Pediatric Congenital Heart Disease CHD Incidence 5 8









































- Slides: 95

Pediatric Congenital Heart Disease

CHD • Incidence: 5 -8 per 1000 live births – Major cause of death in first year of life (after prematurity) – Most common anomaly is VSD – 28% of kids with CHD have another recognized anomaly (trisomy 21, 13, 18, +++ )

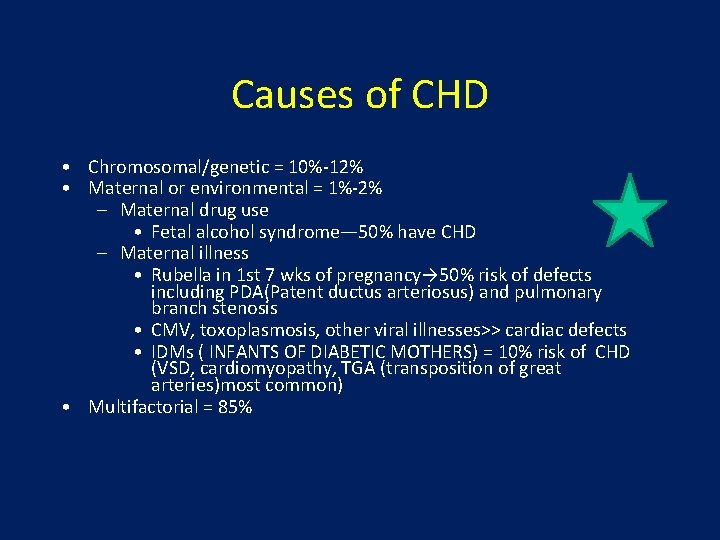
Causes of CHD • Chromosomal/genetic = 10%-12% • Maternal or environmental = 1%-2% – Maternal drug use • Fetal alcohol syndrome— 50% have CHD – Maternal illness • Rubella in 1 st 7 wks of pregnancy→ 50% risk of defects including PDA(Patent ductus arteriosus) and pulmonary branch stenosis • CMV, toxoplasmosis, other viral illnesses>> cardiac defects • IDMs ( INFANTS OF DIABETIC MOTHERS) = 10% risk of CHD (VSD, cardiomyopathy, TGA (transposition of great arteries)most common) • Multifactorial = 85%

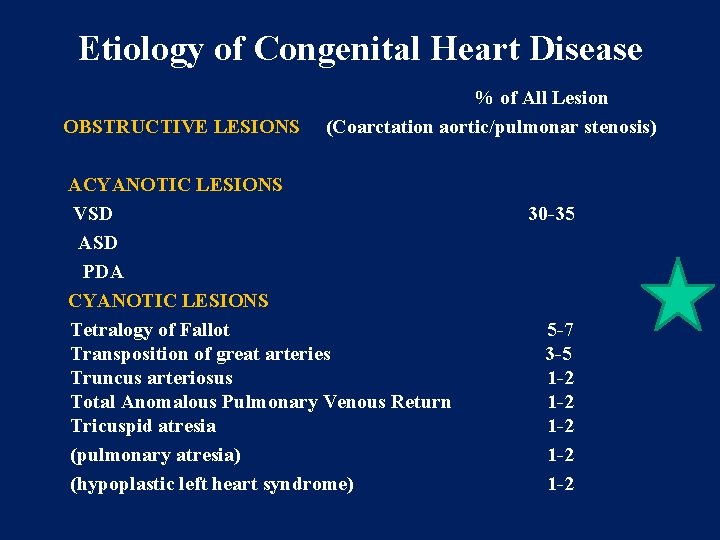
Etiology of Congenital Heart Disease OBSTRUCTIVE LESIONS % of All Lesion (Coarctation aortic/pulmonar stenosis) ACYANOTIC LESIONS VSD ASD PDA CYANOTIC LESIONS Tetralogy of Fallot Transposition of great arteries Truncus arteriosus Total Anomalous Pulmonary Venous Return Tricuspid atresia (pulmonary atresia) (hypoplastic left heart syndrome) 30 -35 5 -7 3 -5 1 -2 1 -2 1 -2

Older Classifications of CHD • Acyanotic – May become cyanotic • Cyanotic – May be pink – May develop CHF

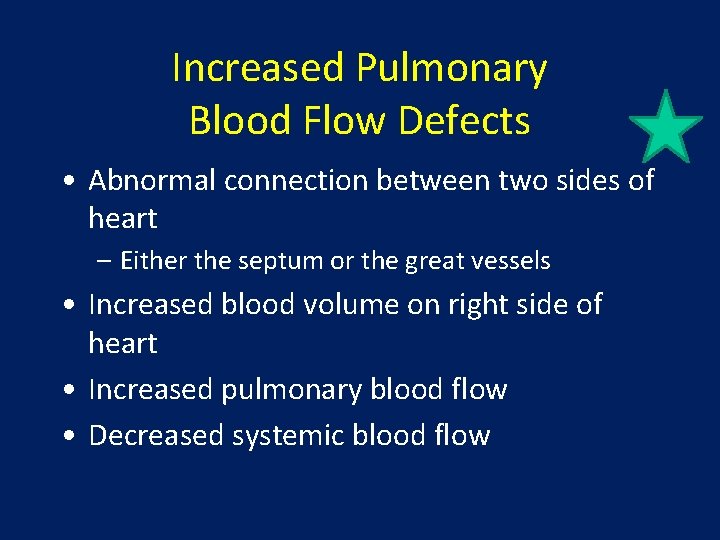
Newer Classification of CHD • Hemodynamic characteristics – Increased pulmonary blood flow – Decreased pulmonary blood flow – Obstruction of blood flow out of the heart – Mixed blood flow

Increased Pulmonary Blood Flow Defects • Abnormal connection between two sides of heart – Either the septum or the great vessels • Increased blood volume on right side of heart • Increased pulmonary blood flow • Decreased systemic blood flow

Increased Pulmonary Blood Flow Defects • Atrial septal defect • Ventricular septal defect • Patent ductus arteriosus

Obstructive Defects • Coarctation of the aorta • Aortic stenosis • Pulmonic stenosis

Decreased Pulmonary Blood Flow Defects • Tetralogy of Fallot • Tricuspid atresia

Mixed Defects • Transposition of great vessels • Total anomalous pulmonary venous connection • Hypoplastic heart syndrome – Right – Left

ATRIAL SEPTAL DEFECT

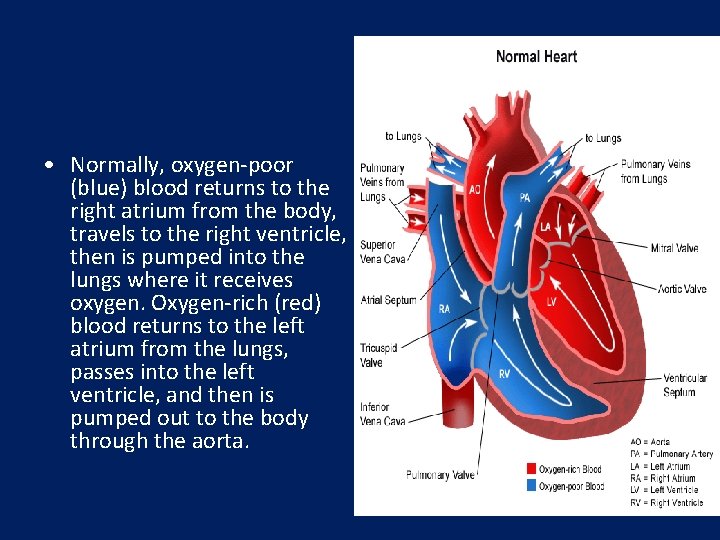
• Normally, oxygen-poor (blue) blood returns to the right atrium from the body, travels to the right ventricle, then is pumped into the lungs where it receives oxygen. Oxygen-rich (red) blood returns to the left atrium from the lungs, passes into the left ventricle, and then is pumped out to the body through the aorta.

