Satish Pradhan Dnyanasadhana College Department Of Chemistry FLAME


- Slides: 23

Satish Pradhan Dnyanasadhana College Department Of Chemistry FLAME EMISSION SPECTROSCOPY Sem-V

Contents � 3. 1 Atomic Spectroscopy (07 L) � 3. 1. 1 Absorption and Emission Spectra � 3. 1. 2 Energy level diagrams � 3. 1. 3 Flame Photometry – Principle, Instrumentation (Flame atomizers, Types of Burners, Wavelength Selectors, Detectors)

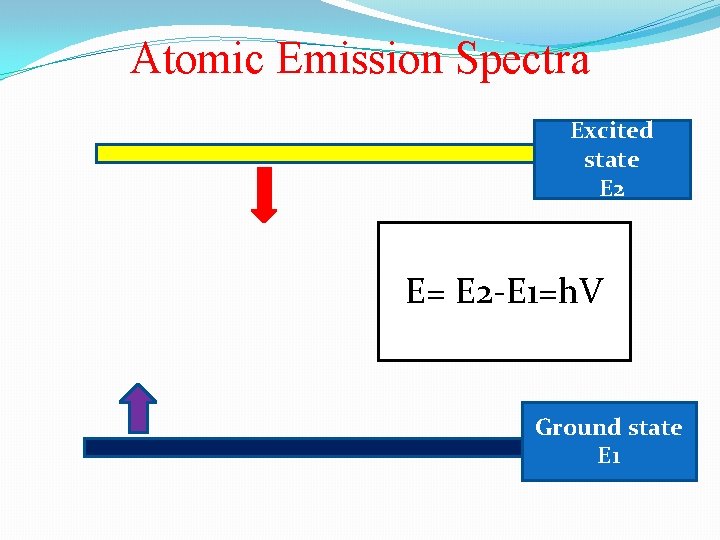
Atomic Emission Spectra Excited state E 2 E= E 2 -E 1=h. V Ground state E 1

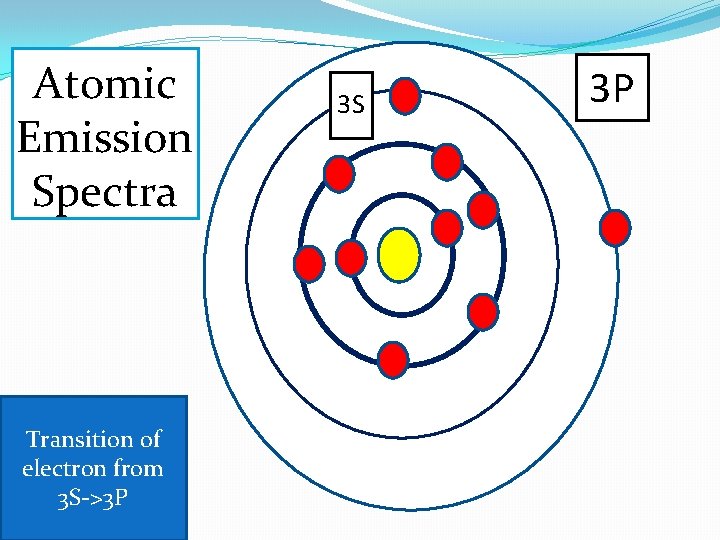
Atomic Emission Spectra Transition of electron from 3 S->3 P 3 S 3 P

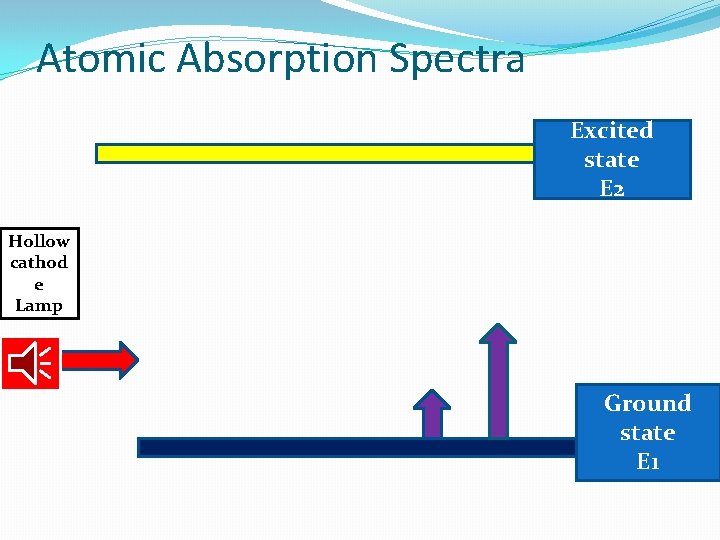
Atomic Absorption Spectra Excited state E 2 Hollow cathod e Lamp Ground state E 1

