LiquidLiquid Extraction Example A liquidliquid ternary phase diagram
- Slides: 18

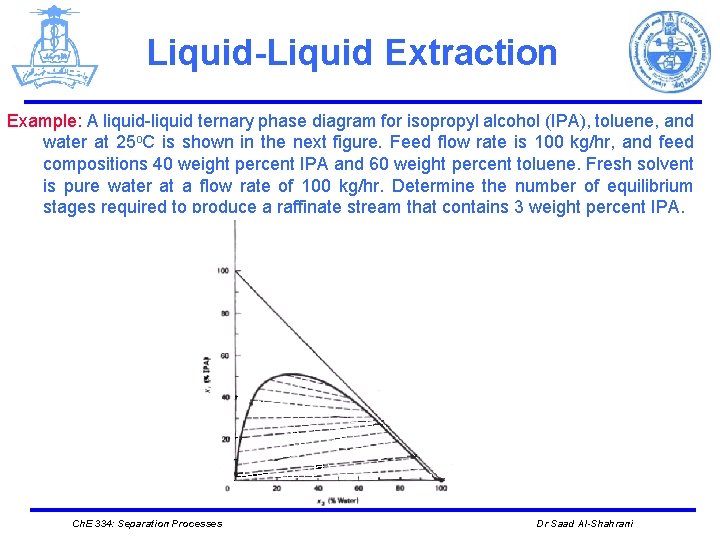
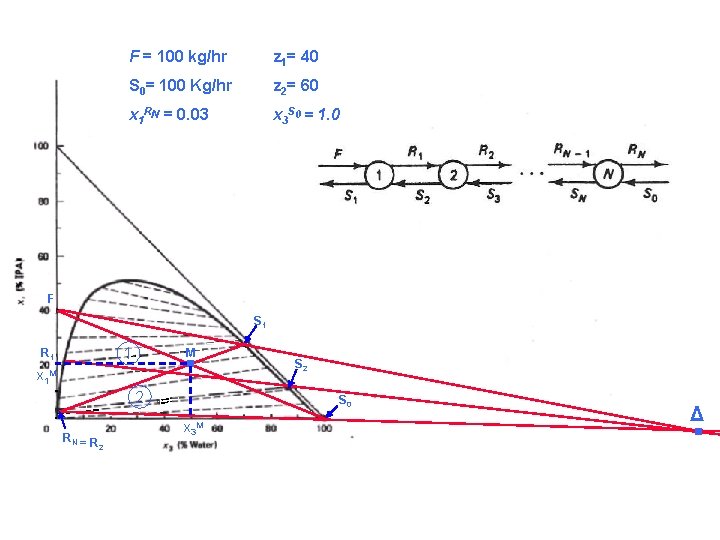
Liquid-Liquid Extraction Example: A liquid-liquid ternary phase diagram for isopropyl alcohol (IPA), toluene, and water at 25 o. C is shown in the next figure. Feed flow rate is 100 kg/hr, and feed compositions 40 weight percent IPA and 60 weight percent toluene. Fresh solvent is pure water at a flow rate of 100 kg/hr. Determine the number of equilibrium stages required to produce a raffinate stream that contains 3 weight percent IPA. Ch. E 334: Separation Processes Dr Saad Al-Shahrani

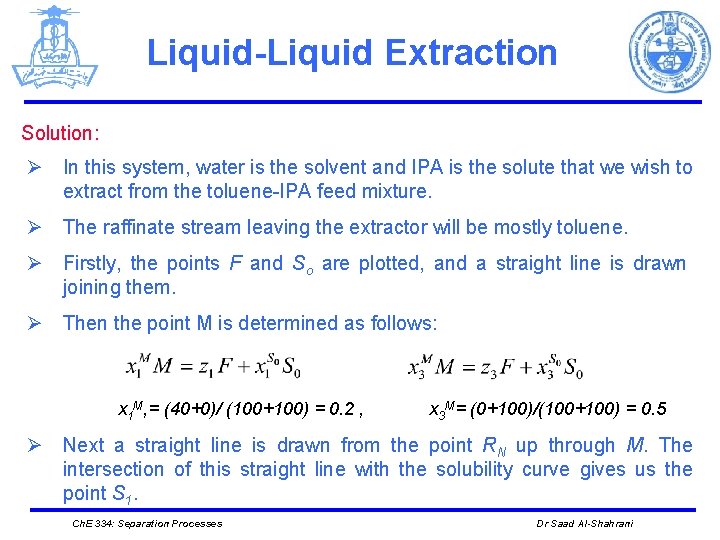
Liquid-Liquid Extraction Solution: Ø In this system, water is the solvent and IPA is the solute that we wish to extract from the toluene-IPA feed mixture. Ø The raffinate stream leaving the extractor will be mostly toluene. Ø Firstly, the points F and So are plotted, and a straight line is drawn joining them. Ø Then the point M is determined as follows: x 1 M, = (40+0)/ (100+100) = 0. 2 , x 3 M= (0+100)/(100+100) = 0. 5 Ø Next a straight line is drawn from the point RN up through M. The intersection of this straight line with the solubility curve gives us the point S 1. Ch. E 334: Separation Processes Dr Saad Al-Shahrani

