8 4 Temperature and Pressure GayLussacs Law GayLussacs


- Slides: 14

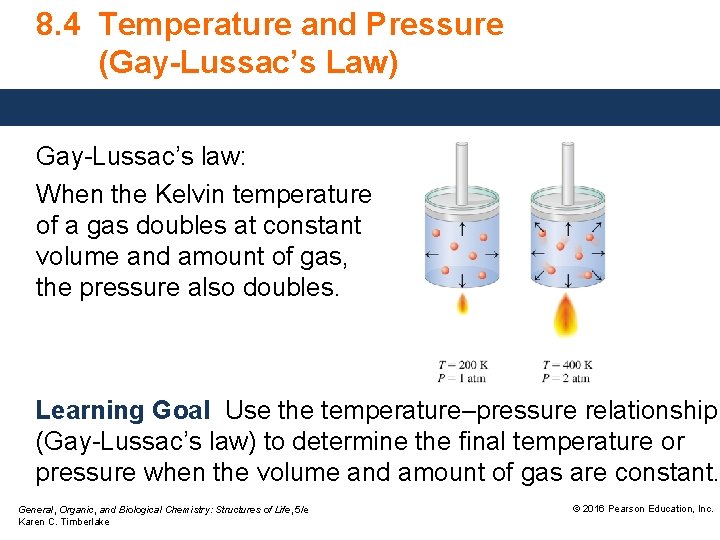
8. 4 Temperature and Pressure (Gay-Lussac’s Law) Gay-Lussac’s law: When the Kelvin temperature of a gas doubles at constant volume and amount of gas, the pressure also doubles. Learning Goal Use the temperature–pressure relationship (Gay-Lussac’s law) to determine the final temperature or pressure when the volume and amount of gas are constant. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

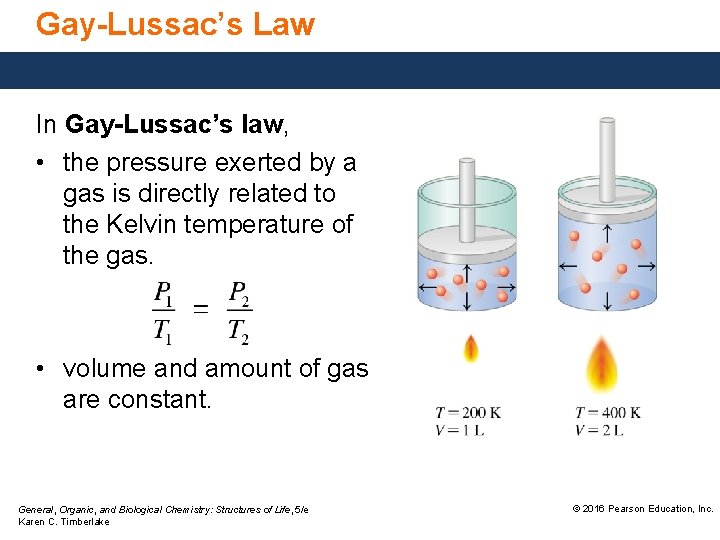
Gay-Lussac’s Law In Gay-Lussac’s law, • the pressure exerted by a gas is directly related to the Kelvin temperature of the gas. • volume and amount of gas are constant. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

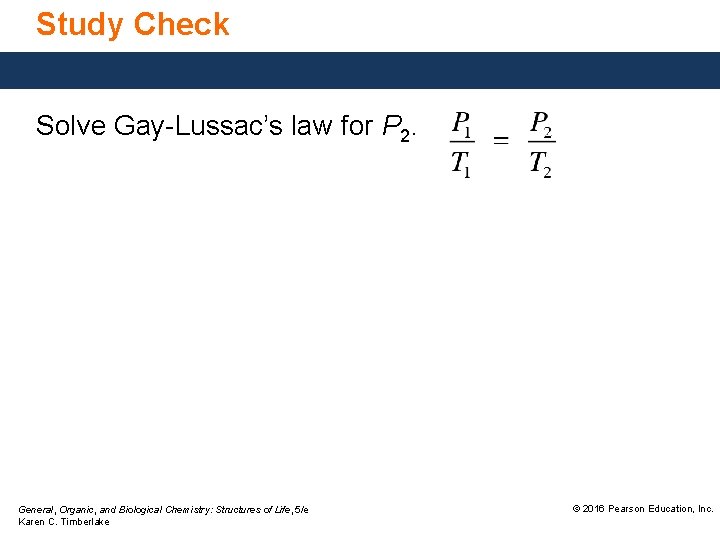
Study Check Solve Gay-Lussac’s law for P 2. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