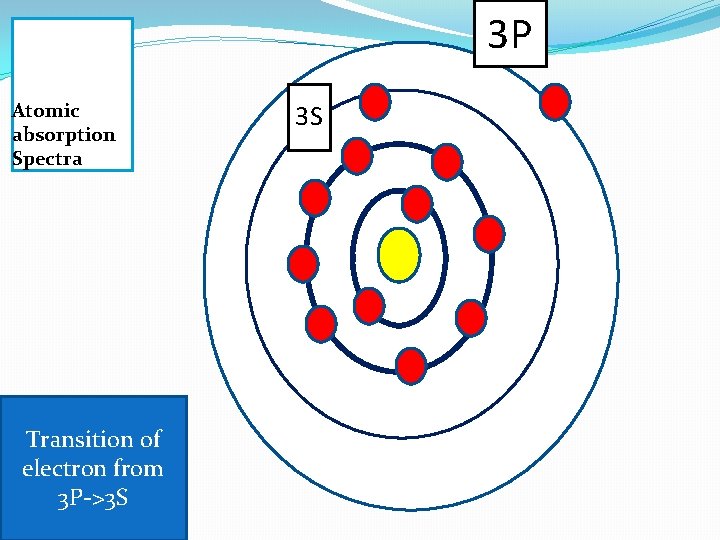
3 P Atomic absorption Spectra Transition of electron from 3 P->3 S 3 S

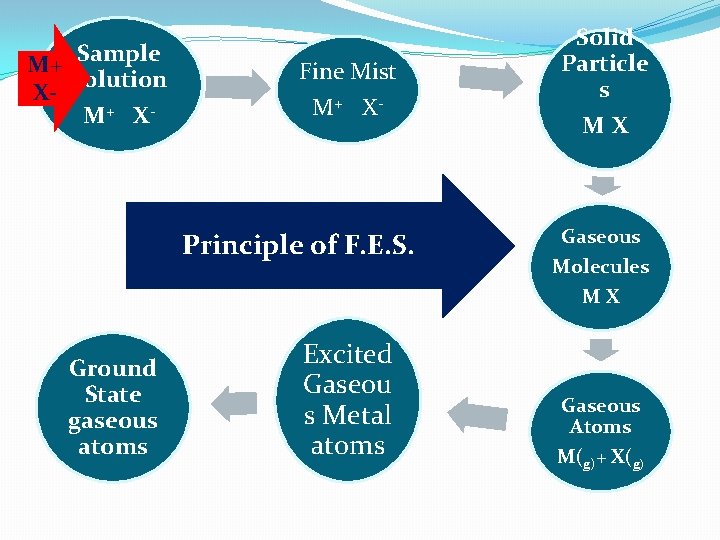
M+ Sample Solution XM+ X - Fine Mist M+ X- Principle of F. E. S. Solid Particle s MX Gaseous Molecules MX Ground State gaseous atoms Excited Gaseou s Metal atoms Gaseous Atoms M(g)+ X(g)