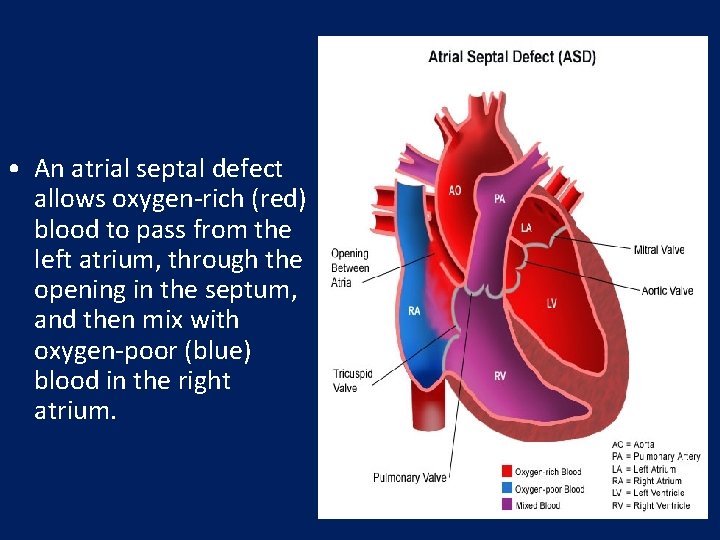
• An atrial septal defect allows oxygen-rich (red) blood to pass from the left atrium, through the opening in the septum, and then mix with oxygen-poor (blue) blood in the right atrium.

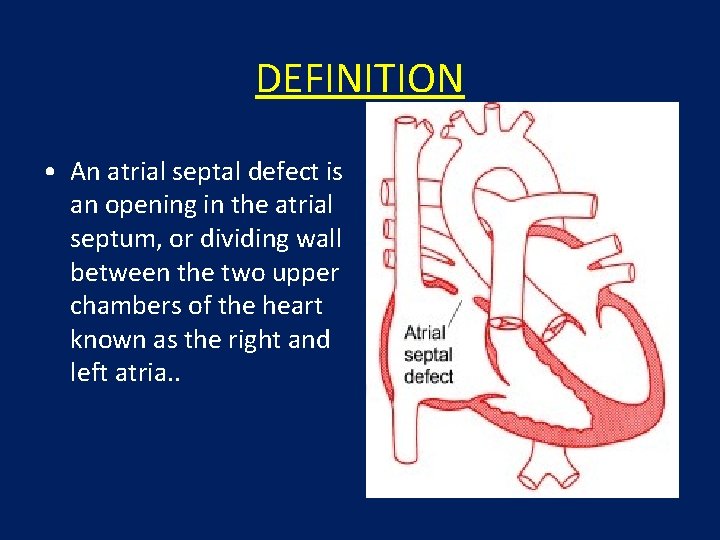
DEFINITION • An atrial septal defect is an opening in the atrial septum, or dividing wall between the two upper chambers of the heart known as the right and left atria. .

HEMODYNAMICS • RT. ATRIUM RECEIVES BLOOD FROM SUP. & INF. VENA CAVA & FROM LT. ATRIUM • RT. ATRIUM ENLARGES

HEMODYNAMICS • LARGE VOL OF BLOOD FROM RT. ATRIUM PASSES THRU NORMAL TRICUSPID VALVE & PULMONARY VALVE • DELAYED DIASTOLIC MURMUR(LOW LT STERNAL BORDER) • RT. VENTRICLE ENLARGES • PULMONARY EJECTION MURMUR

HEMODYNAMICS • PULM. VALVE CLOSES LATE & P 2 IS DELAYED • RV IS FULLY LOADED, SO FURTHER RISE IN RV VOLUME CANNOT OCCUR • WIDELY SPLIT S 2 • FIXED SPLIT S 2 • ACCENTUATED S 2

PRESENTATION • • • recurrent chest infections fatigue sweating rapid breathing shortness of breath poor growth

ON EXAMINATION • INSPECTION • PARASTERNAL IMPULSE • PALPATION • SYSTOLIC THRILL AT 2 ND LT SPACE

AUSCULTATION • • WIDE FIXED SPLIT S 2 ACCENTUATED P 2 ESM AT LT 2 nd & 3 rd INTERSPACES DELAYED DIASTOLIC MURMUR AT LOW LT INTERSPACE

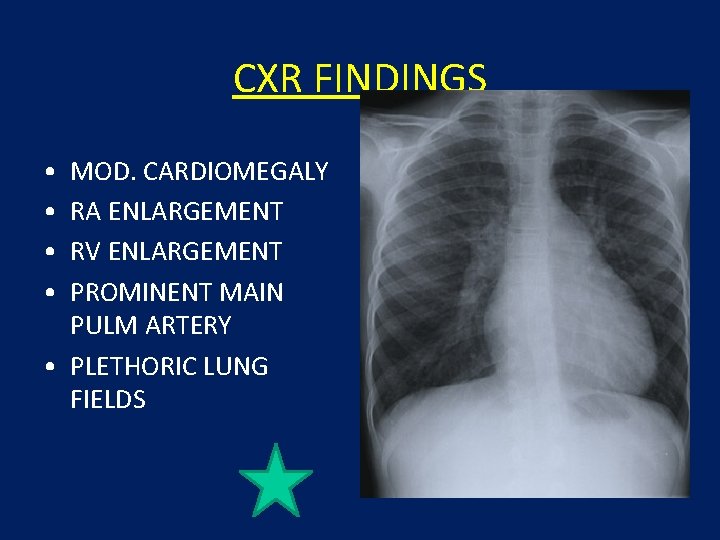
CXR FINDINGS • • MOD. CARDIOMEGALY RA ENLARGEMENT RV ENLARGEMENT PROMINENT MAIN PULM ARTERY • PLETHORIC LUNG FIELDS