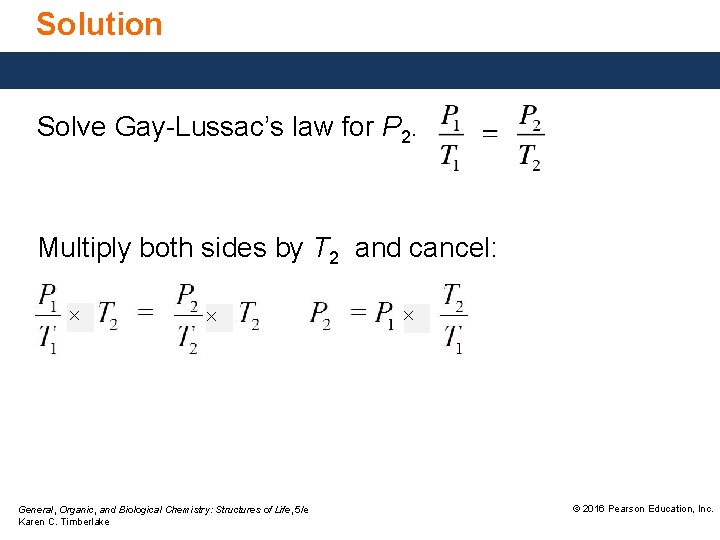
Solution Solve Gay-Lussac’s law for P 2. Multiply both sides by T 2 and cancel: × × General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake × © 2016 Pearson Education, Inc.

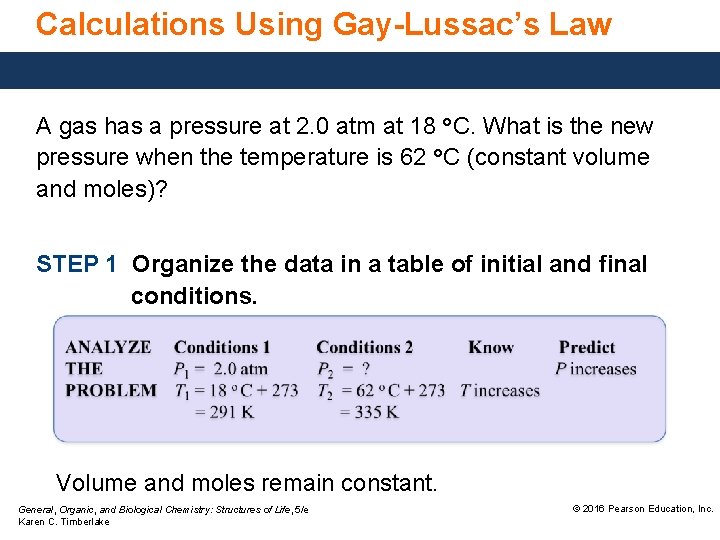
Calculations Using Gay-Lussac’s Law A gas has a pressure at 2. 0 atm at 18 °C. What is the new pressure when the temperature is 62 °C (constant volume and moles)? STEP 1 Organize the data in a table of initial and final conditions. Volume and moles remain constant. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

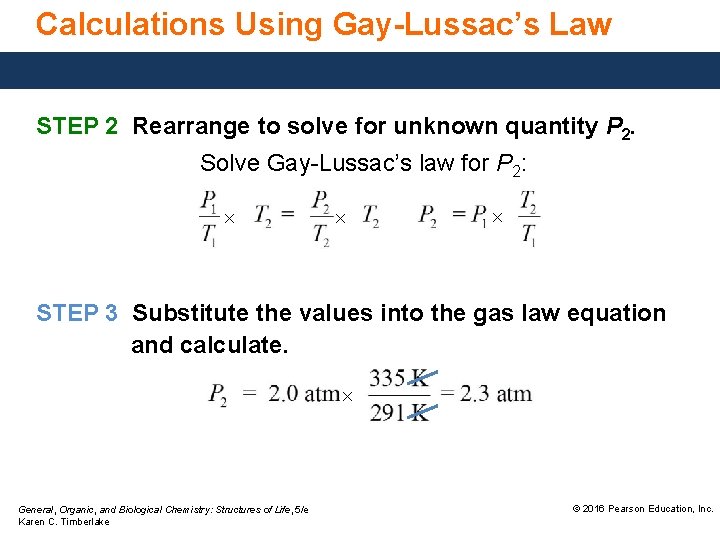
Calculations Using Gay-Lussac’s Law STEP 2 Rearrange to solve for unknown quantity P 2. Solve Gay-Lussac’s law for P 2: × × × STEP 3 Substitute the values into the gas law equation and calculate. × General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Study Check A gas has a pressure of 645 Torr at 128 °C. What is the temperature in Celsius if the pressure increases to 824 Torr (V and n remain constant)? General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Solution A gas has a pressure of 645 Torr at 128 °C. What is the temperature in Celsius if the pressure increases to 824 Torr (V and n remain constant)? STEP 1 Organize the data in a table of initial and final conditions. Volume and moles remain constant. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Solution A gas has a pressure of 645 Torr at 128 °C. What is the temperature in Celsius if the pressure increases to 824 Torr (V and n remain constant)? STEP 2 Rearrange to solve for unknown quantity T 2 Solve Gay-Lussac’s law for T 2: × STEP 3 Substitute the values into the gas law equation and calculate. × General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake - © 2016 Pearson Education, Inc.

