Thermodynamics Thermodynamics Temperature Particles Pressure volume and temperature

























- Slides: 46

Thermodynamics

Thermodynamics • • Temperature Particles Pressure, volume and temperature Energy and Power Heat transfer Measuring Temperature Specific heat capacity Latent heat
ENERGY

Energy (Joule) • Energy can be transferred or transformed • kinetic • potential (chemical, electrical, gravitational, elastic) • radiant (sound, light and other electromagnetic waves) • internal (heat/thermal energy)

TEMPERATURE

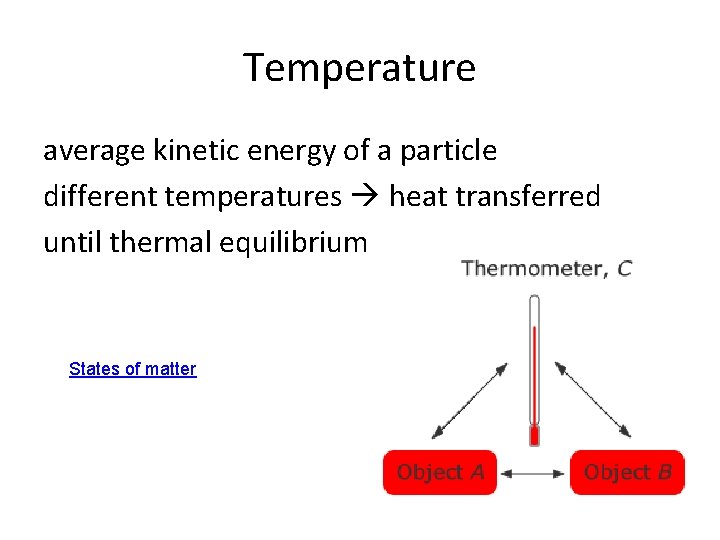
Temperature The temperature of an object is given by the average kinetic energy of its particles.

2. Measuring temperature Which thermometers use thermal expansion as their thermodynamic property? a) liquid in glass b) thermistor c) constant volume gas thermometer d) thermocouple

a) liquid in glass

Temperature Scales use a thermodynamic property scales calibrated at 2 fixed points (often melting ice and boiling water

INTERNAL ENERGY

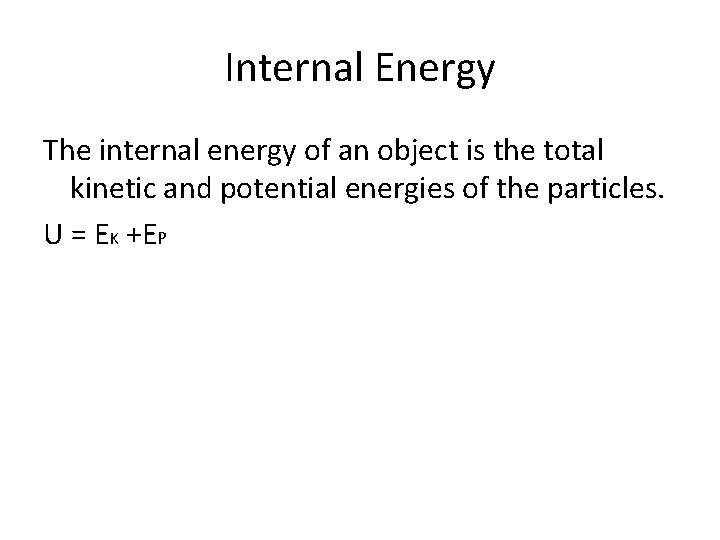
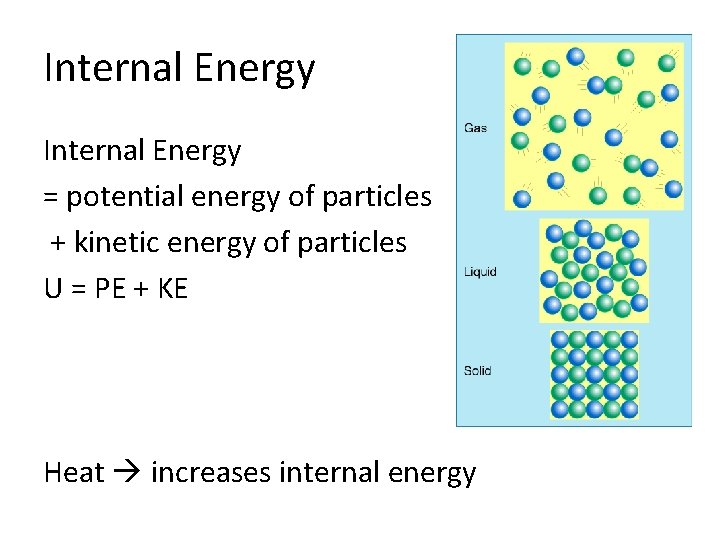
Internal Energy The internal energy of an object is the total kinetic and potential energies of the particles. U = EK +EP