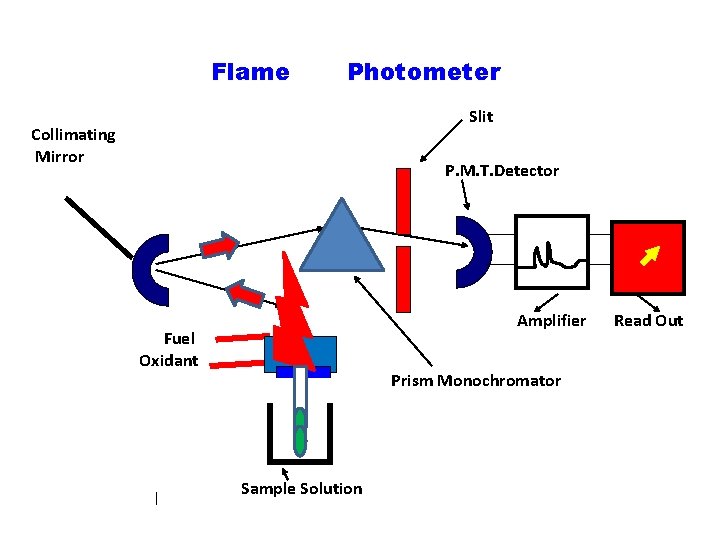
Flame Photometer Slit Collimating Mirror P. M. T. Detector Amplifier Fuel Oxidant Prism Monochromator Sample Solution Read Out

Components of Flame Photometer Burner Total Consumption Burner Premix Burner Monochromator Prism Detector P. M. T.

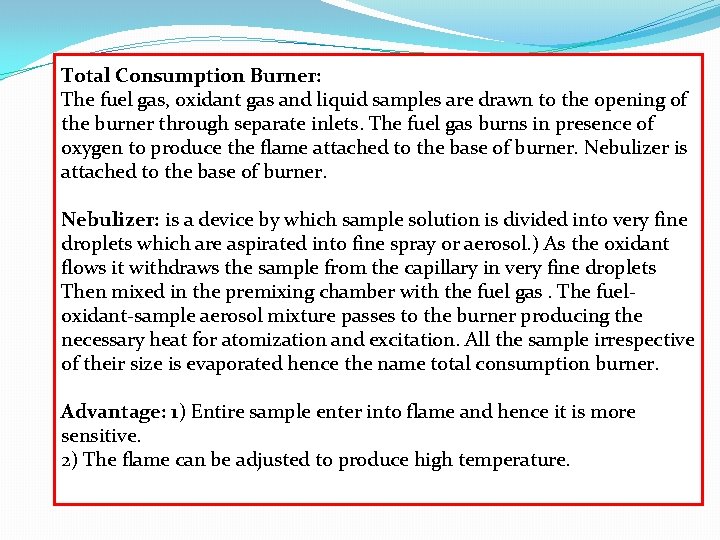
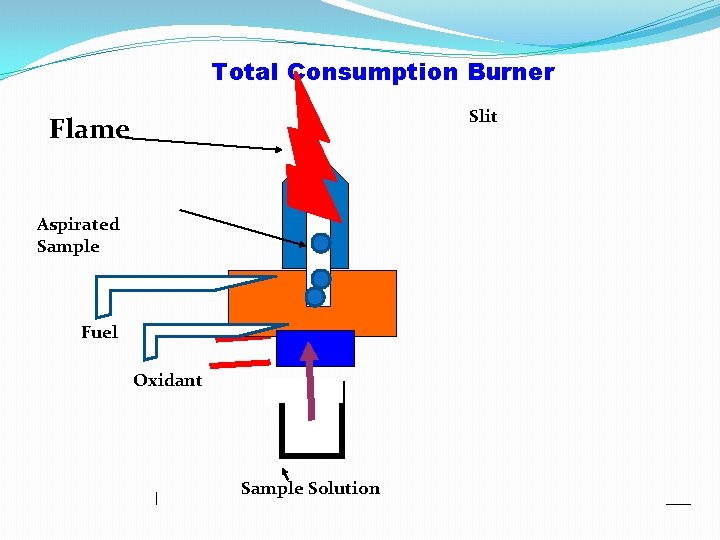
Total Consumption Burner: The fuel gas, oxidant gas and liquid samples are drawn to the opening of the burner through separate inlets. The fuel gas burns in presence of oxygen to produce the flame attached to the base of burner. Nebulizer is attached to the base of burner. Nebulizer: is a device by which sample solution is divided into very fine droplets which are aspirated into fine spray or aerosol. ) As the oxidant flows it withdraws the sample from the capillary in very fine droplets Then mixed in the premixing chamber with the fuel gas. The fueloxidant-sample aerosol mixture passes to the burner producing the necessary heat for atomization and excitation. All the sample irrespective of their size is evaporated hence the name total consumption burner. Advantage: 1) Entire sample enter into flame and hence it is more sensitive. 2) The flame can be adjusted to produce high temperature.

