GayLussacs Law GayLussacs Law The relationship among pressure

- Slides: 8

Gay-Lussac’s Law

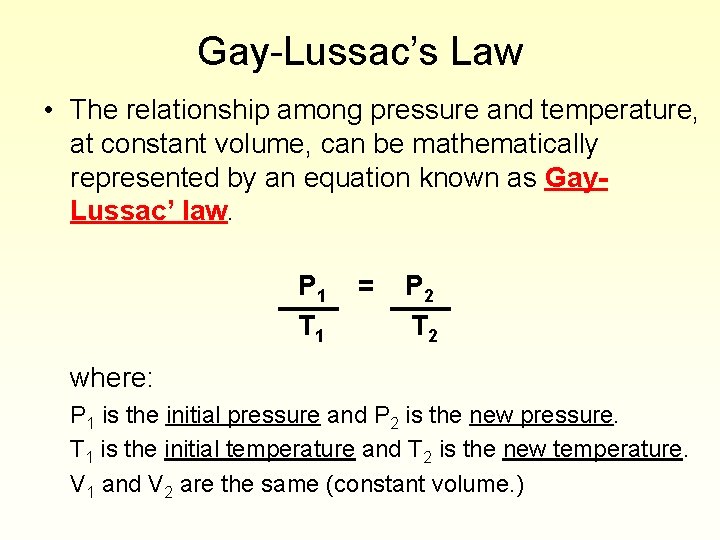
Gay-Lussac’s Law • The relationship among pressure and temperature, at constant volume, can be mathematically represented by an equation known as Gay. Lussac’ law. P 1 T 1 = P 2 T 2 where: P 1 is the initial pressure and P 2 is the new pressure. T 1 is the initial temperature and T 2 is the new temperature. V 1 and V 2 are the same (constant volume. )

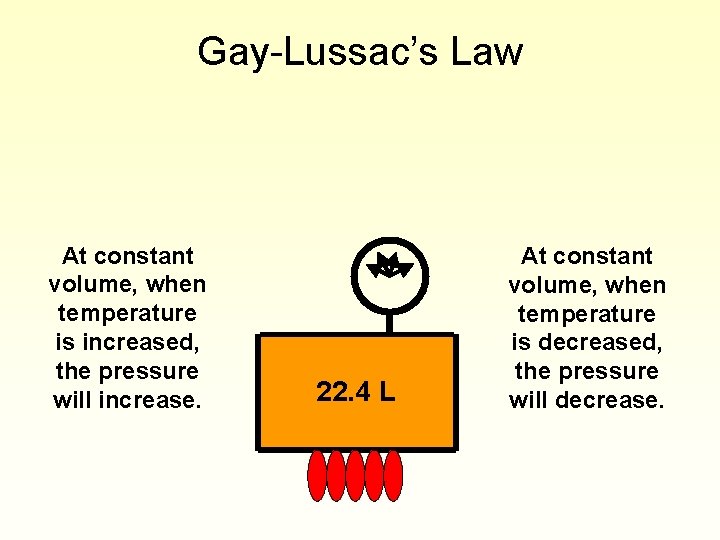
Gay-Lussac’s Law At constant volume, when temperature is increased, the pressure will increase. 22. 4 L At constant volume, when temperature is decreased, the pressure will decrease.

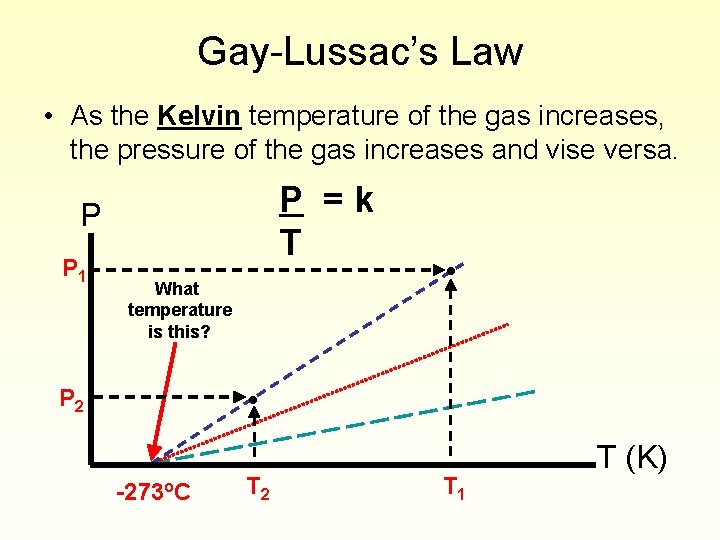
Gay-Lussac’s Law • As the Kelvin temperature of the gas increases, the pressure of the gas increases and vise versa. P =k T P P 1 What temperature is this? P 2 • • -273 o. C T 2 T 1 T (K)

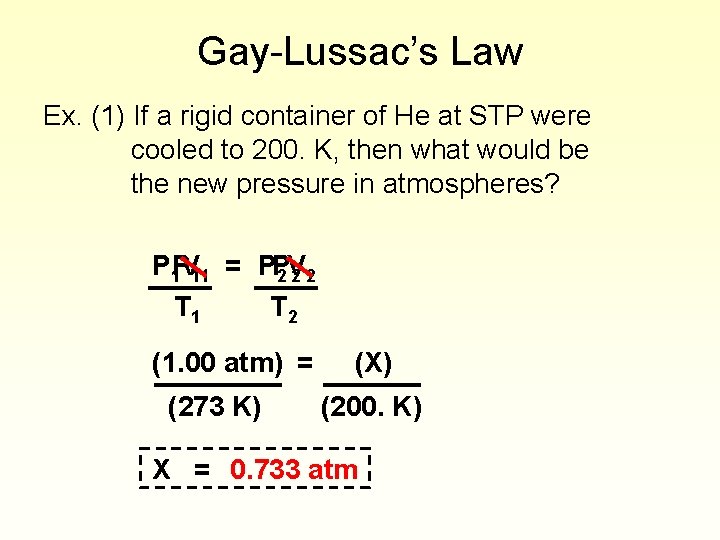
Gay-Lussac’s Law Ex. (1) If a rigid container of He at STP were cooled to 200. K, then what would be the new pressure in atmospheres? P 1 PV 11 = PP 2 V 22 T 1 T 2 (1. 00 atm) = (273 K) (X) (200. K) X = 0. 733 atm