5. Internal Energy During a change of state from solid to liquid at the melting point: a) the temperature of the substance stays the same b) the internal energy of the substance stays the same c) the kinetic energy of the particles stays the same

Internal Energy = potential energy of particles + kinetic energy of particles U = PE + KE Heat increases internal energy

a) the temperature of the substance stays the same and c) the average kinetic energy of the particles stays the same

Which liquid has more internal energy? cup of hot tea 80 o. C water in swimming pool 25 o. C

THERMAL EQUILIBRIUM

Temperature average kinetic energy of a particle different temperatures heat transferred until thermal equilibrium States of matter

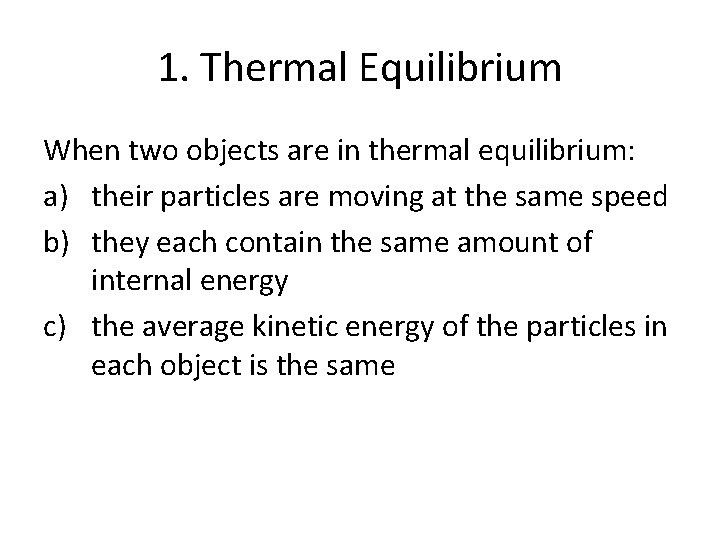
1. Thermal Equilibrium When two objects are in thermal equilibrium: a) their particles are moving at the same speed b) they each contain the same amount of internal energy c) the average kinetic energy of the particles in each object is the same

c) the average kinetic energy of the particles in each object is the same

Heat transfer Which ice cube will melt first?

GASES

The Ideal Gas all collisions between atoms or molecules are perfectly elastic no intermolecular attractive forces Image: http: //kaffee. 50 webs. com/Science/activities/Chem/Activity. Gas_Laws. PSet 1. html

