Gases Pressure and Volume Boyles Law Temperature and

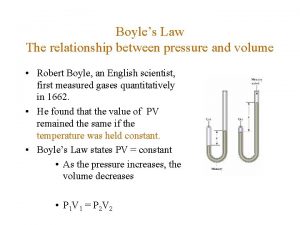
Gases Pressure and Volume (Boyle’s Law) Temperature and Volume (Charles’ Law) Temperature and Pressure (Gay-Lussac’s Law)
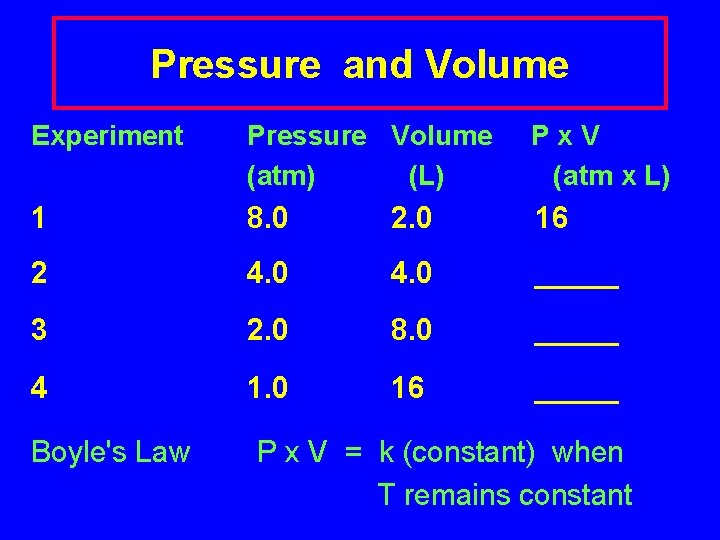
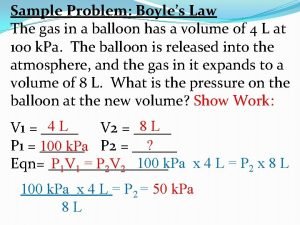
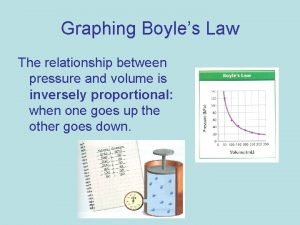
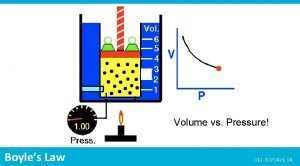
Pressure and Volume Experiment Pressure Volume (atm) (L) Px. V (atm x L) 1 8. 0 2. 0 16 2 4. 0 _____ 3 2. 0 8. 0 _____ 4 1. 0 16 _____ Boyle's Law P x V = k (constant) when T remains constant
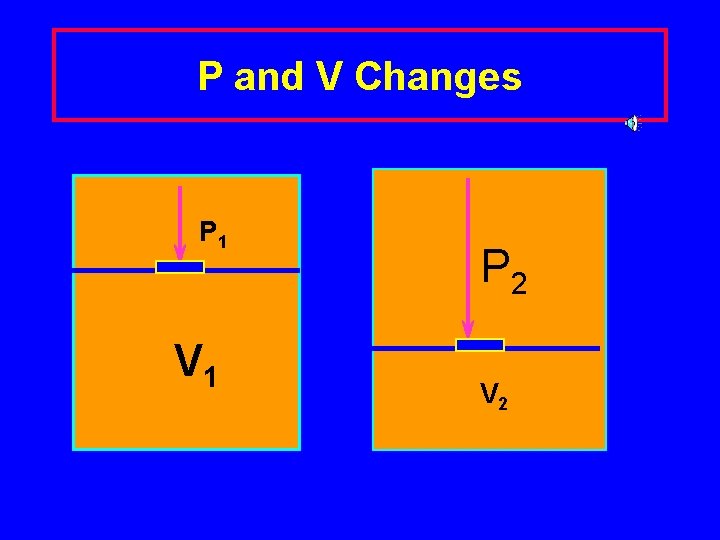
P and V Changes P 1 V 1 P 2 V 2
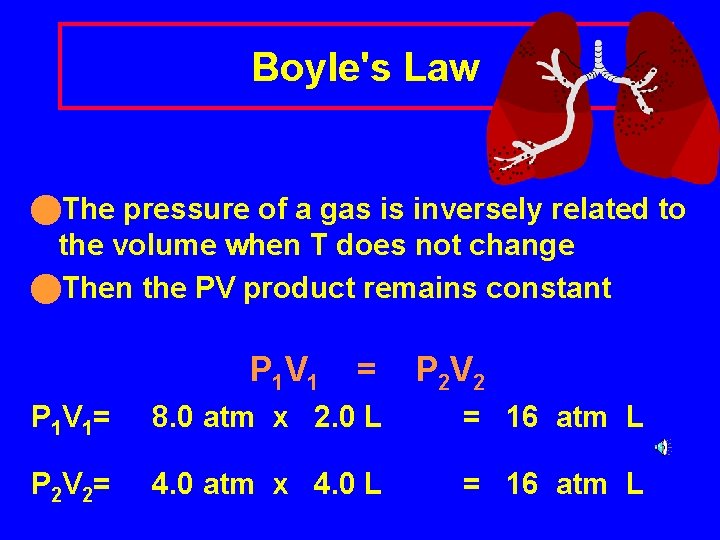
Boyle's Law n. The pressure of a gas is inversely related to the volume when T does not change n. Then the PV product remains constant P 1 V 1 = P 2 V 2 P 1 V 1= 8. 0 atm x 2. 0 L = 16 atm L P 2 V 2= 4. 0 atm x 4. 0 L = 16 atm L