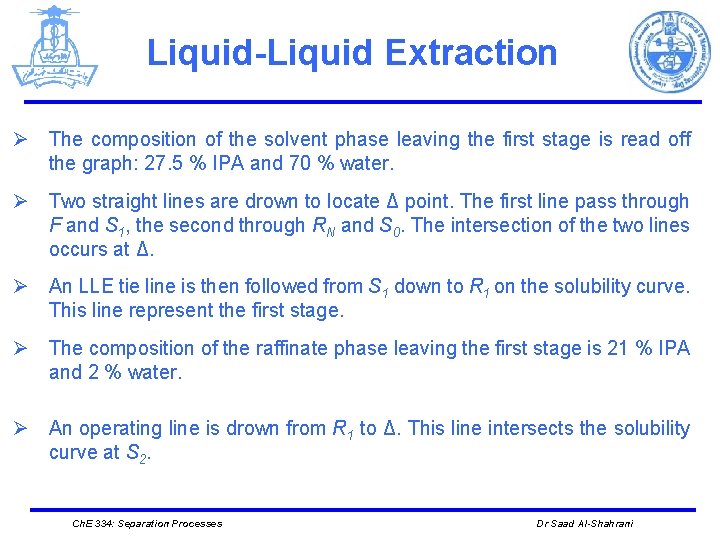
Liquid-Liquid Extraction Ø The composition of the solvent phase leaving the first stage is read off the graph: 27. 5 % IPA and 70 % water. Ø Two straight lines are drown to locate Δ point. The first line pass through F and S 1, the second through RN and S 0. The intersection of the two lines occurs at Δ. Ø An LLE tie line is then followed from S 1 down to R 1 on the solubility curve. This line represent the first stage. Ø The composition of the raffinate phase leaving the first stage is 21 % IPA and 2 % water. Ø An operating line is drown from R 1 to Δ. This line intersects the solubility curve at S 2. Ch. E 334: Separation Processes Dr Saad Al-Shahrani

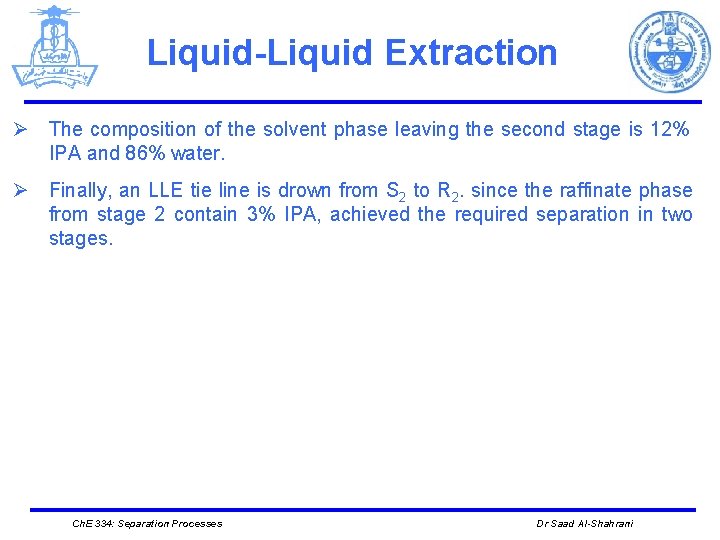
Liquid-Liquid Extraction Ø The composition of the solvent phase leaving the second stage is 12% IPA and 86% water. Ø Finally, an LLE tie line is drown from S 2 to R 2. since the raffinate phase from stage 2 contain 3% IPA, achieved the required separation in two stages. Ch. E 334: Separation Processes Dr Saad Al-Shahrani