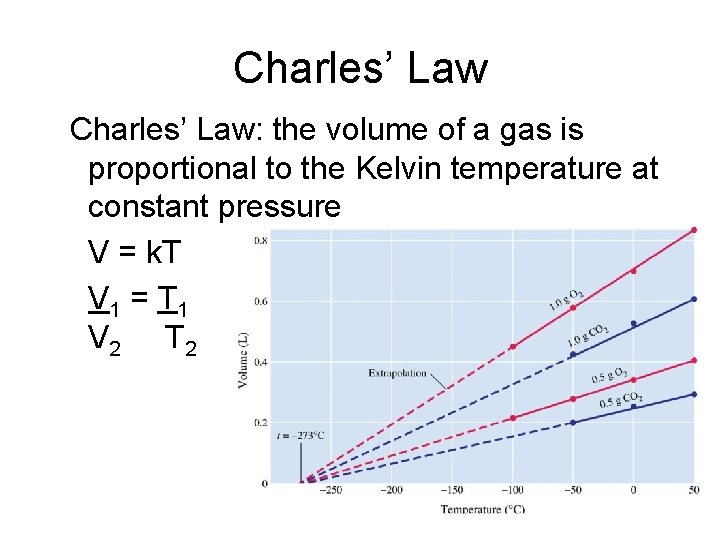
Charles’ Law: the volume of a gas is proportional to the Kelvin temperature at constant pressure V = k. T V 1 = T 1 V 2 T 2

Absolute zero is the temperature at which the particles of a substance have no kinetic energy. This occurs at -273 o. C.

Kelvin temperature scale The Kelvin scale of temperature is defined by absolute zero and is designed so that 1 Kelvin = 1 o. C. This gives absolute zero (0 K) as -273. 15 o. C.

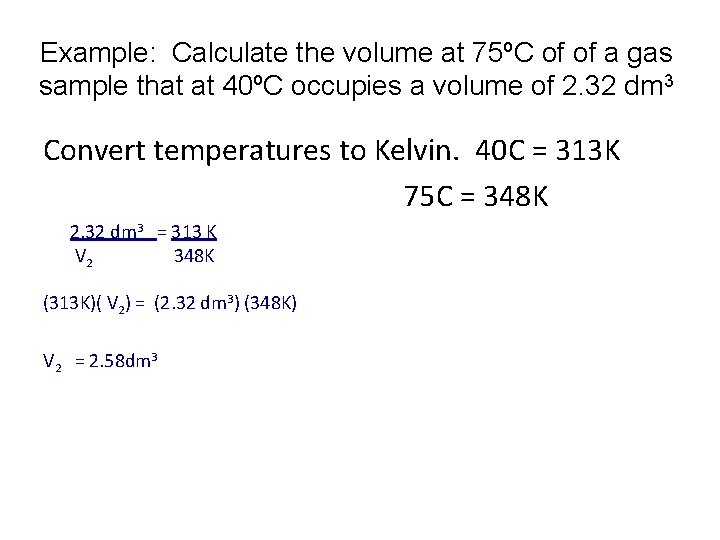
Example: Calculate the volume at 75ºC of of a gas sample that at 40ºC occupies a volume of 2. 32 dm 3 Convert temperatures to Kelvin. 40 C = 313 K 75 C = 348 K 2. 32 dm 3 = 313 K V 2 348 K (313 K)( V 2) = (2. 32 dm 3) (348 K) V 2 = 2. 58 dm 3

Heat Transfer

How is heat transferred? • Conduction • Convection • Radiation

specific heat capacity How much energy is needed to increase temperature?

Heat capacity • Describe what happens to the temperature of liquid coffee at 90°C when it is poured into a cup at room temperature. • Which direction does heat flow? Image: http: //en. wikipedia. org/wiki/Coffee

Heat capacity • The heat capacity of an object is the energy required to raise its temperature by 1°C Image: http: //en. wikipedia. org/wiki/Milk

Heating water It takes 4180 J of heat energy to increase the temperature of 1 kg of water by 1°C. a) how much heat is needed for 0. 5 kg by 1°C? b) how much heat is needed for 1 kg from 20 to 50 C? c) how much heat for 5 kg from 20 to 100 C?

