GayLussacs Law The pressure of an ideal gas





- Slides: 9

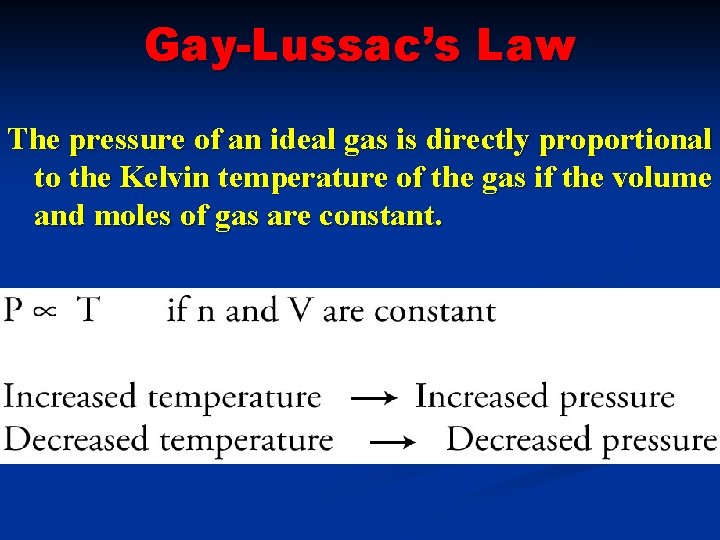
Gay-Lussac’s Law The pressure of an ideal gas is directly proportional to the Kelvin temperature of the gas if the volume and moles of gas are constant.

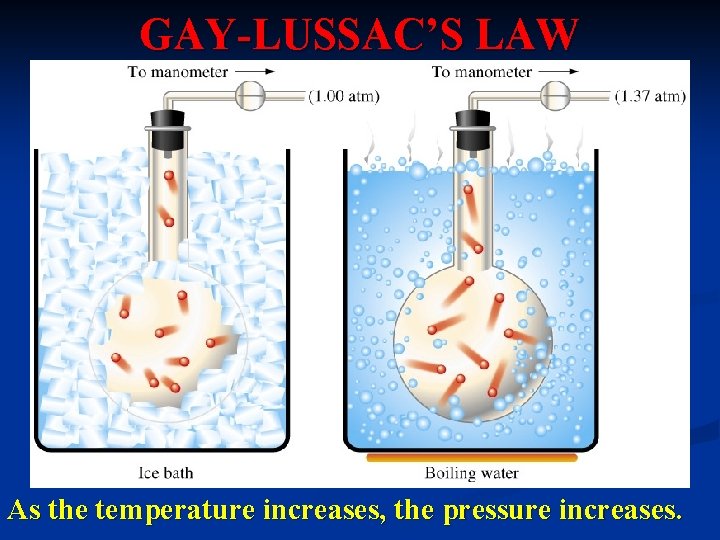
GAY-LUSSAC’S LAW As the temperature increases, the pressure increases.

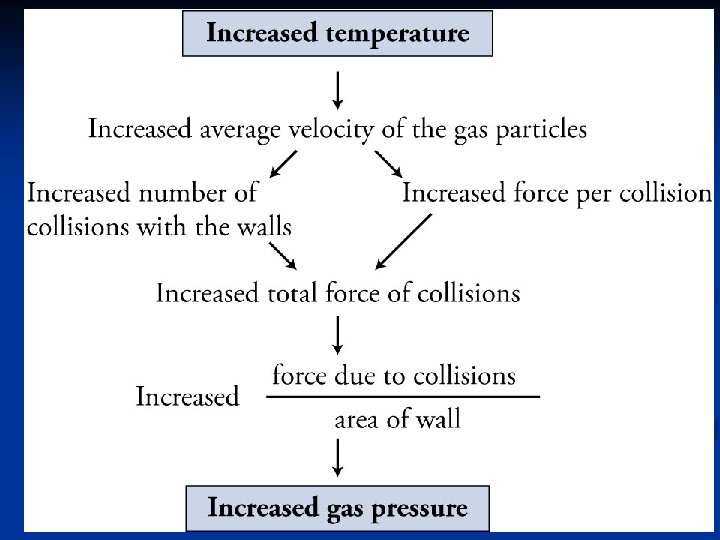
Relationship between P and T


Example 1: 10. 0 L of a gas is found to exert 97. 0 k. Pa at 25. 0°C. What would be the required temperature (in Celsius) to change the pressure to standard pressure? Answer: change 25. 0°C to 298. 0 K and remember that standard pressure in k. Pa is 101. 325. Insert values into the equation (the Chem. Team will use the left-hand one in the graphic above) and get: x = 311. 3 K or 38. 3 o. C

Example 2: 5. 00 L of a gas is collected at 22. 0°C and 745. 0 mm. Hg. When the temperature is changed to standard, what is the new pressure? Answer: convert to Kelvin and insert: 689 mm Hg

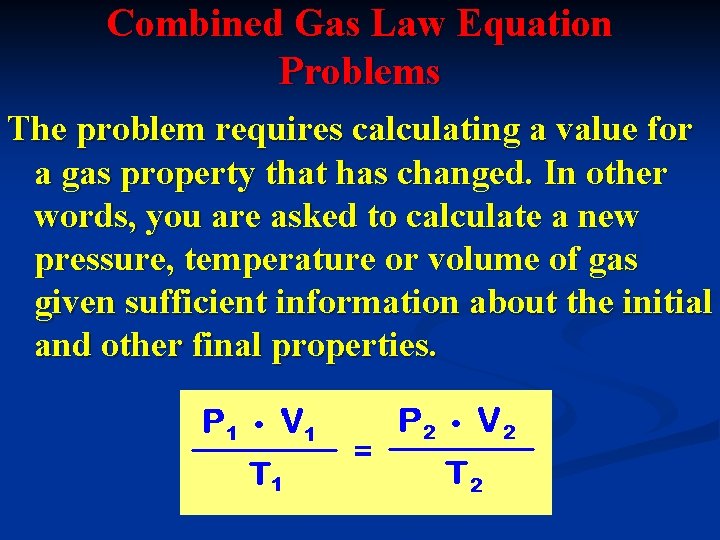
Combined Gas Law Equation Problems The problem requires calculating a value for a gas property that has changed. In other words, you are asked to calculate a new pressure, temperature or volume of gas given sufficient information about the initial and other final properties.

Combined Gas Law Equation Problem Solving Steps Step 1: Assign the variables P, T, and V to the values you are given and to the unknown value. Use the subscripts 1 and 2 to show initial or final conditions. Step 2: Write out the combined gas law equation, but eliminate the variables for any constant properties. (You can assume that the properties not mentioned in the problem remain constant. )

Combined Gas Law Equation Problem Solving Steps Step 3: Rearrange the equation to isolate the unknown property. Step 4: Plug in the values for the given properties. Step 5: Make any necessary unit conversions and cancel your units. Step 6: Calculate your answer and report it with the correct units and significant figures.