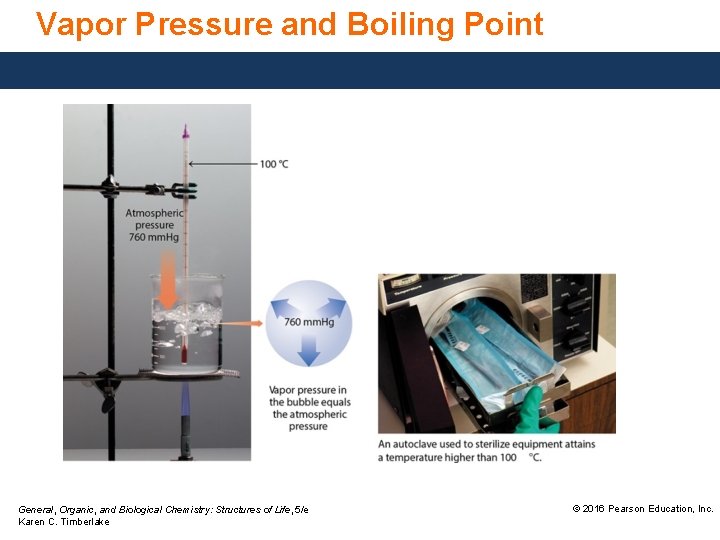
Vapor Pressure and Boiling Point When liquid molecules with sufficient kinetic energy break away from the surface of a liquid, they become a vapor. • In an open container, all the liquid will eventually evaporate. • In a closed container, the vapor accumulates and creates pressure called vapor pressure. A liquid • exerts its own vapor pressure at a given temperature. • boils when its vapor pressure becomes equal to the external pressure. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

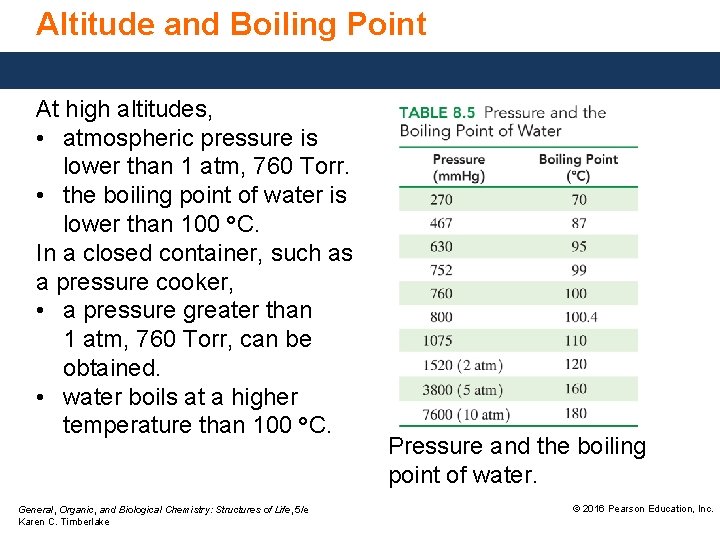
Altitude and Boiling Point At high altitudes, • atmospheric pressure is lower than 1 atm, 760 Torr. • the boiling point of water is lower than 100 °C. In a closed container, such as a pressure cooker, • a pressure greater than 1 atm, 760 Torr, can be obtained. • water boils at a higher temperature than 100 °C. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake Pressure and the boiling point of water. © 2016 Pearson Education, Inc.

Vapor Pressure and Boiling Point General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Study Check Explain why water boils at a lower temperature in the mountains than at sea level. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Solution Explain why water boils at a lower temperature in the mountains than at sea level. Atmospheric pressure in the mountains is less than at sea level. The vapor pressure of the water reaches the atmospheric pressure at a lower temperature. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
 Gaylussacs law
Gaylussacs law Daltons gas law
Daltons gas law Which gas law relates pressure and temperature
Which gas law relates pressure and temperature 13-4 practice problems chemistry
13-4 practice problems chemistry Guy lussacs law
Guy lussacs law Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law and second law and third law
Newton's first law and second law and third law Difference between curie temperature and neel temperature
Difference between curie temperature and neel temperature Difference between curie temperature and neel temperature
Difference between curie temperature and neel temperature Ferromagnetis
Ferromagnetis Relation between temperature and pressure
Relation between temperature and pressure Gas variable relationships
Gas variable relationships Le chateliers principle
Le chateliers principle Gas law constant
Gas law constant What are temperature pulse respirations and blood pressure
What are temperature pulse respirations and blood pressure