Section 3 7Gas Laws How can we calculate






- Slides: 24

Section 3. 7—Gas Laws How can we calculate Pressure, Volume and Temperature of our airbag?

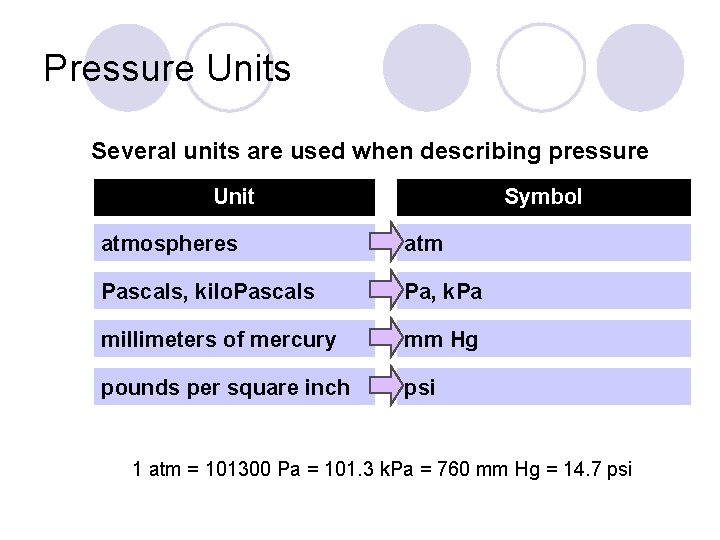
Pressure Units Several units are used when describing pressure Unit Symbol atmospheres atm Pascals, kilo. Pascals Pa, k. Pa millimeters of mercury mm Hg pounds per square inch psi 1 atm = 101300 Pa = 101. 3 k. Pa = 760 mm Hg = 14. 7 psi

Definition Kelvin (K)– temperature scale with an absolute zero Temperatures cannot fall below an absolute zero A temperature scale with absolute zero is needed in Gas Law calculations because you can’t have negative pressures or volumes

Definition Standard Temperature and Pressure (STP) – 1 atm (or the equivalent in another unit) and 0°C (273 K) Problems often use “STP” to indicate quantities…don’t forget this “hidden” information when making your list!

Gas Laws

KMT and Gas Laws The Gas Laws are the experimental observations of the gas behavior that the Kinetic Molecular Theory explains.

“Before” and “After” in Gas Laws This section has 4 gas laws which have “before” and “after” conditions. For example: Where P 1 and n 1 are pressure and # of moles “before” and P 2 and n 2 are pressure and # of moles “after” Both sides of the equation are talking about the sample of gas —with the “ 1” variables before a change, and the “ 2” variables after the change

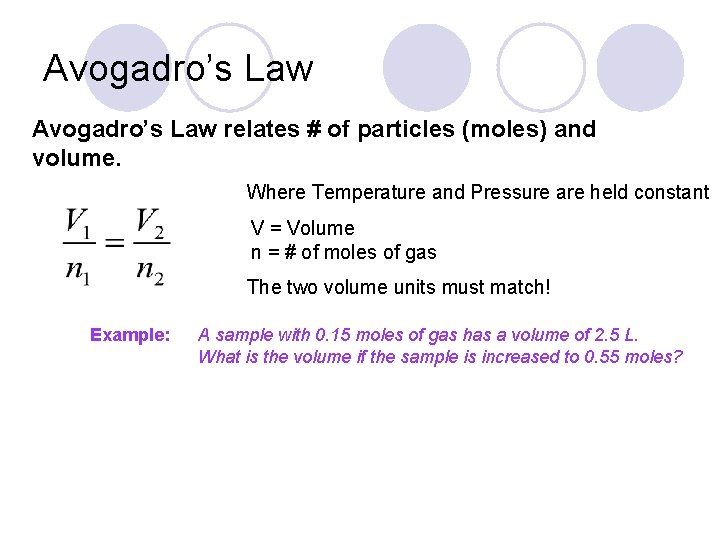
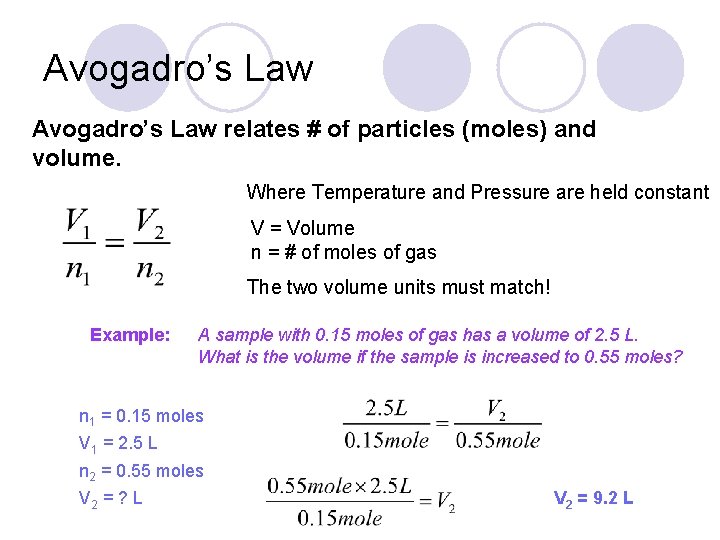
Avogadro’s Law relates # of particles (moles) and volume. Where Temperature and Pressure are held constant V = Volume n = # of moles of gas The two volume units must match! Example: A sample with 0. 15 moles of gas has a volume of 2. 5 L. What is the volume if the sample is increased to 0. 55 moles?