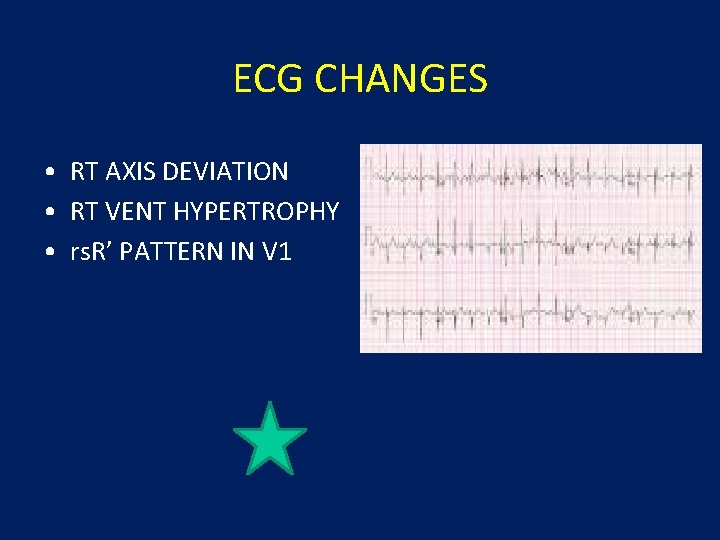
ECG CHANGES • RT AXIS DEVIATION • RT VENT HYPERTROPHY • rs. R’ PATTERN IN V 1

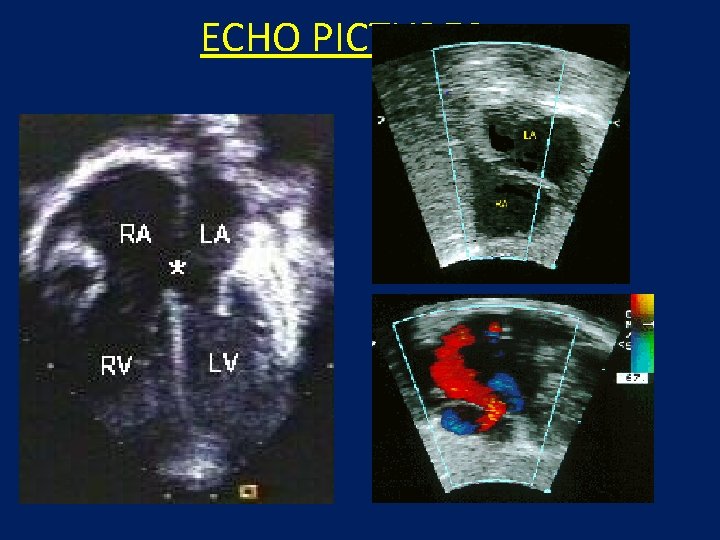
ECHO PICTURES

SEVERITY ASSESMENT • INTENSITY OF THE TWO MURMURS • THE HEART SIZE

COMPLICATION • PULMONARY HYPERTENSION (ABOVE 20 YEARS) • DISAPPEARANCE OF DIASTOLIC MURMUR • APPEARANCE OF PULM EJECN CLICK • LOUD PALPABLE P 2 • P 2_STILL WIDELY SPLIT

MANAGEMENT • • MEDICAL ANTIBIOTICS FOR CHEST INFECTIONS DIGOXIN TO INCREASE WORK OF HEART DIURETICS TO REDUCE PRELOAD

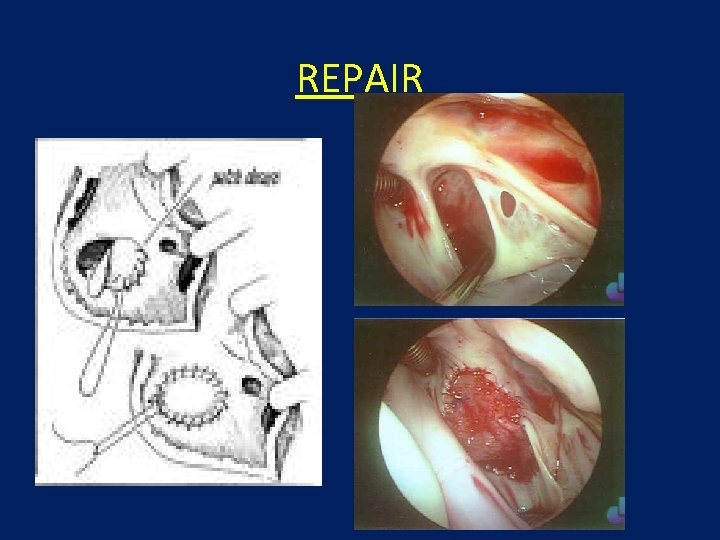
REPAIR

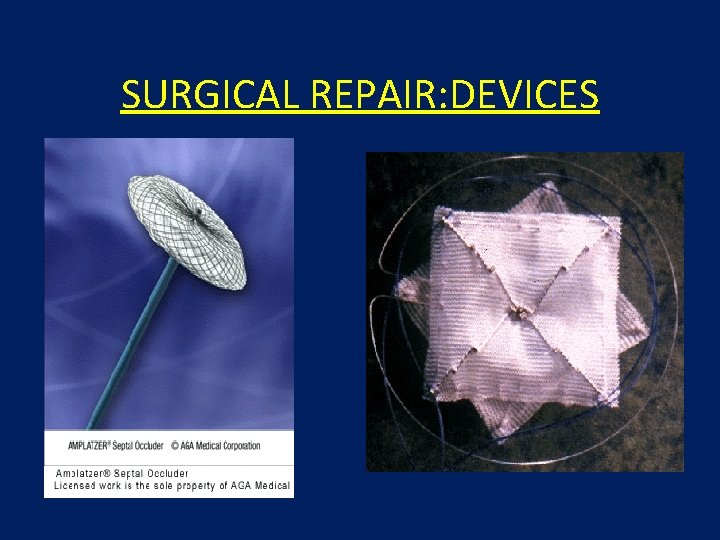
SURGICAL REPAIR: DEVICES

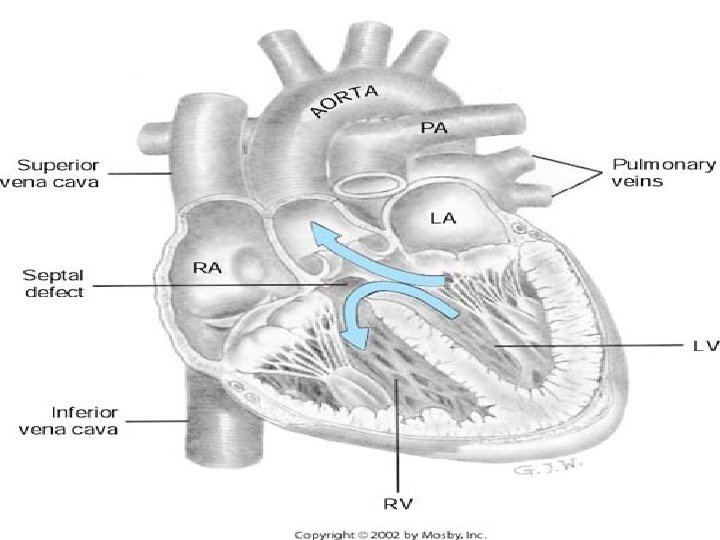
VENTRICULAR SEPTAL DEFECT • 2 nd most common CHD(32%): CONGENITAL HEART DISEASE • SYNONYMS * Roger’s disease * Interventricular septal defect * congenital cardiac anomaly

PATHOPHYSIOLOGY • primarily depends on size & status of pulm. vascular bed rather than location • Small communication (less than 0. 5 cm`) VSD is restrictive & rt. ventricular pressure is normal – does not cause significant hemodynamic derangement (Qp: Qs=1. 75: 1. 0)= RATIO OF PULMONARY/SYSTHEMIC BLOOD FLOW • Moderately restrictive VSD with a moderate shunt (Qp: Qs=1. 5 -2. 5: 1. 0) &poses hemodynamic burden on LV • Large nonrestrictive VSDs (more than 1. 0 cm`) Rt&Lt ventricular pressure are equalised(Qp: Qs is more than 2: 1) • Large VSDs at birth , PVR ( pulmonary vascular resistance) may remain higher than normal and Lt to Rt shunt may intially limited – involution of media of small pulm. arterioles, PVR decreases—large Lt to Rt shunt ensues • In some infants large VSDs , pulm. arteriolar thickness never decreases – pulm. obstructive disease develops. when Qp: Qs=1: 1 shunt becomes bidirectional, signs of heart failure abate &pt. becomes cyanotic. (Eisenmenger syndrome)