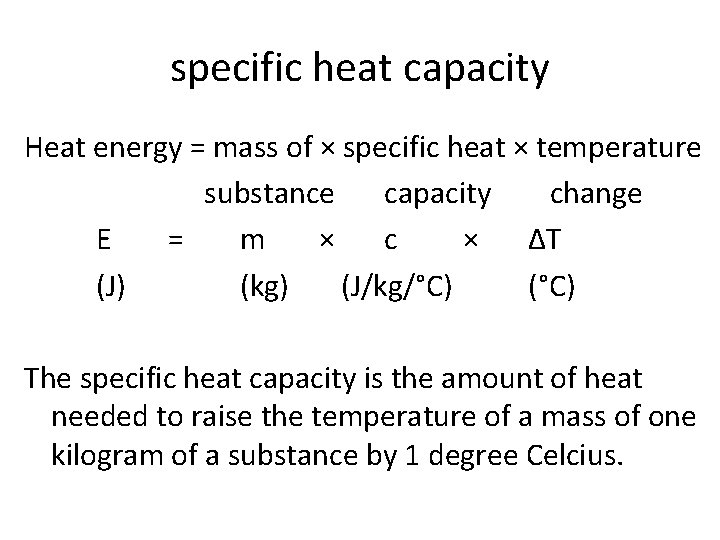
specific heat capacity Heat energy = mass of × specific heat × temperature substance capacity change E = m × c × ∆T (J) (kg) (J/kg/°C) (°C) The specific heat capacity is the amount of heat needed to raise the temperature of a mass of one kilogram of a substance by 1 degree Celcius.

Coffee example

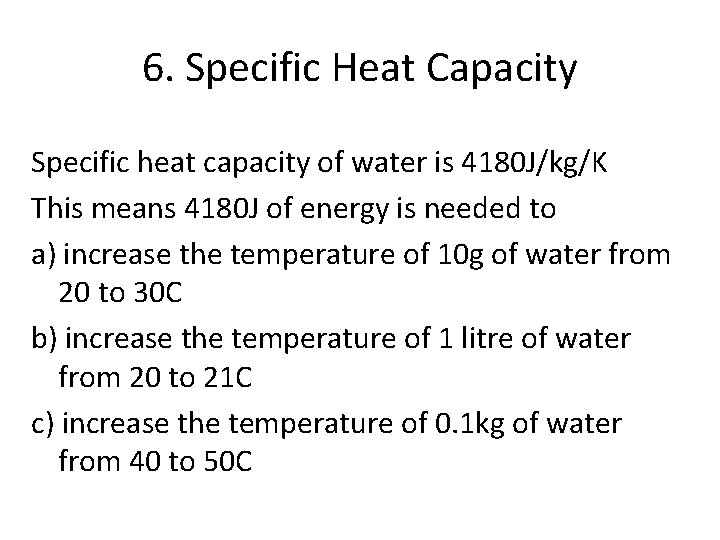
6. Specific Heat Capacity Specific heat capacity of water is 4180 J/kg/K This means 4180 J of energy is needed to a) increase the temperature of 10 g of water from 20 to 30 C b) increase the temperature of 1 litre of water from 20 to 21 C c) increase the temperature of 0. 1 kg of water from 40 to 50 C

b) increase the temperature of 1 litre of water from 20 to 21 C and c) increase the temperature of 0. 1 kg of water from 40 to 50 C

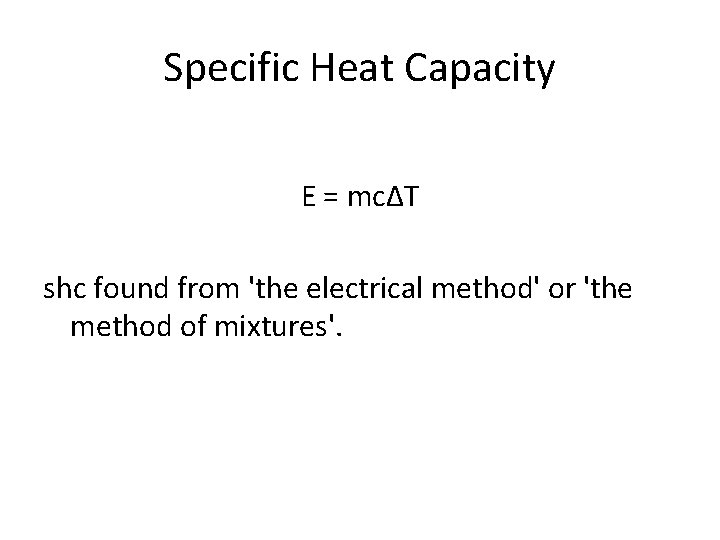
Specific Heat Capacity E = mcΔT shc found from 'the electrical method' or 'the method of mixtures'.