Avogadro’s Law relates # of particles (moles) and volume. Where Temperature and Pressure are held constant V = Volume n = # of moles of gas The two volume units must match! Example: A sample with 0. 15 moles of gas has a volume of 2. 5 L. What is the volume if the sample is increased to 0. 55 moles? n 1 = 0. 15 moles V 1 = 2. 5 L n 2 = 0. 55 moles V 2 = ? L V 2 = 9. 2 L

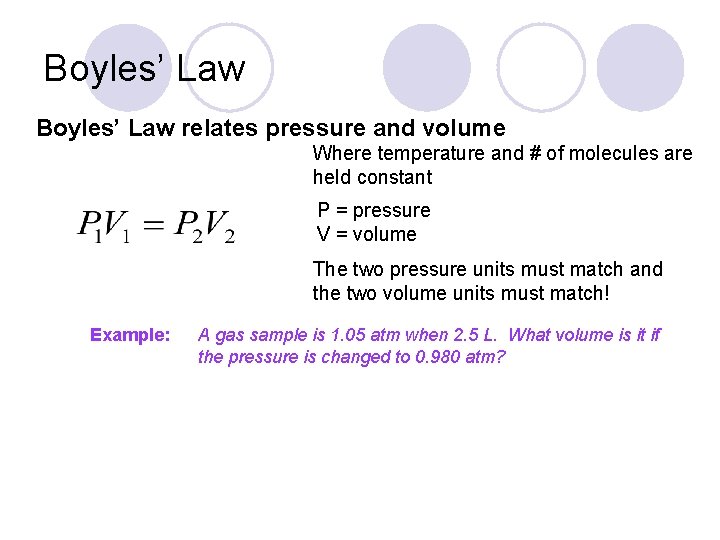
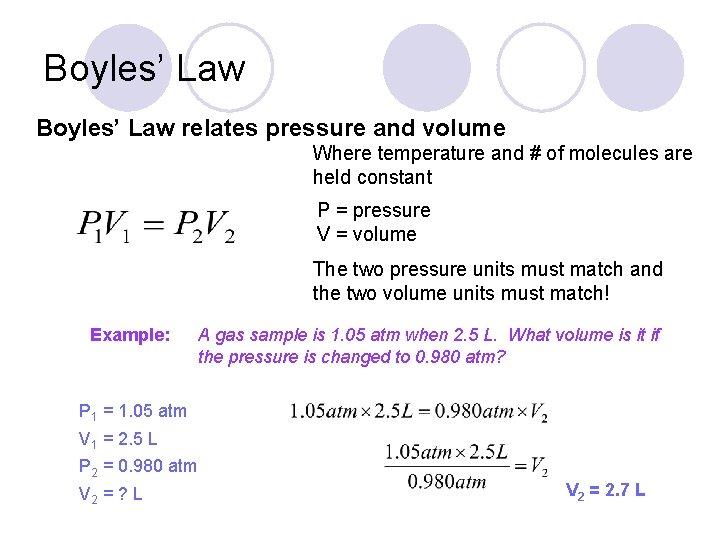
Boyles’ Law relates pressure and volume Where temperature and # of molecules are held constant P = pressure V = volume The two pressure units must match and the two volume units must match! Example: A gas sample is 1. 05 atm when 2. 5 L. What volume is it if the pressure is changed to 0. 980 atm?