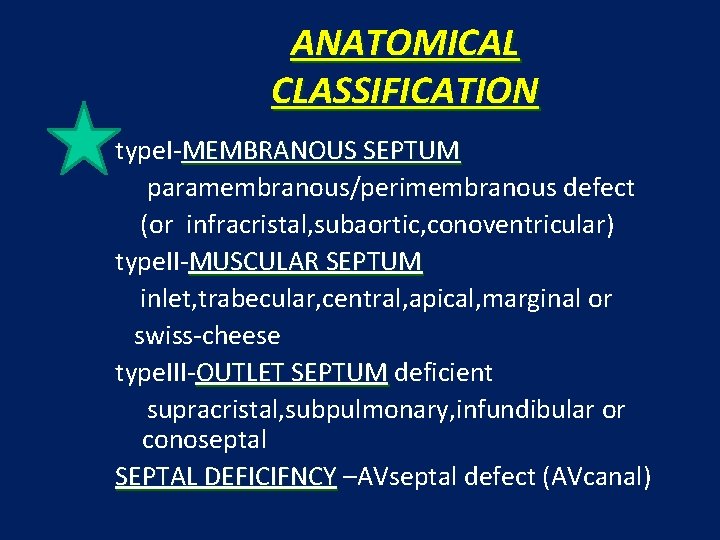
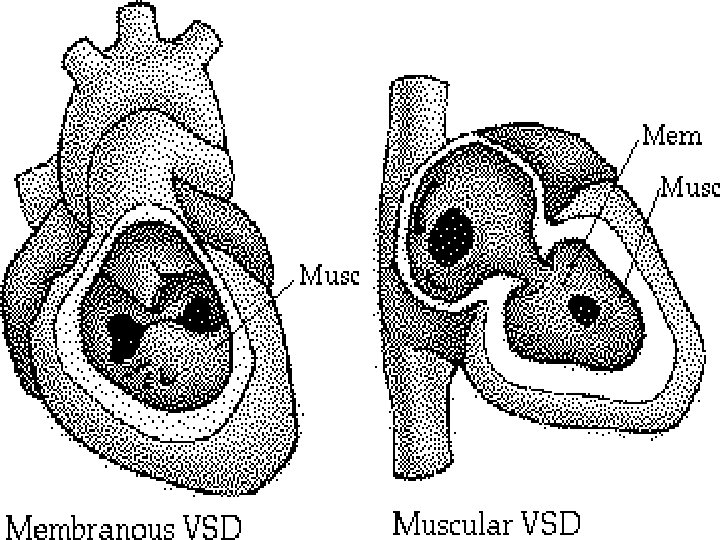
ANATOMICAL CLASSIFICATION type. I-MEMBRANOUS SEPTUM paramembranous/perimembranous defect (or infracristal, subaortic, conoventricular) type. II-MUSCULAR SEPTUM inlet, trabecular, central, apical, marginal or swiss-cheese type. III-OUTLET SEPTUM deficient supracristal, subpulmonary, infundibular or conoseptal SEPTAL DEFICIFNCY –AVseptal defect (AVcanal)



CLINICAL FEATURES • Race : no particular racial predilection • Sex : no particular sex preference • Age : infants– infants difficult in postnatal period, although ccf during first 6 mths is frequent, X-ray&ECG are normal. children—after first year variable clinical children picture emerges. small VSD – asymptomatic large VSD – common symptoms -palpitation, dyspnoea on exertion, feeding difficulties , poor growth -frequent chest infections

PHYSICAL FINDINGS • Pulse pressure is relatively wide • Precordium is hyperkinetic with a systolic thrill at LSB( LEFT STERNAL BORDER) • S 1&S 2 are masked by a PSM at Lt. sternal border , max. intensity of the murmur is best heard at 3 rd, 4 th&5 th Lt interspace. Also well heard at the 2 nd space but not conducted beyond apex • Presence of mid-diastolic , low pitched rumble at the apex is caused by increased flow across the mitral valve &indicates Qp: Qs=2: 1/greater • Maladie de Roger –small VSD presenting in older children as a loud PSM w/o other significant hemodynamic changes

INVESTIGATIONS • ECHOCARDIOGRAPHY two-dimensional &doppler colour flow • CHEST RADIOGRAPHY - normal - biventricular hypertrophy - pulmonary plethora • ELECTROCARDIOGRAPHY -small. VSD ~ normal tracing -mod. VSD ~ broad, notched P wave characteristic of Lt. Atrial overload as well as LV overload, namely, deep Q waves & tall R waves in leads V 5 and V 6 and often AF -large VSD ~RVH with rt. axis deviation. With further progression biventricular hypertrophy; P waves may be notched/peaked.

Other investigations • CAT SCAN (Computed Axial Tomography) • MRI • ULTRASOUND • ANGIOGRAPHY (cardiac catheterization and angiography)

COMPLICATIONS • • • Congestive cardiac failure Infective endocarditis on rt. ventricular side Aortic insufficiency Complete heart block Delayed growth & development (FTT) in infancy Damage to electrical conduction system during surgery(causing arrythmias) • Pulmonary hypertension

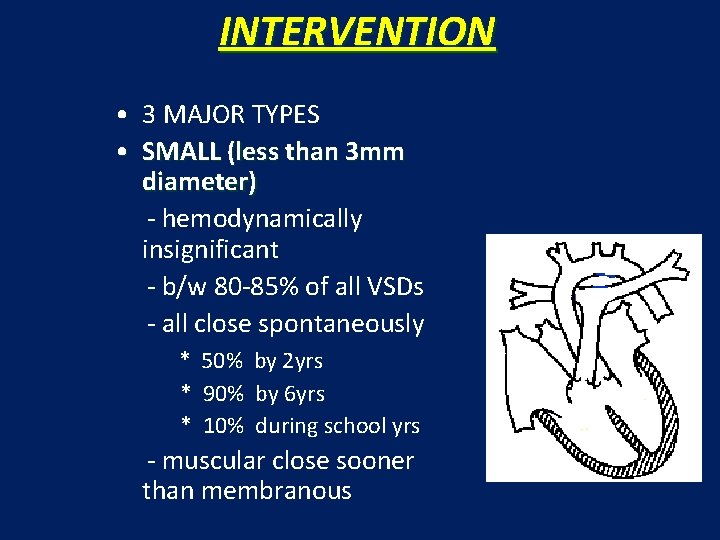
INTERVENTION • 3 MAJOR TYPES • SMALL (less than 3 mm diameter) - hemodynamically insignificant - b/w 80 -85% of all VSDs - all close spontaneously * 50% by 2 yrs * 90% by 6 yrs * 10% during school yrs - muscular close sooner than membranous

NEWBORN VSD • Most common lesion • 2/3 rds close spontaneously • Small VSD • Definite murmur • Will probably close • Large VSD • No murmur • No problems • Home with Mom


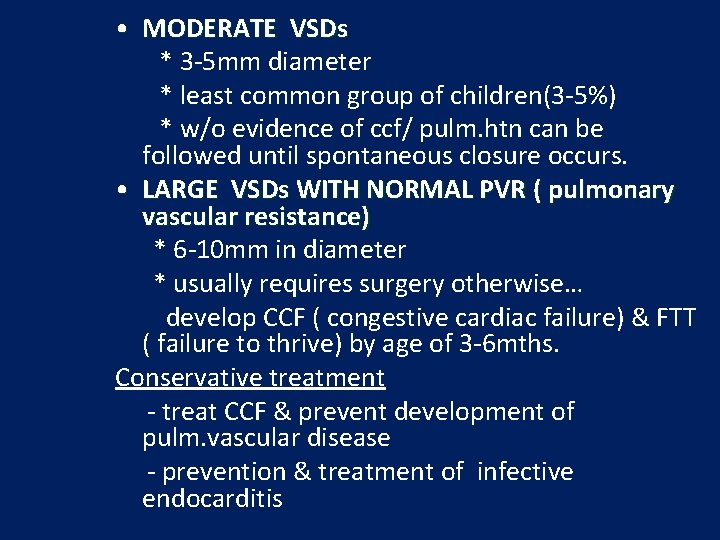
• MODERATE VSDs * 3 -5 mm diameter * least common group of children(3 -5%) * w/o evidence of ccf/ pulm. htn can be followed until spontaneous closure occurs. • LARGE VSDs WITH NORMAL PVR ( pulmonary vascular resistance) * 6 -10 mm in diameter * usually requires surgery otherwise… develop CCF ( congestive cardiac failure) & FTT ( failure to thrive) by age of 3 -6 mths. Conservative treatment - treat CCF & prevent development of pulm. vascular disease - prevention & treatment of infective endocarditis