Latent heat

Latent Heat Latent heat of fusion: energy needed to melt a solid without a temperature rise Latent heat of vaporization: energy needed to boil a liquid without a temperature rise. Energy = mass × spedific latent heat E = m. L

Ideal gases

3. Temperature and Pressure The temperature of an ideal gas (in Kelvin) is proportional to its pressure so a) at absolute zero the pressure is zero b) at absolute zero the particles have no kinetic energy c) below absolute zero the pressure is negative

a) at absolute zero the pressure is zero and b) at absolute zero the particles have no kinetic

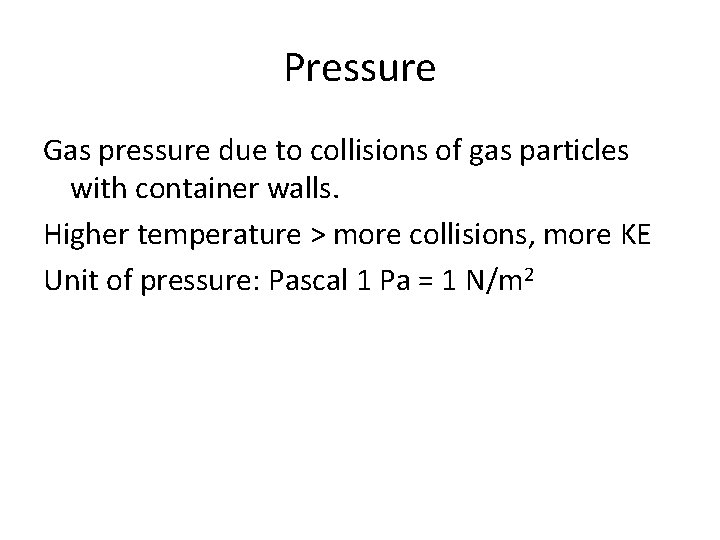
Pressure Gas pressure due to collisions of gas particles with container walls. Higher temperature > more collisions, more KE Unit of pressure: Pascal 1 Pa = 1 N/m 2

4. Pressure, Volume and Temperature When the pressure of an ideal gas is doubled a) the volume is half if the temperature is kept constant b) the volume is double if the temperature is kept constant c) the temperature is double if the volume is kept constant

a) the volume is half if the temperature is kept constant and c) the temperature is double

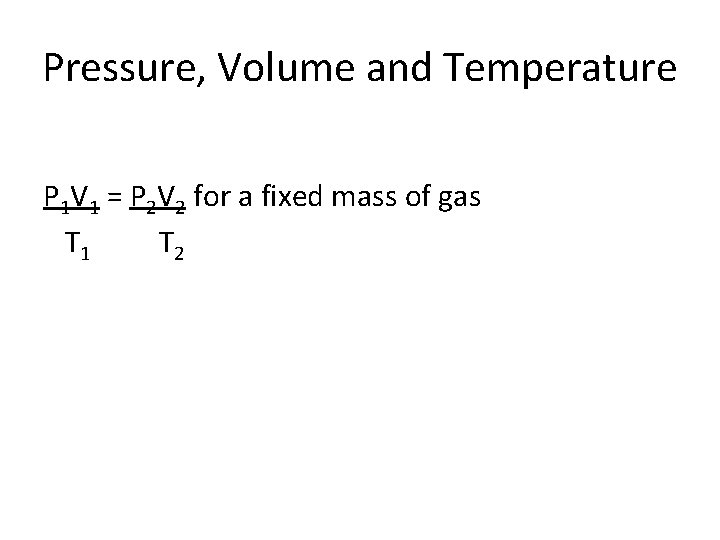
Pressure, Volume and Temperature P 1 V 1 = P 2 V 2 for a fixed mass of gas T 1 T 2