Boyles’ Law relates pressure and volume Where temperature and # of molecules are held constant P = pressure V = volume The two pressure units must match and the two volume units must match! Example: A gas sample is 1. 05 atm when 2. 5 L. What volume is it if the pressure is changed to 0. 980 atm? P 1 = 1. 05 atm V 1 = 2. 5 L P 2 = 0. 980 atm V 2 = ? L V 2 = 2. 7 L

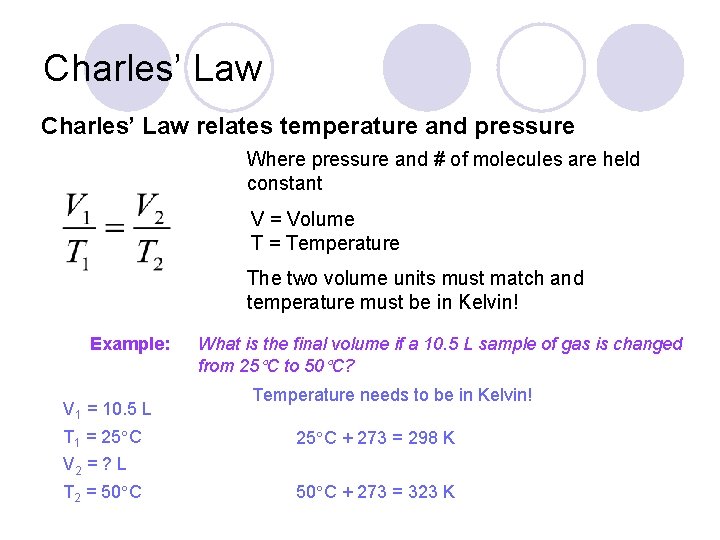
Charles’ Law relates temperature and pressure Where pressure and # of molecules are held constant V = Volume T = Temperature The two volume units must match and temperature must be in Kelvin! Example: V 1 = 10. 5 L T 1 = 25 C What is the final volume if a 10. 5 L sample of gas is changed from 25 C to 50 C? Temperature needs to be in Kelvin! 25 C + 273 = 298 K V 2 = ? L T 2 = 50 C + 273 = 323 K

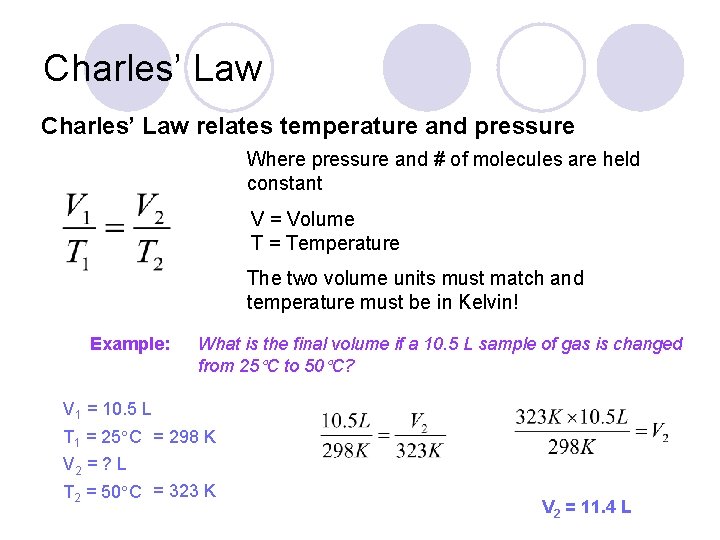
Charles’ Law relates temperature and pressure Where pressure and # of molecules are held constant V = Volume T = Temperature The two volume units must match and temperature must be in Kelvin! Example: What is the final volume if a 10. 5 L sample of gas is changed from 25 C to 50 C? V 1 = 10. 5 L T 1 = 25 C = 298 K V 2 = ? L T 2 = 50 C = 323 K V 2 = 11. 4 L