Evaluation of Cyanotic Heart Disease

Physical Examination Central Cyanosis vs. Peripheral cyanosis Vital signs Lung and CNS examination to rule these out Cardiac Examination Heaves, thrills, abnormal or increased precordial activity Absent or diminished femoral pulses Abnormal first or second heart sound (abnormal splitting) Extra heart sounds (gallop, ejection click, opening snap) Murmurs that are loud, harsh, blowing

Initial evaluation of child’s heart Physical exam • Listen to heart first when/if infant quiet (warm stethoscope) • First concentrate on S 1 and especially S 2 Louder than normal? Split normally? • Systolic murmur: Starts after or obscures S 1? • Diastolic murmur? • Widely radiating murmur? • Palpate liver • BP in arm and leg • Tongue - cyanosis

History Difficulty feeding, irritablility, diaphoresis (perspiring profusely), failure to thrive (FTT) Prenatal history: maternal diabetes, SLE Congenital Infections (TORCH) Drugs taken in pregnancy Family history: heart problem before 50 y. o. Chromosomal Abnormalities

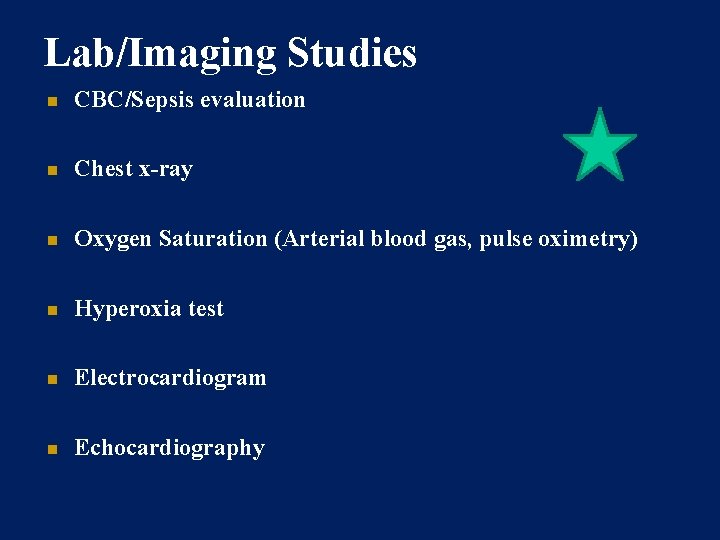
Lab/Imaging Studies CBC/Sepsis evaluation Chest x-ray Oxygen Saturation (Arterial blood gas, pulse oximetry) Hyperoxia test Electrocardiogram Echocardiography

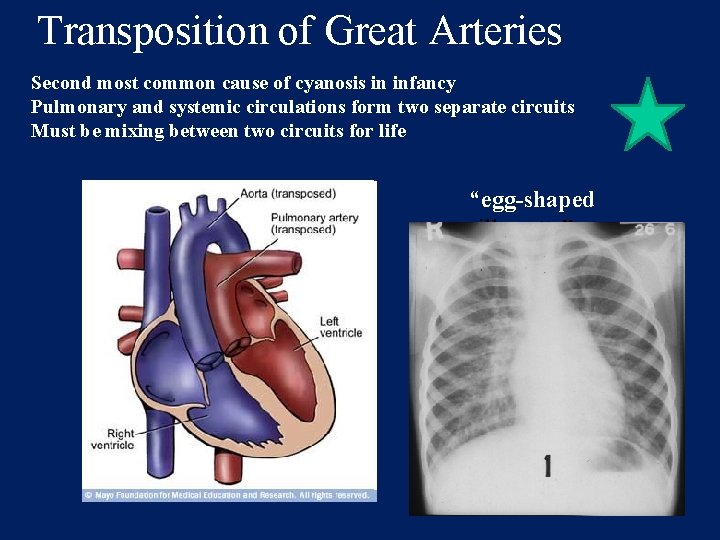
Transposition of Great Arteries Second most common cause of cyanosis in infancy Pulmonary and systemic circulations form two separate circuits Must be mixing between two circuits for life “egg-shaped silhouette”

Clinical Findings Severe cyanosis present at birth 1/3 have VSD, some have ASD Some have subpulmonic stenosis Loud, single S 2 Systolic murmur indicates VSD or pulmonic stenosis ECG reveals right ventricular hypertrophy

Transposition of Great Arteries: Tx PGE 1 administration necessary Balloon atrial septostomy necessary (Rashkind procedure) Arterial Switch procedure performed first week of life

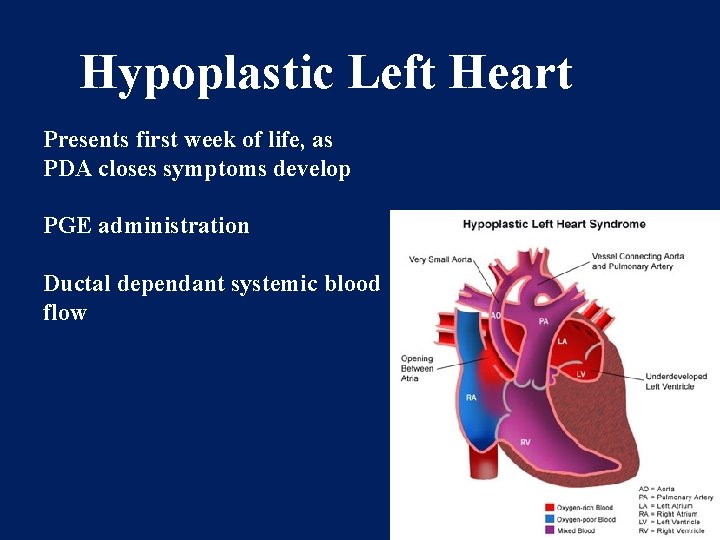
Hypoplastic Left Heart Presents first week of life, as PDA closes symptoms develop PGE administration Ductal dependant systemic blood flow

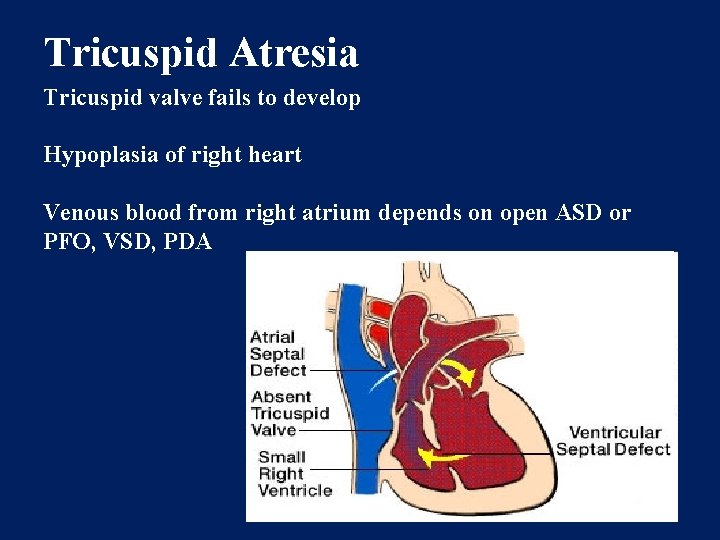
Tricuspid Atresia Tricuspid valve fails to develop Hypoplasia of right heart Venous blood from right atrium depends on open ASD or PFO, VSD, PDA