Total Consumption Burner Slit Flame Aspirated Sample Fuel Oxidant Sample Solution

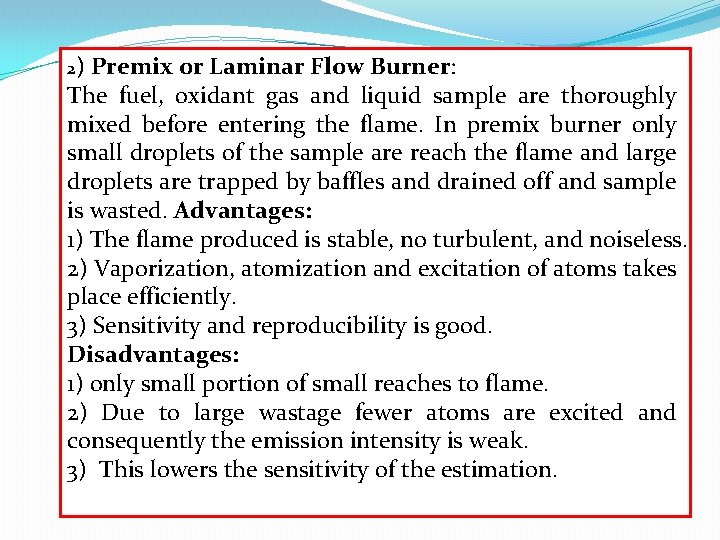
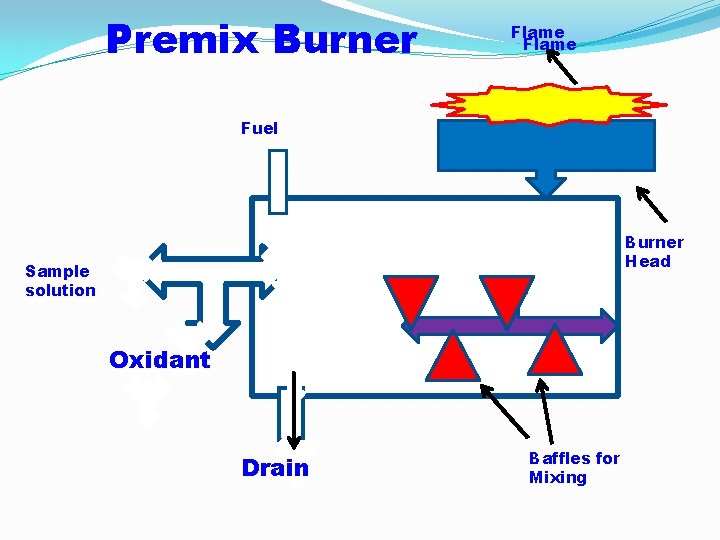
2) Premix or Laminar Flow Burner: The fuel, oxidant gas and liquid sample are thoroughly mixed before entering the flame. In premix burner only small droplets of the sample are reach the flame and large droplets are trapped by baffles and drained off and sample is wasted. Advantages: 1) The flame produced is stable, no turbulent, and noiseless. 2) Vaporization, atomization and excitation of atoms takes place efficiently. 3) Sensitivity and reproducibility is good. Disadvantages: 1) only small portion of small reaches to flame. 2) Due to large wastage fewer atoms are excited and consequently the emission intensity is weak. 3) This lowers the sensitivity of the estimation.

Premix Burner Flame Fuel Burner Head Sample solution Oxidant Drain Baffles for Mixing

Application of Flame Emission Spectroscopy F. E. S. Used for Qualitative analysis and Quantitative analysis of Alkali and alkaline earth metals. q q q q • Soil analysis: Na, K, Ca, Mg. Fertilizer : Na, K, Ca, Mg. Biological Fluids : Na, K, Ca, Mg. Petrol : Lead Cement: Ca and Mg. Glass Analysis: Na, Ca, Mg, K, and Li. Organic Compounds : Boron Physiology and Clinical Studies : Electrolyte Balance.