F = 100 kg/hr z 1= 40 S 0= 100 Kg/hr z 2= 60 x 1 RN = 0. 03 x 3 S 0 = 1. 0 F . 1 R 1 M x 1 M 2 RN = R S 2 S 0 x 3 M 2 S 1 . Δ

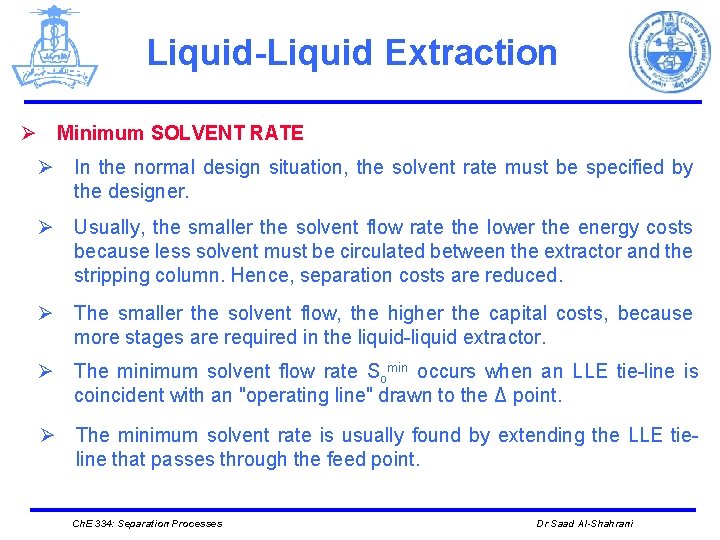
Liquid-Liquid Extraction Ø Minimum SOLVENT RATE Ø In the normal design situation, the solvent rate must be specified by the designer. Ø Usually, the smaller the solvent flow rate the lower the energy costs because less solvent must be circulated between the extractor and the stripping column. Hence, separation costs are reduced. Ø The smaller the solvent flow, the higher the capital costs, because more stages are required in the liquid-liquid extractor. Ø The minimum solvent flow rate Somin occurs when an LLE tie-line is coincident with an "operating line" drawn to the Δ point. Ø The minimum solvent rate is usually found by extending the LLE tieline that passes through the feed point. Ch. E 334: Separation Processes Dr Saad Al-Shahrani

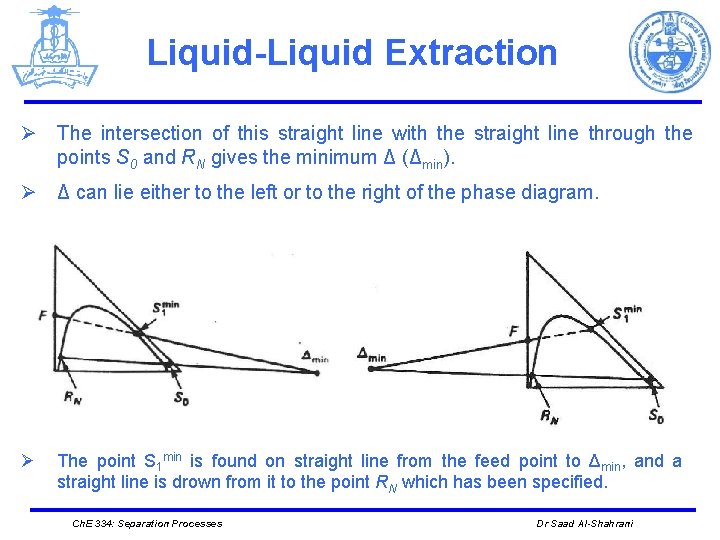
Liquid-Liquid Extraction Ø The intersection of this straight line with the straight line through the points S 0 and RN gives the minimum Δ (Δmin). Ø Δ can lie either to the left or to the right of the phase diagram. Ø The point S 1 min is found on straight line from the feed point to Δmin, and a straight line is drown from it to the point RN which has been specified. Ch. E 334: Separation Processes Dr Saad Al-Shahrani