Tricuspid Atresia-Clinical Findings Progressive cyanosis as PDA closes 30% transposition of great arteries 70% some degree of Pulmonic stenosis Tacypneic, single S 2 Systolic murmur along left lower sternal border (VSD) ECG reveals left ventricular hypertrophy

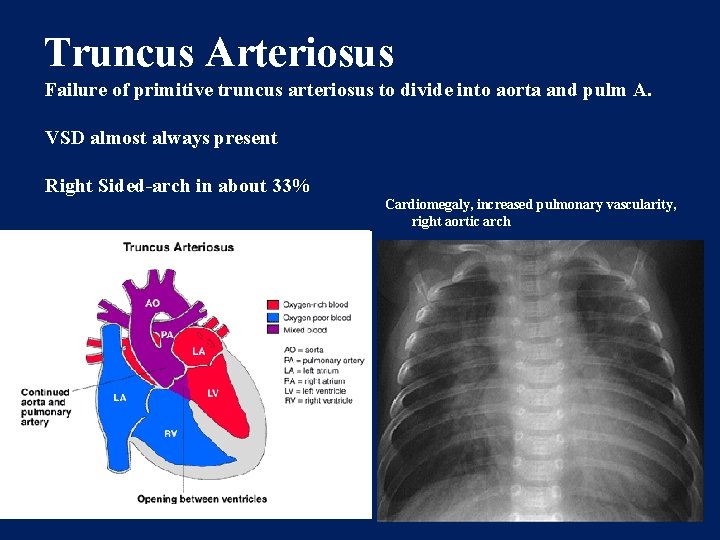
Truncus Arteriosus Failure of primitive truncus arteriosus to divide into aorta and pulm A. VSD almost always present Right Sided-arch in about 33% Cardiomegaly, increased pulmonary vascularity, right aortic arch

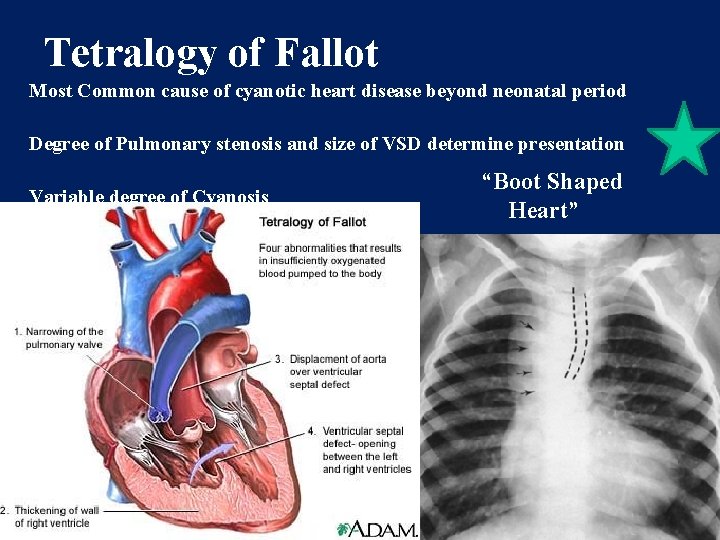
TETRALOGY OF FALLOT • COMMONEST CYANOTIC CONGENITAL HEART DISEASE • 10 % OF ALL CONGENITAL HEART DISEASES

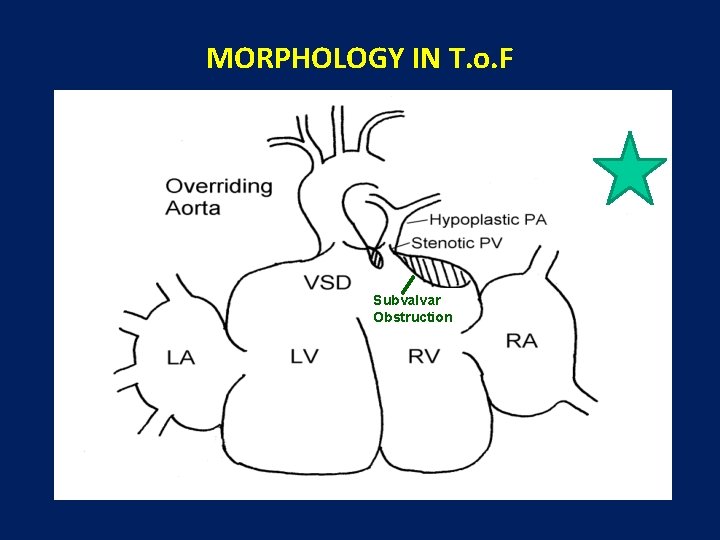
MORPHOLOGY FOUR MORPHOLOGICAL DEFECTS 1. VENTRICULAR SEPTAL DEFECT 2. RIGHT VENTRICULAR OUTFLOW TRACT OBSTRUCTION • SUBVALVAR • SUPRAVALVAR 3. OVERRIDING OF THE AORTA 4. RIGHT VENTRICULAR HYPERTROPHY

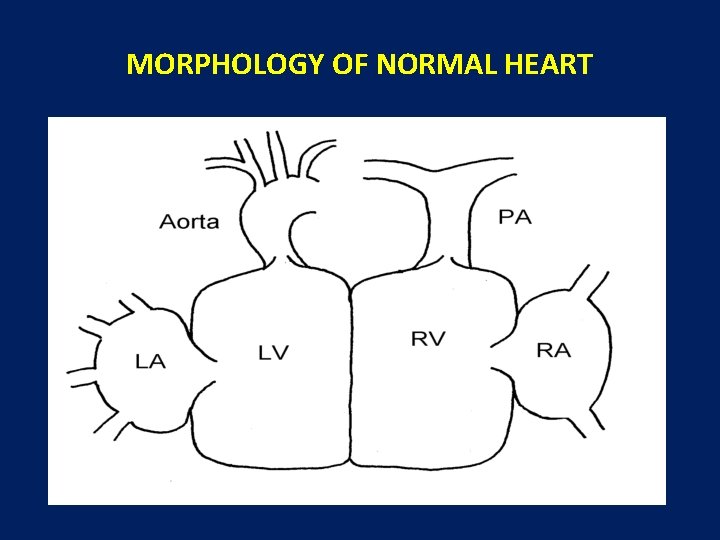
MORPHOLOGY OF NORMAL HEART

MORPHOLOGY IN T. o. F Subvalvar Obstruction

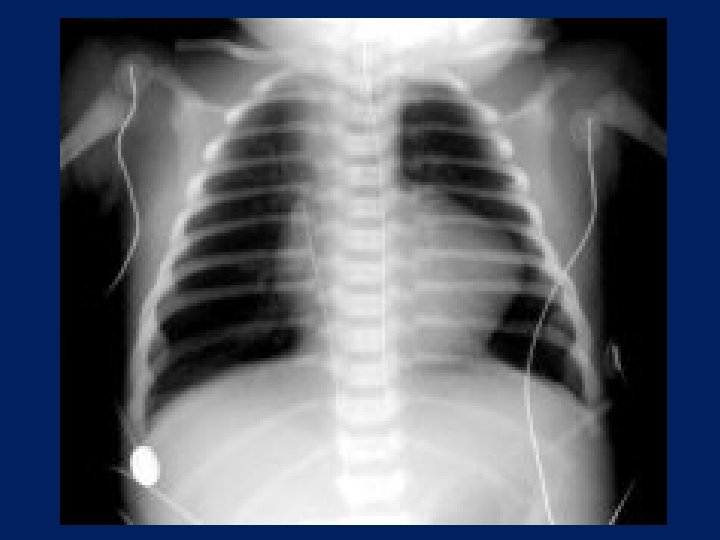
Tetralogy of Fallot Most Common cause of cyanotic heart disease beyond neonatal period Degree of Pulmonary stenosis and size of VSD determine presentation Variable degree of Cyanosis “Boot Shaped Heart”