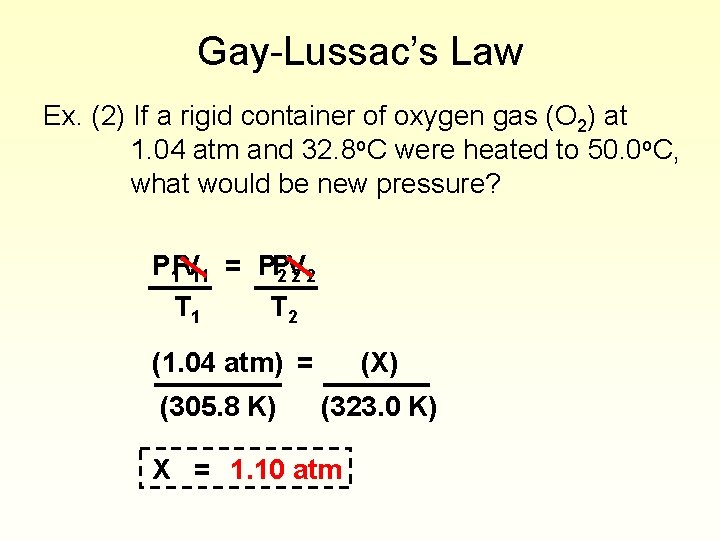
Gay-Lussac’s Law Ex. (2) If a rigid container of oxygen gas (O 2) at 1. 04 atm and 32. 8 o. C were heated to 50. 0 o. C, what would be new pressure? P 1 PV 11 = PP 2 V 22 T 1 T 2 (1. 04 atm) = (305. 8 K) (X) (323. 0 K) X = 1. 10 atm

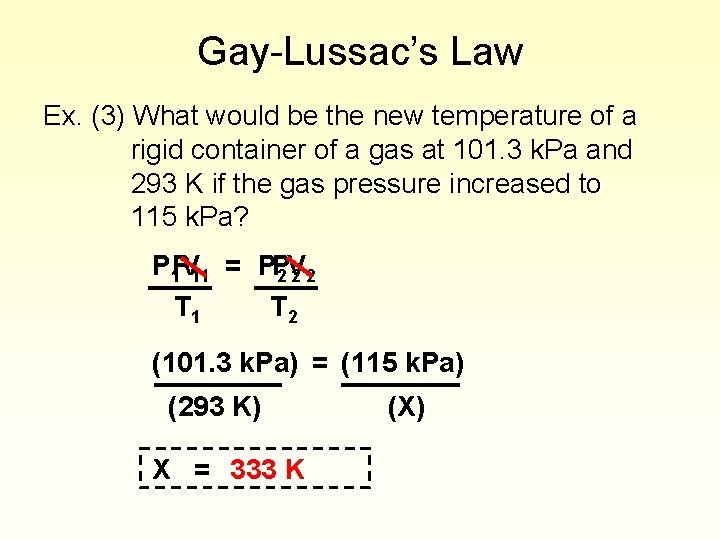
Gay-Lussac’s Law Ex. (3) What would be the new temperature of a rigid container of a gas at 101. 3 k. Pa and 293 K if the gas pressure increased to 115 k. Pa? P 1 PV 11 = PP 2 V 22 T 1 T 2 (101. 3 k. Pa) = (115 k. Pa) (293 K) X = 333 K (X)

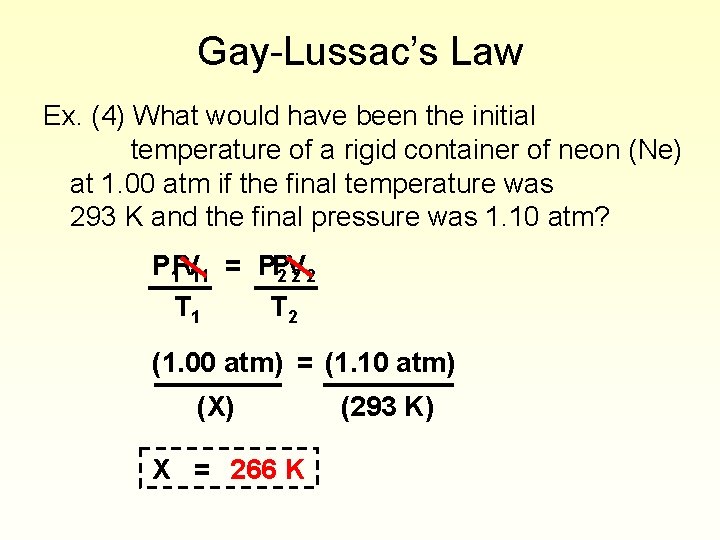
Gay-Lussac’s Law Ex. (4) What would have been the initial temperature of a rigid container of neon (Ne) at 1. 00 atm if the final temperature was 293 K and the final pressure was 1. 10 atm? P 1 PV 11 = PP 2 V 22 T 1 T 2 (1. 00 atm) = (1. 10 atm) (X) X = 266 K (293 K)