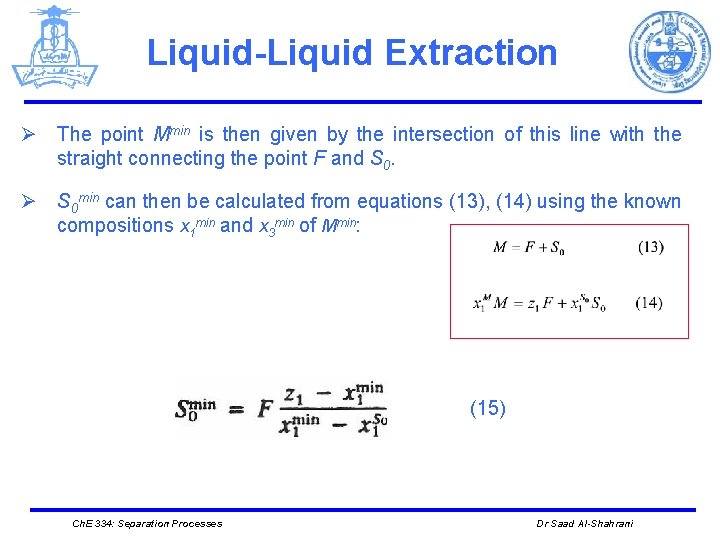
Liquid-Liquid Extraction Ø The point Mmin is then given by the intersection of this line with the straight connecting the point F and S 0. Ø S 0 min can then be calculated from equations (13), (14) using the known compositions x 1 min and x 3 min of Mmin: (15) Ch. E 334: Separation Processes Dr Saad Al-Shahrani

Liquid-Liquid Extraction Ø DESIGN PROCEDURE Ø The summarized specific sequence of steps to design a countercurrent liquid-liquid extractor for a ternary system are as follow: 1. Parameters specified. (a) Feed flow rate and compositions (F, z 1, z 3). (b) Composition of solute in the raffinate stream leaving the last stage (x 1 RN). (c) LLE data for the ternary system. (d) Economic ratio of actual to minimum solvent flow rates (S 0/ S 0 min). (e) Composition of fresh solvent (x 1 S 0, x 3 S 0) Ch. E 334: Separation Processes Dr Saad Al-Shahrani

Liquid-Liquid Extraction 2. Calculate the minimum solvent flow rate S 0 min. (a) Extend the tie-line through the point F. (b) Draw a line through the points S 0 and RN. (c) Locate S 1 min on the tie-line through F. (d) Draw a line from S 1 min to RN. (e) The intersection of this line with a line through F and S 0 gives the point Mmin. (f) Read off the composition (x 1 min) at Mmin. (g) Calculate S 0 min from equation (15). 3. Set the actual fresh solvent flow rate 4. Calculate the point M from equations (13) and (14). 5. Draw a line from RN through M to find S 1 on the solubility curve. Ch. E 334: Separation Processes Dr Saad Al-Shahrani

Liquid-Liquid Extraction 6. Locate the Δ point by drawing two lines, one through S 0 and RN and one through the other through S 1 and F. 7. Step off stages, alternately using the LLE tie-lines and the A point, as follows: Ch. E 334: Separation Processes Dr Saad Al-Shahrani

Liquid-Liquid Extraction Example: Design a countercurrent liquid-liquid extractor to separate acetone from methyl isobutyl ketone (MIK) using water as a solvent. The column is to operate at 25°C. The organic feed rate is 10, 000 kg/h, and the composition is 45 weight percent acetone and 55 weight percent ketone. The fresh solvent is pure water, and twice the minimum solvent rate is to be used. The concentration of acetone in the organic raffinate leaving the top of the extractor is to be 2. 5 weight percent acetone. Solution: acetone is the solute to be separated from MIK using water as the solvent. Thus, component 1 is acetone and component 3 is water. The point RN is plotted on the solubility curve at x 1 RN = 2. 5 weight percent acetone. The point S 0 is plotted at the right lower comer of the diagram, since x 3 So = 100 weight percent water. The point F is plotted on the vertical axis at z 1 = 45 weight percent acetone. Ch. E 334: Separation Processes Dr Saad Al-Shahrani