PV Problem Freon-12, CCl 2 F 2, is used in refrigeration systems. What is the new volume (L) of a 1. 6 L sample of Freon gas initially at 50 mm Hg after its pressure is changed to 200 mm Hg at constant T?
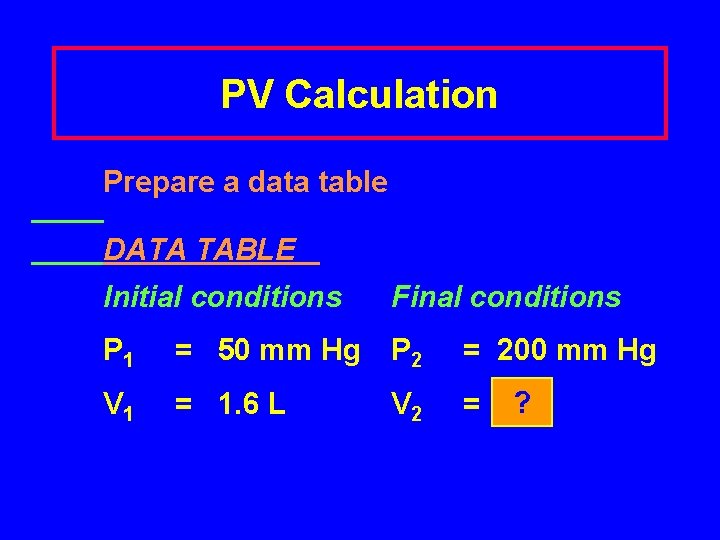
PV Calculation Prepare a data table DATA TABLE Initial conditions Final conditions P 1 = 50 mm Hg P 2 = 200 mm Hg V 1 = 1. 6 L V 2 = ? ?

Find New Volume (V 2) Solve for V 2: V 2 = P 1 V 2 = P 2 V 2 V 1 x P 1 /P 2 V 2 = 1. 6 L x 50 mm Hg = 200 mm Hg 0. 4 L

Learning Check GL 1 A sample of nitrogen gas is 6. 4 L at a pressure of 0. 70 atm. What will the new volume be if the pressure is changed to 1. 40 atm? (T constant) Explain. 1) 3. 2 L 2) 6. 4 L 3) 12. 8 L

Solution GL 1 A sample of nitrogen gas is 6. 4 L at a pressure of 0. 70 atm. What will the new volume be if the pressure is changed to 1. 40 atm? (T constant) 6. 4 L x 0. 70 atm = 3. 2 L (1) 1. 40 atm Volume must decrease to cause an increase in the pressure

Learning Check GL 2 A sample of helium gas has a volume of 12. 0 L at 600. mm Hg. What new pressure is needed to change the volume to 36. 0 L? (T constant) Explain. 1) 200. mm. Hg 2) 400. mm. Hg 3) 1200 mm. Hg