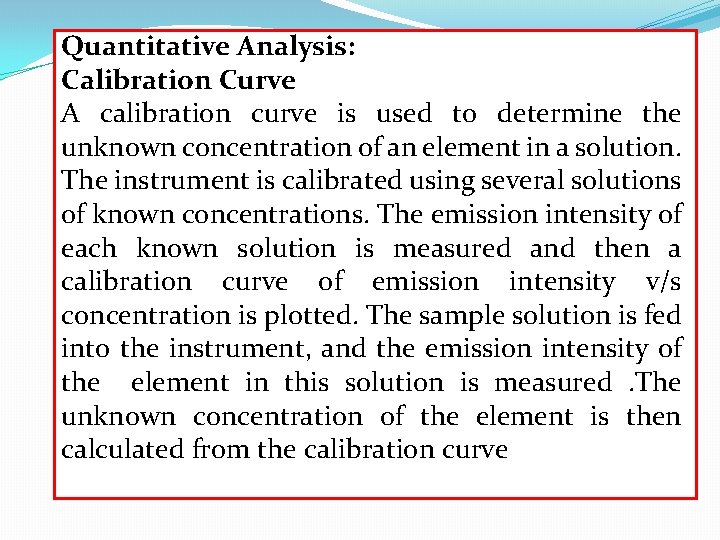
Quantitative Analysis: Calibration Curve A calibration curve is used to determine the unknown concentration of an element in a solution. The instrument is calibrated using several solutions of known concentrations. The emission intensity of each known solution is measured and then a calibration curve of emission intensity v/s concentration is plotted. The sample solution is fed into the instrument, and the emission intensity of the element in this solution is measured. The unknown concentration of the element is then calculated from the calibration curve

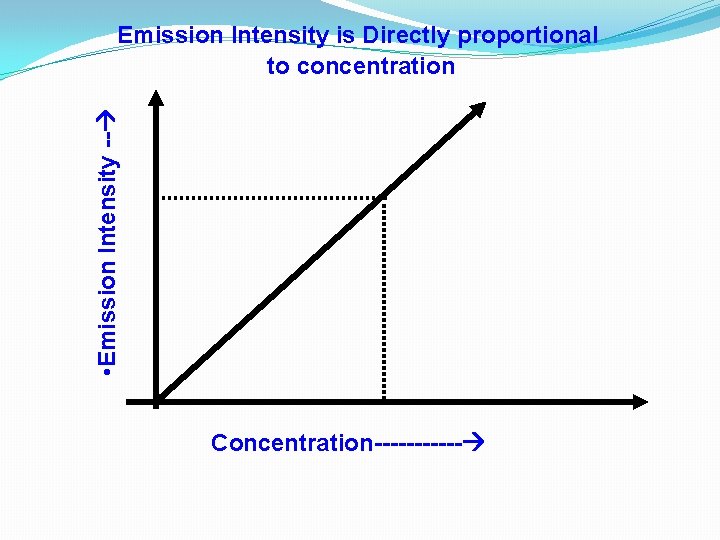
• Emission Intensity -- Emission Intensity is Directly proportional to concentration Concentration------