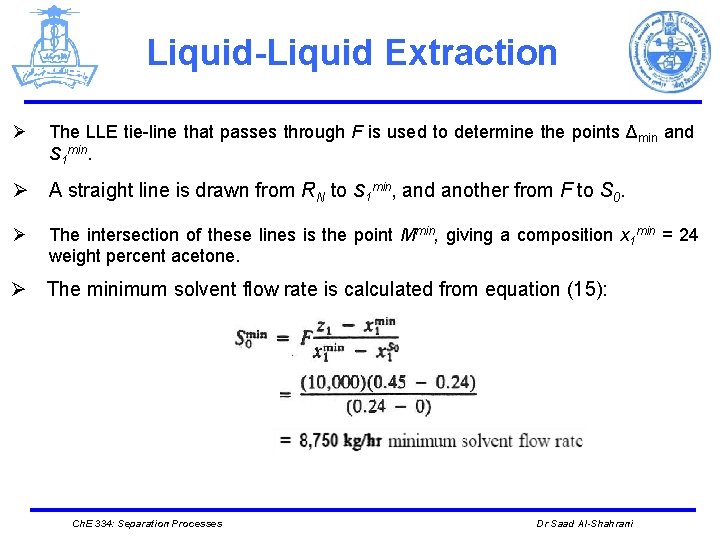
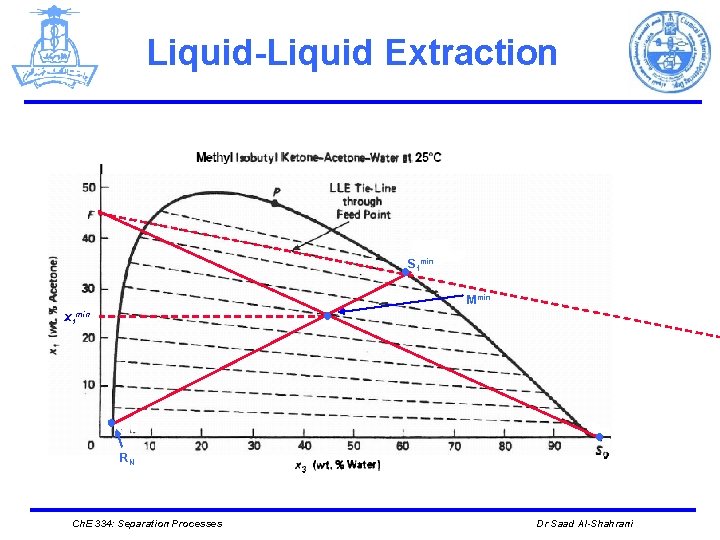
Liquid-Liquid Extraction Ø The LLE tie-line that passes through F is used to determine the points Δmin and S 1 min. Ø A straight line is drawn from RN to S 1 min, and another from F to S 0. Ø The intersection of these lines is the point Mmin, giving a composition x 1 min = 24 weight percent acetone. Ø The minimum solvent flow rate is calculated from equation (15): Ch. E 334: Separation Processes Dr Saad Al-Shahrani

Liquid-Liquid Extraction S min ● 1 ● x 1 min ● Mmin ● RN Ch. E 334: Separation Processes Dr Saad Al-Shahrani

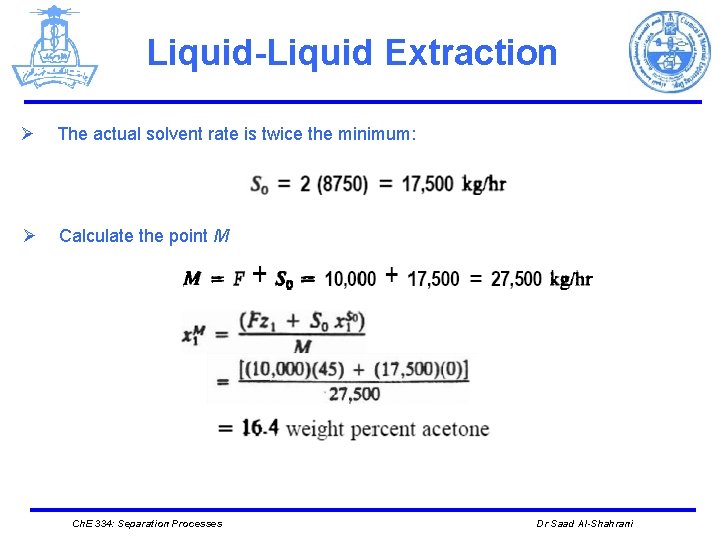
Liquid-Liquid Extraction Ø The actual solvent rate is twice the minimum: Ø Calculate the point M Ch. E 334: Separation Processes Dr Saad Al-Shahrani