ALTERED PHYSIOLOGY • OBSTRUCTION TO FLOW OF DEOXYGENATED BLOOD FROM THE RIGHT VENTRICLE TO THE PULMONARY ARTERY • DECREASED OXYGENATION DUE TO POOR PERFUSION OF THE BLOOD

ALTERED PHYSIOLOGY • SHUNTING OF DEOXYGENATED BLOOD FROM THE RIGHT VENTRICLE TO THE AORTA ACROSS THE VENTRICULAR SEPTAL DEFECT (FACILITATED BY AORTIC OVERRIDE) • POOR SYSTEMIC OXYGENATION, LOW HEMOGLOBIN SATURATION AND CYANOSIS

CLINICAL PRESENTATION • CYANOSIS NOT USUALLY NOTICED AT BIRTH • CAUSES – CHILD LESS ACTIVE IN THE INITIAL FEW MONTHS – FOETAL HEMOGLOBIN HAS MORE AFFINITY FOR OXYGEN THAN ADULT HEMOGLOBIN

CLINICAL PRESENTATION • CYANOSIS MANIFESTS MORE AS CHILD BECOMES MORE ACTIVE • PHYSICAL GROWTH IS > GOOD < • MENTAL DEVELOPMENT MAY BE DELAYED IN SEVERE CASES DUE TO CHRONIC HYPOXIA OF THE BRAIN

Clinical Findings Squatting “Tet spells” due to pulmonary outflow tract spasm Severe cases -at birth severe PS( pulmonary stenosis Mild cases – much later- mild PS Cyanosis usually ECG reveals right ventricular hypertrophy

CYANOTIC SPELLS • TYPICAL OF FALLOT’S TETRALOGY • USUALLY OCCURS WHEN THE CHILD CRIES OR IS VERY ACTIVE AS WHEN THE CHILD WAKES UP FROM SLEEP

CYANOTIC SPELLS • ACTIVITY RESULTS IN – INCREASES OXYGEN DEMAND – DECREASES SYSTEMIC VASCULAR RESISTANCE – INCREASES SYMPATHETIC ACTIVITY WHICH CAUSES INFUNDIBULAR SPASM, I. E. , INCREASE IN THE MUSCULAR OBSTRUCTION TO THE RIGHT VENTRICULAR OUTFLOW AT THE SUBVALVAR LEVEL

SQUATTING • TYPICAL OF FALLOT’S TETRALOGY • CHILD ASSUMES SQUATTING POSTURE VERY FREQUENTLY • SOME POSTURES MAY BE CALLED ‘SQUATTING EQUIVALENTS’ • REASON IS THAT SQUATTING CAUSES AN INCREASE IN RESISTANCE TO SYSTEMIC FLOW – DECREASED SHUNTING ACROSS THE VSD – LESS DESATURATION OF SYSTEMIC BLOOD

NATURAL HISTORY • WIDE SPECTRUM OF CLINICAL MANIFESTATIONS DEPENDING ON SEVERITY OF ABNORMALITIES, I. E. , DEGREE OF OBSTRUCTION TO RIGHT VENTRICULAR OUTFLOW, AND SIZE OF VSD


TREATMENT OPTIONS • ONLY SURGICAL – PALLIATIVE SURGERY – DEFINITIVE SURGERY

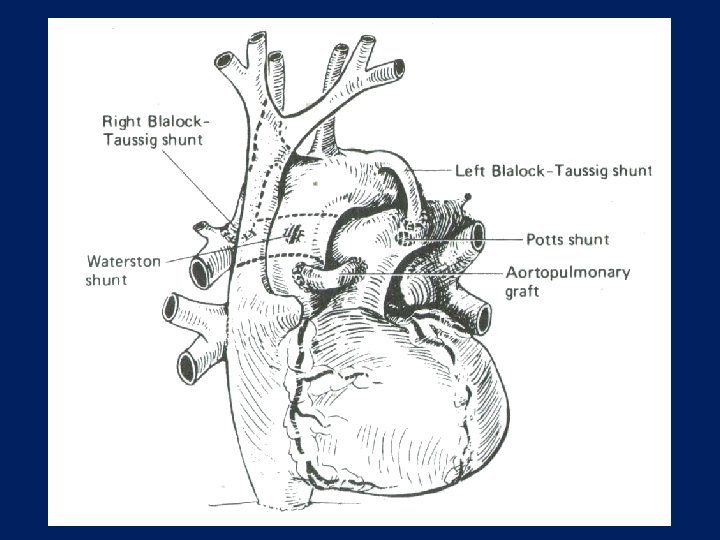
PALLIATIVE SURGERY • AIMED TO DIVERT SYSTEMIC BLOOD INTO THE PULMONARY CIRCULATION AND THUS ENHANCE PULMONARY FLOW AND OXYGENATION • STANDARD OPERATION IS THE MODIFIED BLALOCK-TAUSSIG SHUNT OR OTHER SYSTEMIC PULMONARY SHUNTS SUCH AS POTT’S SHUNT AND WATERSTON-COOLEY SHUNT

Systemic to Pulmonary Shunts

Tetralogy Of Fallot Treatment of Tet Spell • Knee-chest position • O 2 • Morphine 0. 1 -0. 2 mg/kg IM, IV • Phenylephrine gtts : increase systolic BP 20 -40 mm. Hg • Beta blockade, e. g. propanolol: titrate to 0. 1 mg/kg • ABG: Na. HCO 3 if necessary • Surgery

DEFINITIVE SURGERY • RELIEF OF RIGHT VENTRICULAR OUTFLOW TRACT OBSTRUCTION • SEPARATION OF SYSTEMIC AND PULMONARY CIRCULATIONS BY CLOSURE OF THE VSD

TREATMENT STRATEGIES • PALLIATIVE SURGERY IN EARLY CHILDHOOD FOLLOWED BY DEFINITIVE SURGERY IN THE LATER YEARS, USUALLY AFTER 3 – 4 YEARS OF AGE • DEFINITIVE SURGERY IN THE NEONATAL PERIOD OR EARLY CHILDHOOD

T. O. F IN ADULT CARDIAC SURGICAL HOSPITAL • DELAYED DEFINITIVE REPAIR FOLLOWING SHUNT IN EARLY CHILDHOOD • DELAYED PRESENTATION, FOR DEFINITIVE REPAIR • RE-OPERATION FOR DELAYED COMPLICATIONS AFTER DEFINITVE REPAIR