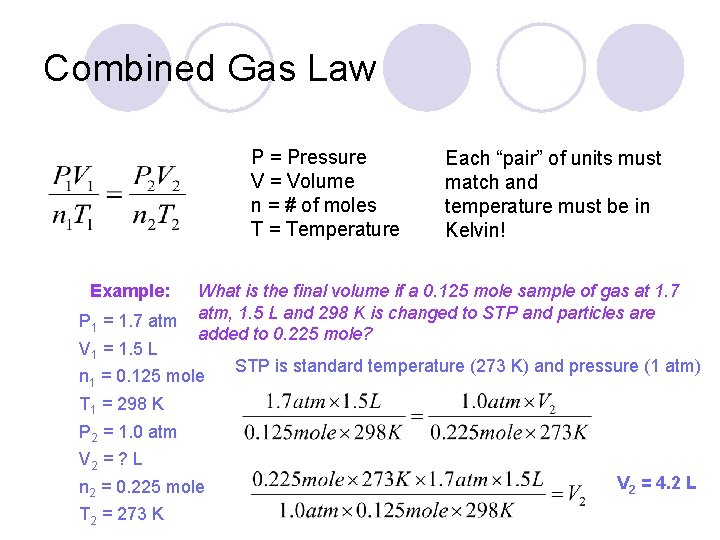
Combined Gas Law P = Pressure V = Volume n = # of moles T = Temperature Example: Each “pair” of units must match and temperature must be in Kelvin! What is the final volume if a 0. 125 mole sample of gas at 1. 7 atm, 1. 5 L and 298 K is changed to STP and particles are added to 0. 225 mole?

Combined Gas Law P = Pressure V = Volume n = # of moles T = Temperature Example: P 1 = 1. 7 atm V 1 = 1. 5 L Each “pair” of units must match and temperature must be in Kelvin! What is the final volume if a 0. 125 mole sample of gas at 1. 7 atm, 1. 5 L and 298 K is changed to STP and particles are added to 0. 225 mole? n 1 = 0. 125 mole STP is standard temperature (273 K) and pressure (1 atm) T 1 = 298 K P 2 = 1. 0 atm V 2 = ? L n 2 = 0. 225 mole T 2 = 273 K V 2 = 4. 2 L

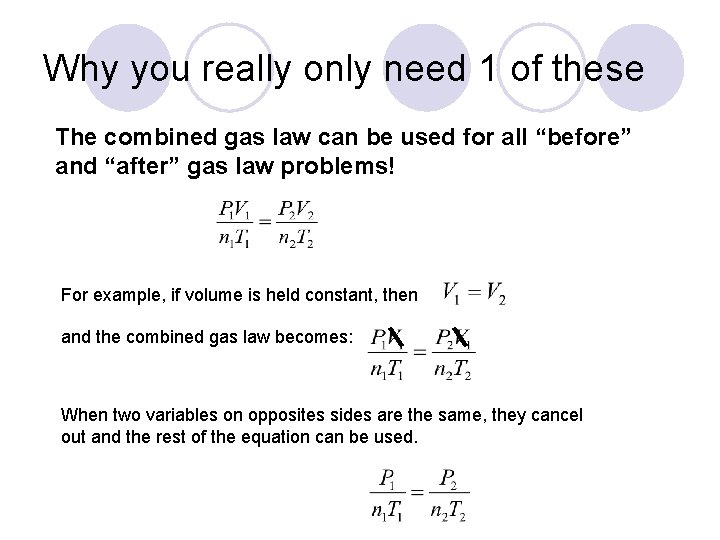
Why you really only need 1 of these The combined gas law can be used for all “before” and “after” gas law problems! For example, if volume is held constant, then and the combined gas law becomes: When two variables on opposites sides are the same, they cancel out and the rest of the equation can be used.

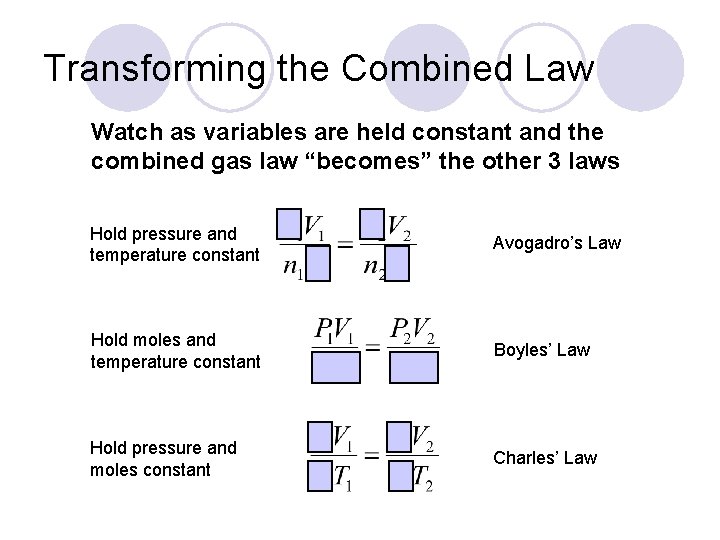
Transforming the Combined Law Watch as variables are held constant and the combined gas law “becomes” the other 3 laws Hold pressure and temperature constant Avogadro’s Law Hold moles and temperature constant Boyles’ Law Hold pressure and moles constant Charles’ Law