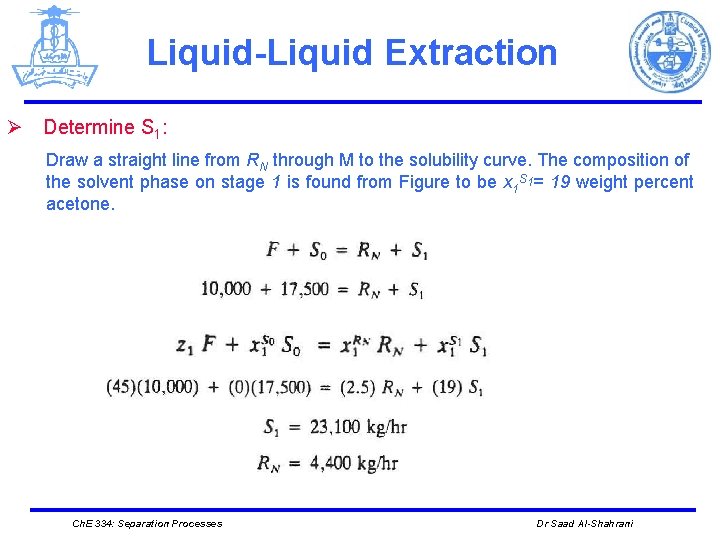
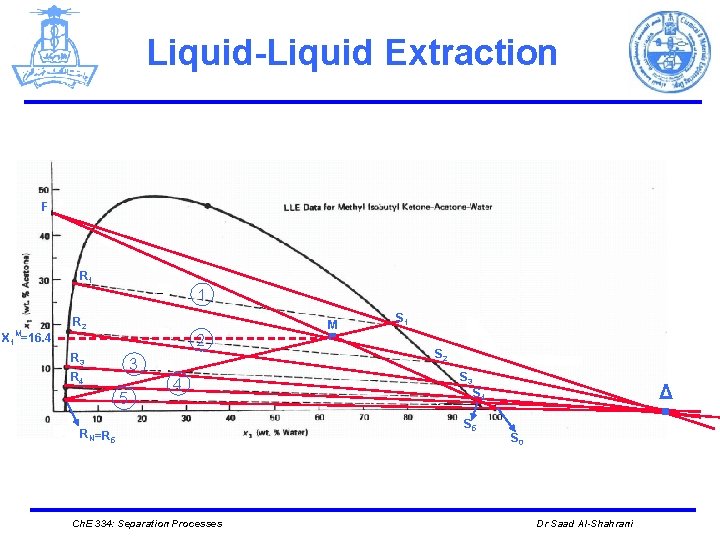
Liquid-Liquid Extraction Ø Determine S 1: Draw a straight line from RN through M to the solubility curve. The composition of the solvent phase on stage 1 is found from Figure to be x 1 S 1= 19 weight percent acetone. Ch. E 334: Separation Processes Dr Saad Al-Shahrani

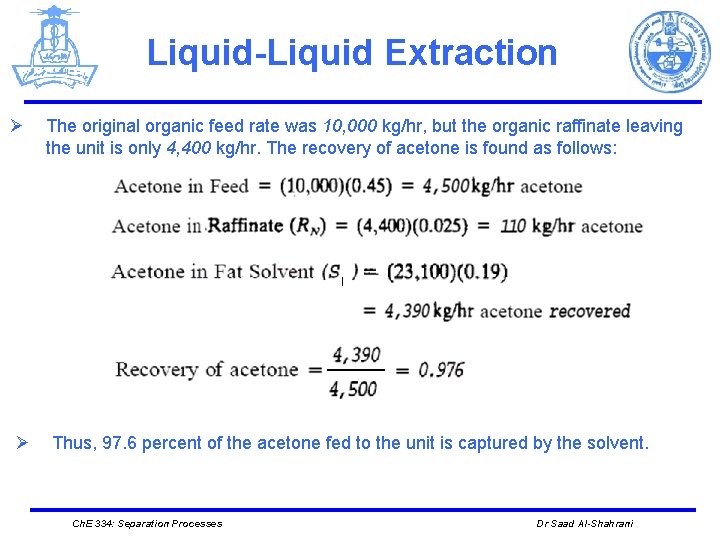
Liquid-Liquid Extraction Ø Ø The original organic feed rate was 10, 000 kg/hr, but the organic raffinate leaving the unit is only 4, 400 kg/hr. The recovery of acetone is found as follows: Thus, 97. 6 percent of the acetone fed to the unit is captured by the solvent. Ch. E 334: Separation Processes Dr Saad Al-Shahrani

Liquid-Liquid Extraction F R 1 1 X 1 M=16. 4 R 2 2 R 3 3 R 4 5 4 RN =R 5 Ch. E 334: Separation Processes . M S 1 S 2 S 3 . Δ S 4 S 5 S 0 Dr Saad Al-Shahrani