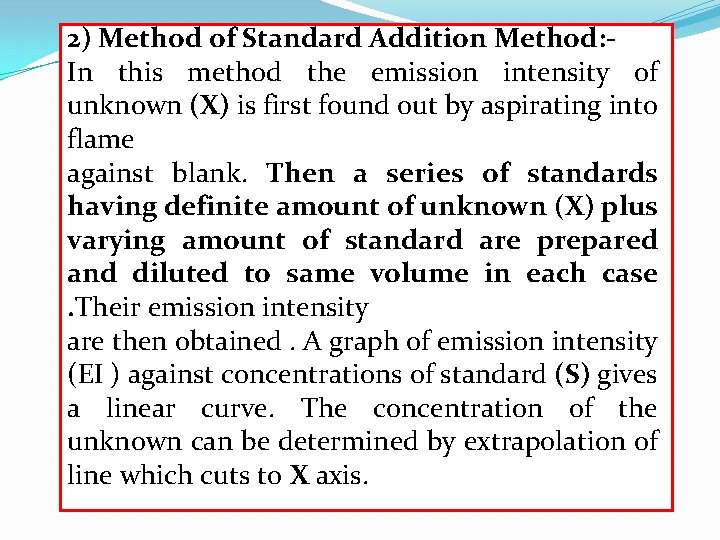
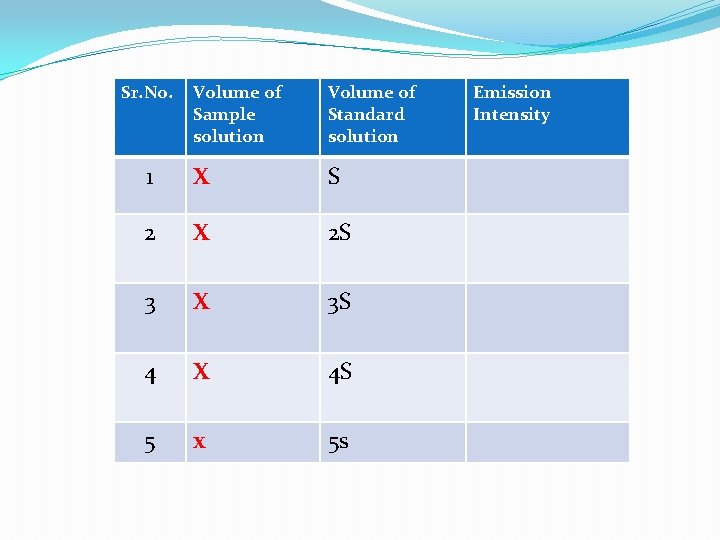
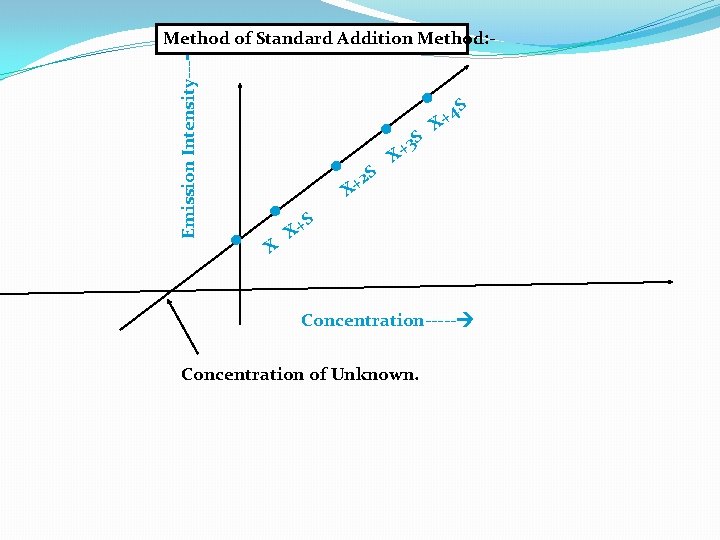
2) Method of Standard Addition Method: In this method the emission intensity of unknown (X) is first found out by aspirating into flame against blank. Then a series of standards having definite amount of unknown (X) plus varying amount of standard are prepared and diluted to same volume in each case. Their emission intensity are then obtained. A graph of emission intensity (EI ) against concentrations of standard (S) gives a linear curve. The concentration of the unknown can be determined by extrapolation of line which cuts to X axis.

Sr. No. Volume of Sample solution Volume of Standard solution 1 X S 2 X 2 S 3 X 3 S 4 X 4 S 5 x 5 s Emission Intensity

Emission Intensity--- Method of Standard Addition Method: - . . X . 2 + X . . S 3 S + X 4 S + X S X+ Concentration----- Concentration of Unknown.

