatography; the stationary phase is bonded chemically to the support surfaces.

Columns for bonded-phase chromatography Supports: silica or silica-based composition. These solids are formed as uniform, porous, mechanically sturdy particles commonly having diameters of 3, 5, or 10 μm.

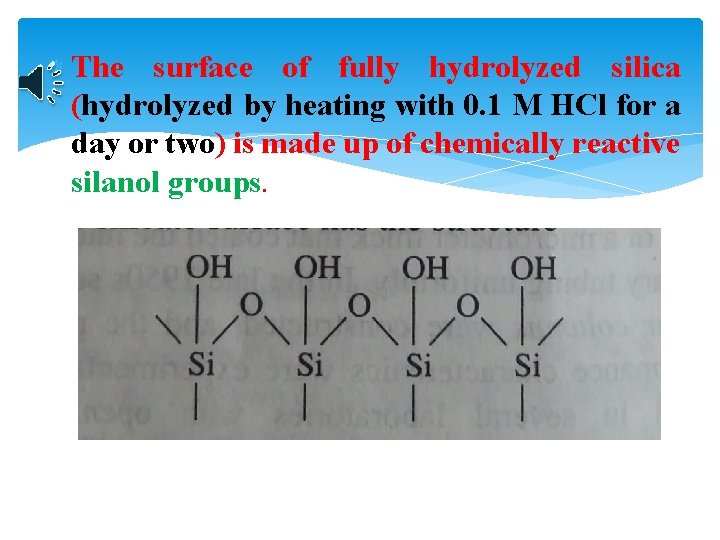
The surface of fully hydrolyzed silica (hydrolyzed by heating with 0. 1 M HCl for a day or two) is made up of chemically reactive silanol groups.

The most useful bonded-phase coating are siloxanes formed by reaction of the hydrolyzed surface with an organochlorosilane. For example, Where R is an alkyl group or a substituted alkyl qroup.

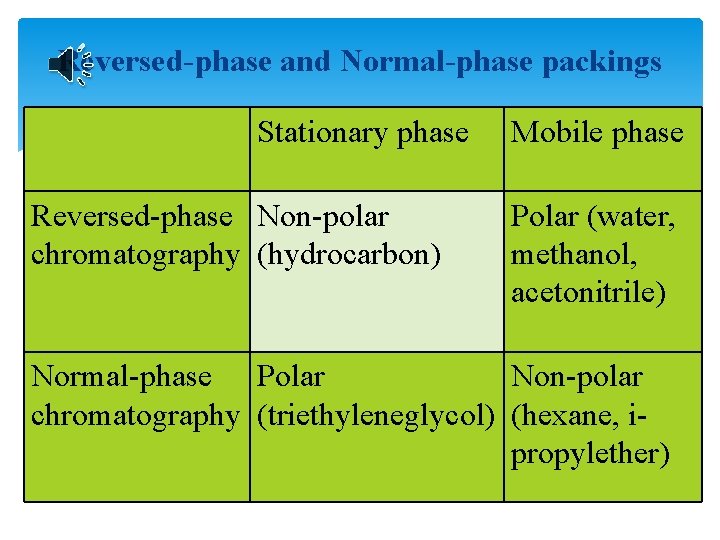
Reversed-phase and Normal-phase packings Stationary phase Reversed-phase Non-polar chromatography (hydrocarbon) Mobile phase Polar (water, methanol, acetonitrile) Normal-phase Polar Non-polar chromatography (triethyleneglycol) (hexane, ipropylether)

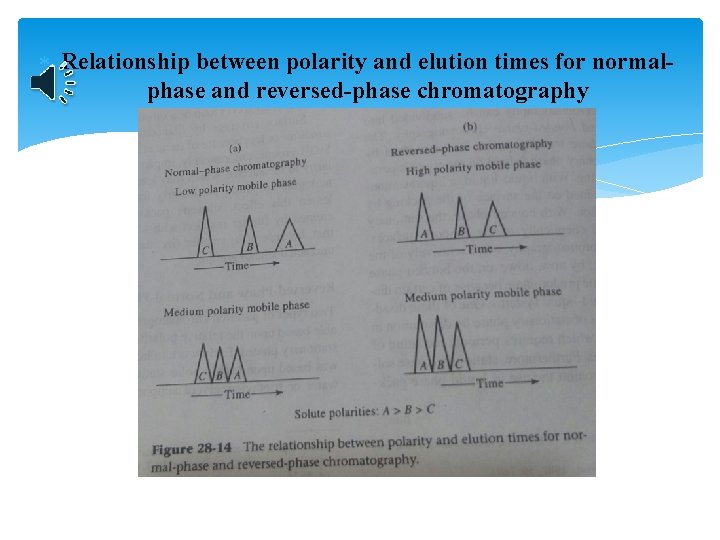
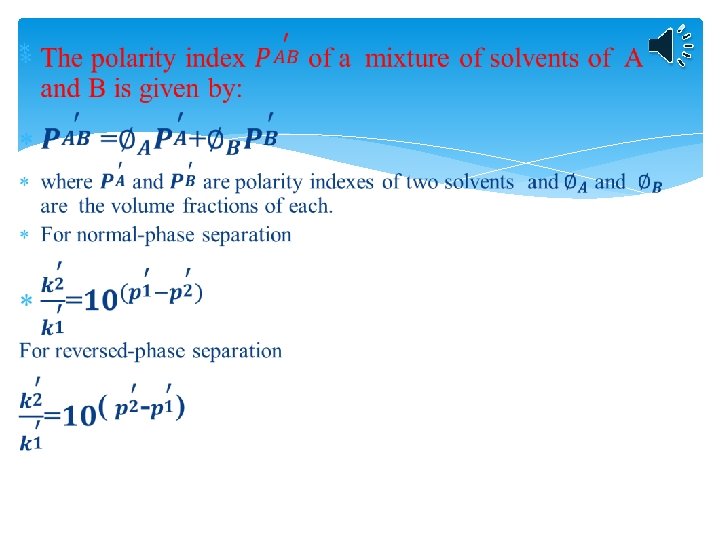
Relationship between polarity and elution times for normalphase and reversed-phase chromatography