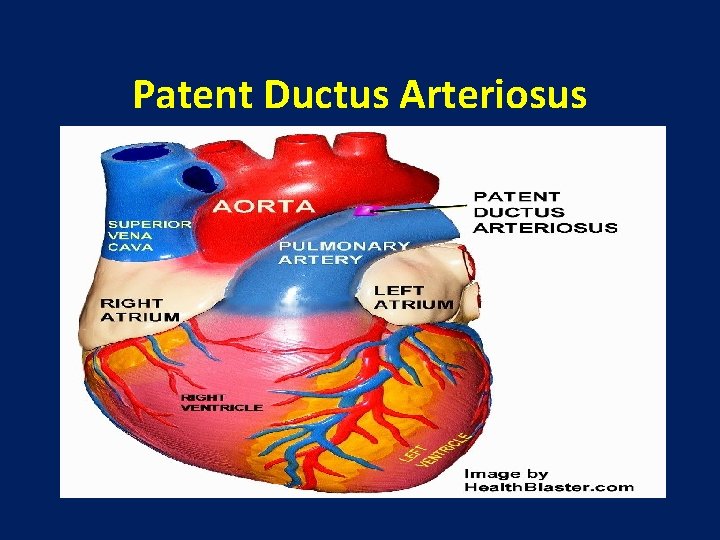
Patent Ductus Arteriosus

Patent Ductus Arteriosus ●The ductus arteriosus in the fetus is an important conduit that allows deoxygenated blood to bypass the collapsed lungs and enter the placenta through the descending aorta and umbilical arteries.

Patent Ductus Arteriosus ● The placenta acts as an oxygenator and returns oxygen rich blood through the umbilical vein and ductus venosus to the fetal heart. ● The placenta produces prostaglandins, which maintain prenatal patency of the ductus and, in early gestation, inhibit the ability of the ductus to contract in response to oxygen.

Patent Ductus Arteriosus ● During the postnatal period, final closure of the ductus arteriosus results from increased production of local vasoconstrictors (like endothelin) in response to higher arterial oxygen, removal of placental prostaglandin and a decrease in the number of prostaglandin E 2 receptors in the ductal wall.

Patent Ductus Arteriosus • The direction of blood flow across the PDA depends on the balance of pulmonary and systemic vascular resistance.

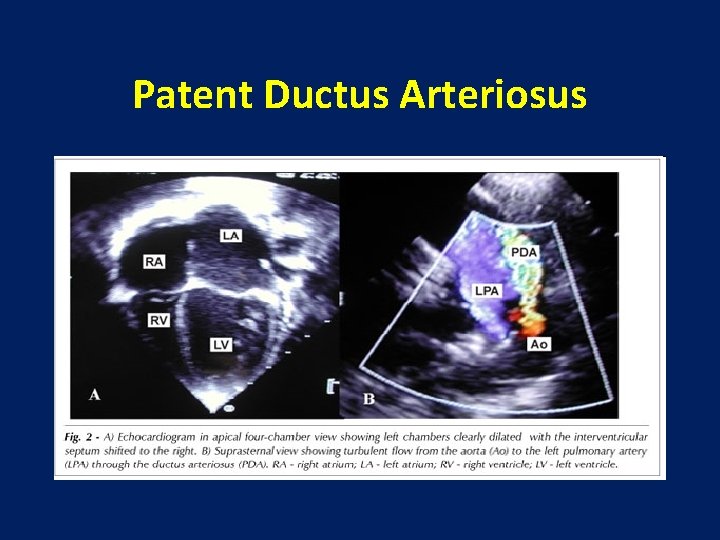
Patent Ductus Arteriosus • The most reliable non-invasive diagnostic tool is echocardiography with Doppler ultrasound. • In most infants, a modified parasternal short axis view offers the best window for PDA visualization. • This view offers the best opportunity to directly measure the PDA.

Patent Ductus Arteriosus

Patent Ductus Arteriosus • The secondary effects of the increased flow can estimate the volume load from the left to right ductal shunt. • A large shunt leads to dilation of the left atrium and left ventricle, as well as holodiastolic reversal of blood flow distal to the ductus in the descending aorta due to run off into the pulmonary bed.

Patent Ductus Arteriosus • The clinical features depend on the magnitude of left-to-right shunt through the PDA and the ability of the infant to initiate compensatory mechanisms to handle the extra volume load.

Relationship to Systemic Organ Perfusion with PDA • Redistribution of systemic blood flow occurs even with moderate shunts. • Retrograde aortic flow, decreased systemic blood flow, and moderate hypotension are common in premature infants with a PDA and may lead to decreased perfusion in many organs, with potential clinical consequences to each.

Relationship to Systemic Organ Perfusion with PDA • Reduced cerebral blood flow or changes in cerebral blood flow velocity patterns have been implicated in the occurrence of intraventricular hemorrhage. • Renal function may be compromised, and myocardial perfusion, particularly subendocardial blood flow, may be reduced.

PDA Consequences • • • Pulmonary Edema Pulmonary Hemorrhage Bronchopulmonary Dysplasia incidence of Necrotizing Enterocolitis incidence of Intraventricular Hemorrhage

Signs and Symptoms • 2 -3 days after birth up to 1 week of life • • o later in those treated with surfactant o o o can be initially silent initially systolic becoming continuous machinery like prominent left ventricular impulse bounding pulses widened pulse pressure (>25 mm. Hg) murmur

Treatment of PDA • Simple fluid restriction along with diuretic use is often recommended to control the symptoms of a PDA. • Furosemide is commonly used. Although furosemide is a prostaglandin agonist, it does not interfere with PDA closure.

Treatment of PDA • The use of oral or, preferably, intravenous (lyophilized) indomethacin to constrict the ductus arteriosus has led to successful nonsurgical closure in a large proportion of treated infants; the effects of indomethacin apparently are best when it is administered before 10 days of age and in less mature infants.

Treatment of PDA • Dose schedules vary, but commonly a first dose of 0. 2 mg/kg is given by nasogastric tube or intravenously. • For intravenous indomethacin, subsequent doses depend on the age at initial treatment if <48 hours, the subsequent two doses are 0. 10 mg/kg; if 2 to 7 days, 0. 20 mg/kg; and if >7 days, 0. 25 mg/kg. A total of three doses usually is given 12 to 24 hours apart depending on urinary output; if urine flow decreases, fewer doses may be used or the time between doses may be extended.

Treatment of PDA Surgical ligation • In small infants that are not a candidate for, or who have failed, medical therapy, surgical ligation remains an effective alternative.

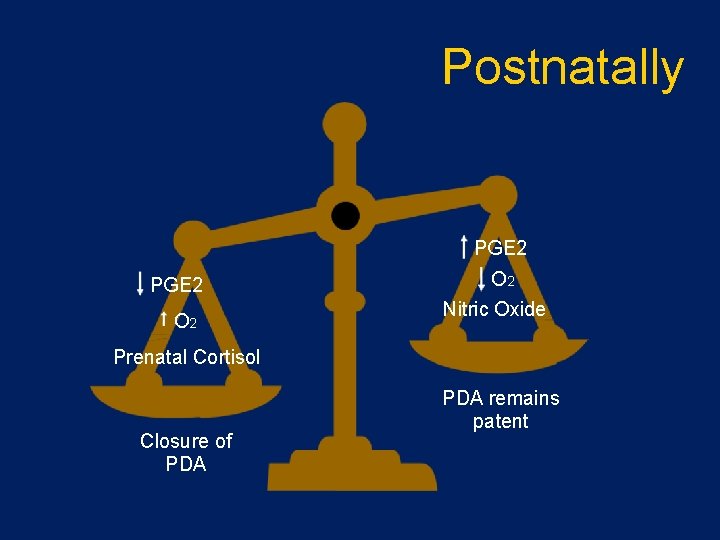
Postnatally PGE 2 O 2 Nitric Oxide Prenatal Cortisol Closure of PDA remains patent