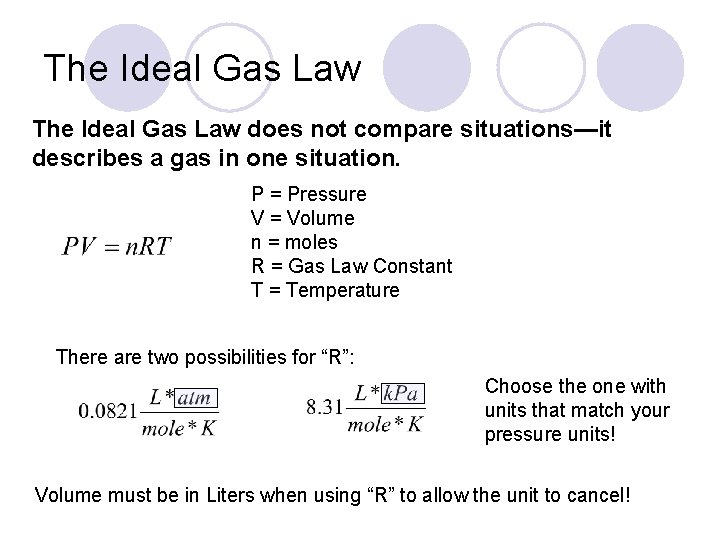
The Ideal Gas Law does not compare situations—it describes a gas in one situation. P = Pressure V = Volume n = moles R = Gas Law Constant T = Temperature There are two possibilities for “R”: Choose the one with units that match your pressure units! Volume must be in Liters when using “R” to allow the unit to cancel!

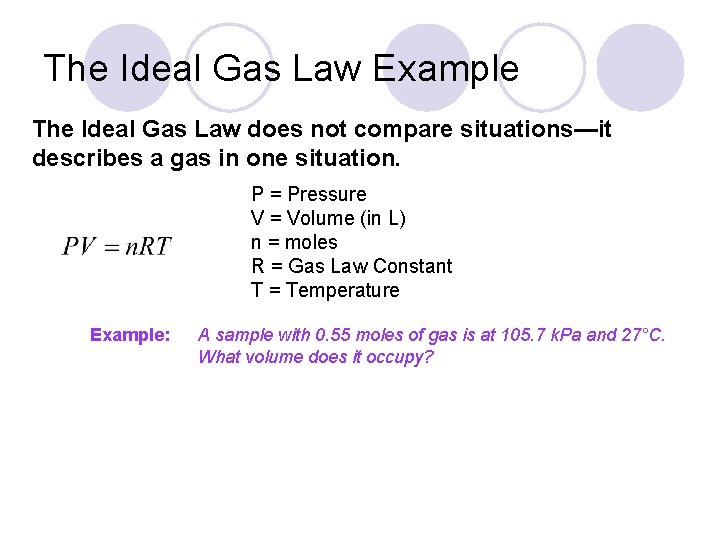
The Ideal Gas Law Example The Ideal Gas Law does not compare situations—it describes a gas in one situation. P = Pressure V = Volume (in L) n = moles R = Gas Law Constant T = Temperature Example: A sample with 0. 55 moles of gas is at 105. 7 k. Pa and 27°C. What volume does it occupy?