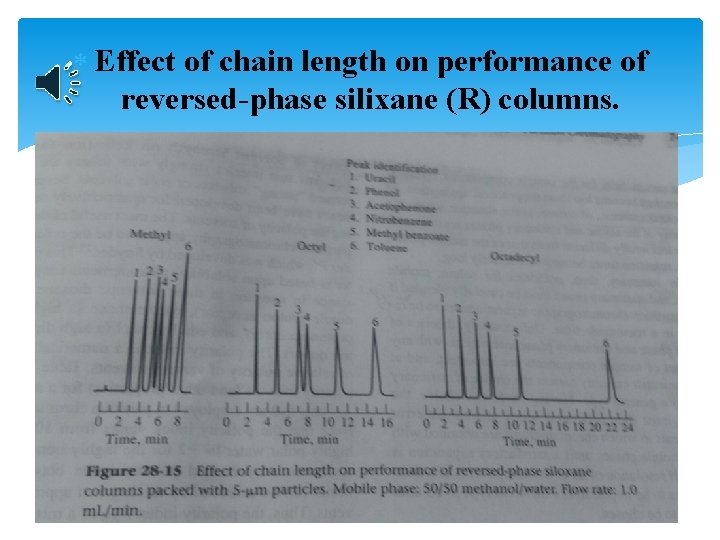
Effect of chain length on performance of reversed-phase silixane (R) columns.

Method development in partition chromatography

Column selection in partition chromatographic separations The polarities of various analyte functional groups in increasing order are: hydrocarbons<ethers<esters<ketones<aldehydes<amides< amines<alcohols. Water is very polar. Often in choosing a column for partition chromatographic separation, the polarity of stationary phase is matched roughly with that of the analytes; a mobile phase of considerably different polarity is then used for elution

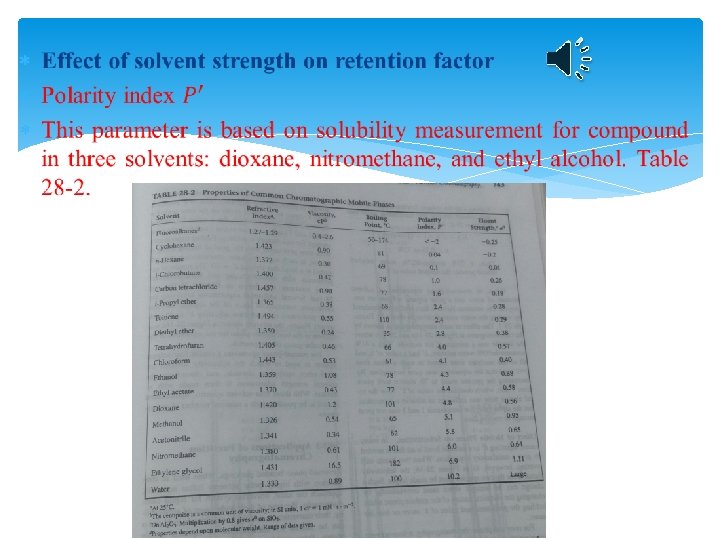
Mobile-phase selection in partition chromatography N, k’, α k’ (2<k’<5 for complex compounds 0. 5<k’<20) is very important in liquid chromatography because strong dependence of this constant upon the composition of mobile phase. Off course α is important.





Applications of partition chromatography
 Adsorption chromatography
Adsorption chromatography Principle gas chromatography
Principle gas chromatography Stationary phase and mobile phase in hplc
Stationary phase and mobile phase in hplc Stationary phase chromatography
Stationary phase chromatography Application of size exclusion chromatography
Application of size exclusion chromatography What is partition coefficient
What is partition coefficient What is chromatography
What is chromatography Disadvantages of liquid chromatography
Disadvantages of liquid chromatography Partition chromatography
Partition chromatography Mikhail s. tswett
Mikhail s. tswett Hplc chromatography introduction
Hplc chromatography introduction High performance liquid chromatography hplc machine
High performance liquid chromatography hplc machine Gas liquid chromatography
Gas liquid chromatography Fast protein liquid chromatography applications
Fast protein liquid chromatography applications High performance liquid chromatography introduction
High performance liquid chromatography introduction Example of pure liquid dielectric
Example of pure liquid dielectric