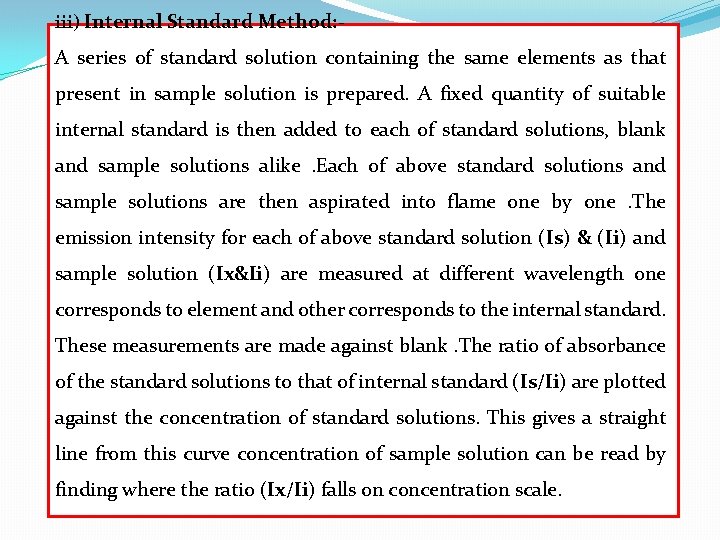
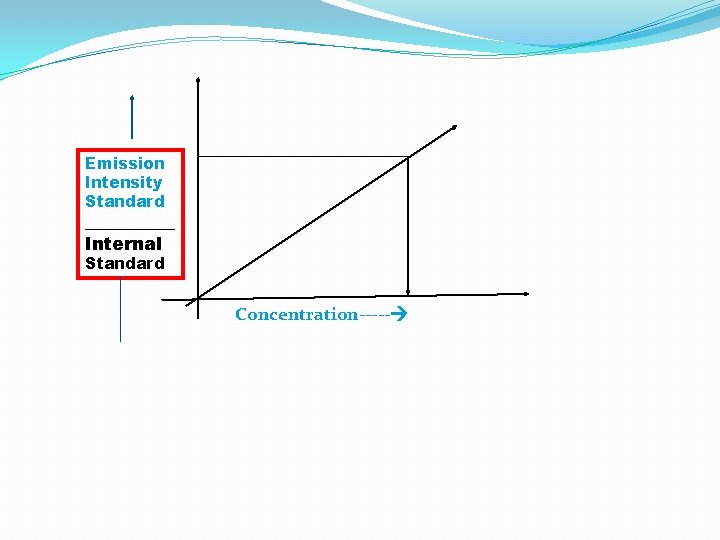
iii) Internal Standard Method: - A series of standard solution containing the same elements as that present in sample solution is prepared. A fixed quantity of suitable internal standard is then added to each of standard solutions, blank and sample solutions alike. Each of above standard solutions and sample solutions are then aspirated into flame one by one. The emission intensity for each of above standard solution (Is) & (Ii) and sample solution (Ix&Ii) are measured at different wavelength one corresponds to element and other corresponds to the internal standard. These measurements are made against blank. The ratio of absorbance of the standard solutions to that of internal standard (Is/Ii) are plotted against the concentration of standard solutions. This gives a straight line from this curve concentration of sample solution can be read by finding where the ratio (Ix/Ii) falls on concentration scale.

Emission Intensity Standard _____ Internal Standard Concentration-----

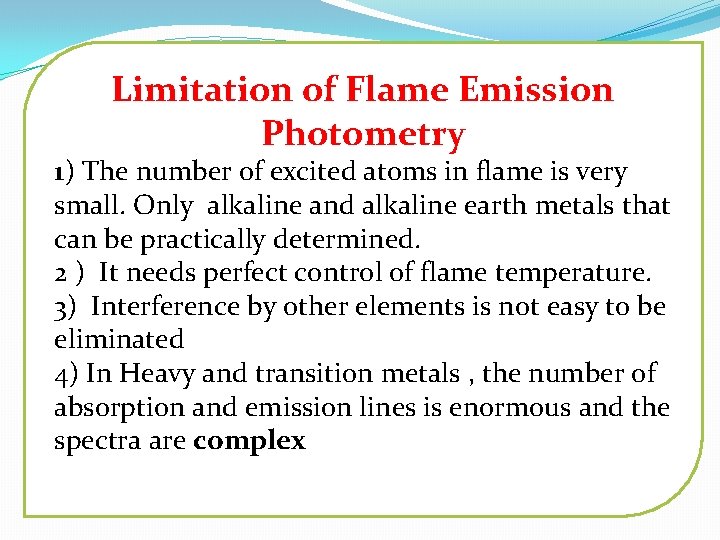
Limitation of Flame Emission Photometry 1) The number of excited atoms in flame is very small. Only alkaline and alkaline earth metals that can be practically determined. 2 ) It needs perfect control of flame temperature. 3) Interference by other elements is not easy to be eliminated 4) In Heavy and transition metals , the number of absorption and emission lines is enormous and the spectra are complex

Thank you