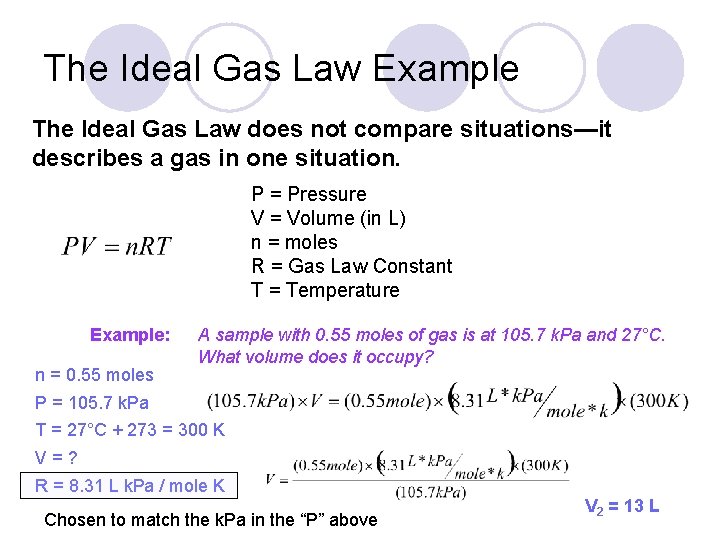
The Ideal Gas Law Example The Ideal Gas Law does not compare situations—it describes a gas in one situation. P = Pressure V = Volume (in L) n = moles R = Gas Law Constant T = Temperature Example: n = 0. 55 moles A sample with 0. 55 moles of gas is at 105. 7 k. Pa and 27°C. What volume does it occupy? P = 105. 7 k. Pa T = 27°C + 273 = 300 K V=? R = 8. 31 L k. Pa / mole K Chosen to match the k. Pa in the “P” above V 2 = 13 L

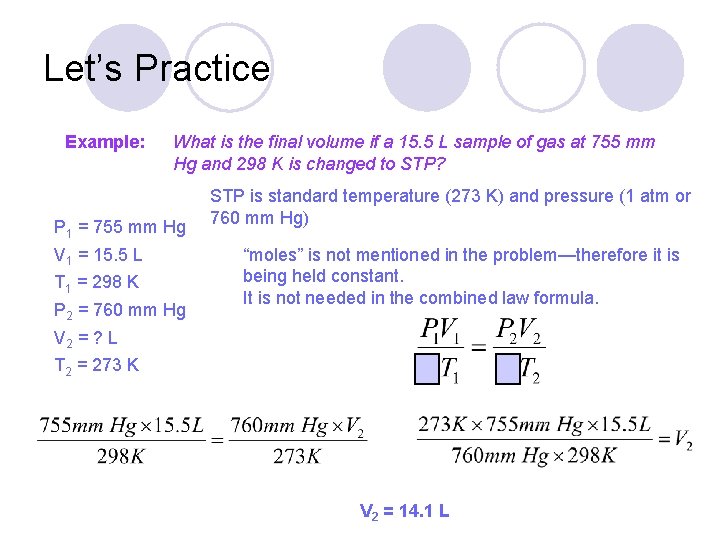
Let’s Practice Example: What is the final volume if a 15. 5 L sample of gas at 755 mm Hg and 298 K is changed to STP?

Let’s Practice Example: What is the final volume if a 15. 5 L sample of gas at 755 mm Hg and 298 K is changed to STP? P 1 = 755 mm Hg V 1 = 15. 5 L T 1 = 298 K P 2 = 760 mm Hg STP is standard temperature (273 K) and pressure (1 atm or 760 mm Hg) “moles” is not mentioned in the problem—therefore it is being held constant. It is not needed in the combined law formula. V 2 = ? L T 2 = 273 K V 2 = 14. 1 L

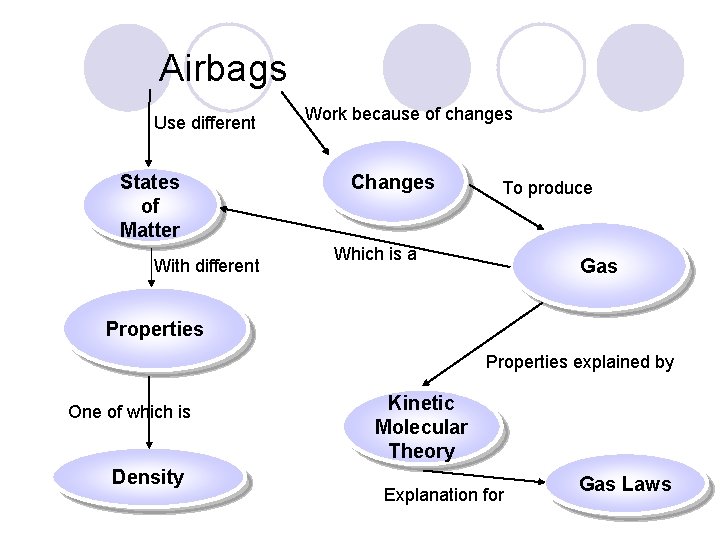
What did you learn about airbags?

Airbags Use different States of Matter With different Work because of changes Changes To produce Which is a Gas Properties explained by One of which is Density Kinetic Molecular Theory Explanation for Gas Laws