Solution GL 2 A sample of helium gas has a volume of 12. 0 L at 600. mm Hg. What new pressure is needed to change the volume to 36. 0 L? (T constant) Explain. 600. mm Hg x 12. 0 L = 200. mm. Hg (1) 36. 0 L Pressure decrease when volume increases.
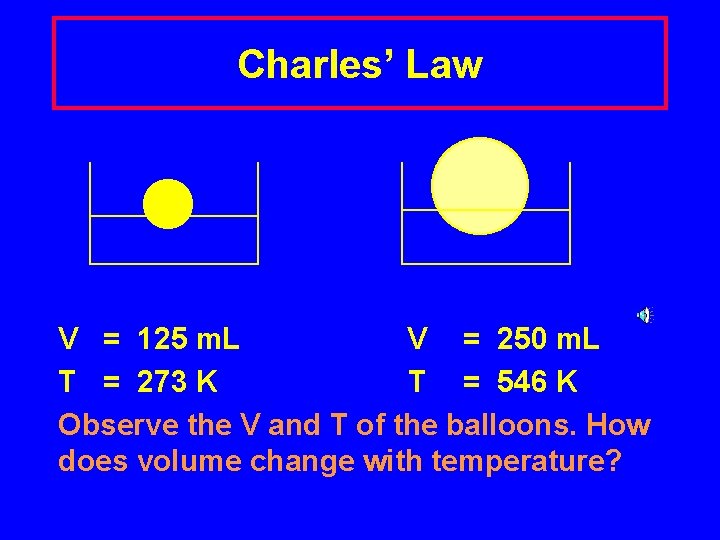
Charles’ Law V = 125 m. L V = 250 m. L T = 273 K T = 546 K Observe the V and T of the balloons. How does volume change with temperature?
Charles’ Law: V and T At constant pressure, the volume of a gas is directly related to its absolute (K) temperature V 1 = V 2 T 1 T 2
Learning Check GL 3 Use Charles’ Law to complete the statements below: 1. If final T is higher than initial T, final V is (greater, or less) than the initial V. 2. If final V is less than initial V, final T is (higher, or lower) than the initial T.

Solution GL 3 V 1 T 1 = V 2 T 2 1. If final T is higher than initial T, final V is (greater) than the initial V. 2. If final V is less than initial V, final T is (lower) than the initial T.

V and T Problem A balloon has a volume of 785 m. L on a Fall day when the temperature is 21°C. In the winter, the gas cools to 0°C. What is the new volume of the balloon?
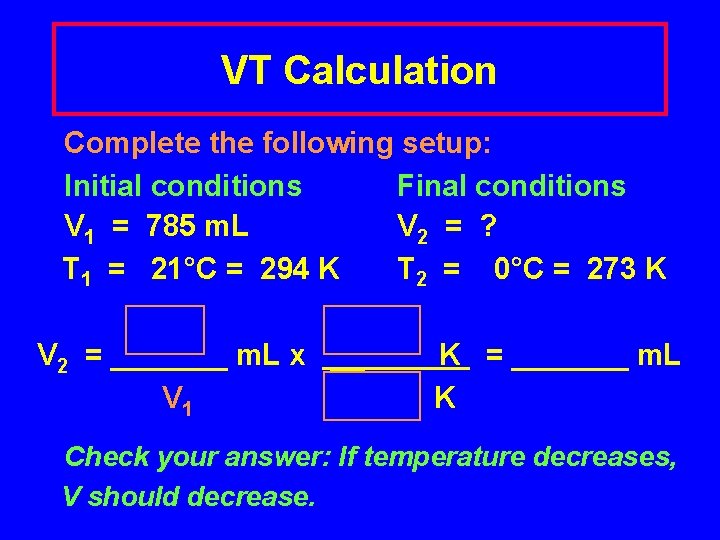
VT Calculation Complete the following setup: Initial conditions Final conditions V 1 = 785 m. L V 2 = ? T 1 = 21°C = 294 K T 2 = 0°C = 273 K V 2 = _______ m. L x __ V 1 K = _______ m. L K Check your answer: If temperature decreases, V should decrease.
Learning Check GL 4 A sample of oxygen gas has a volume of 420 m. L at a temperature of 18°C. What temperature (in °C) is needed to change the volume to 640 m. L? 1) 443°C 2) 170°C 3) - 82°C

Solution GL 4 A sample of oxygen gas has a volume of 420 m. L at a temperature of 18°C. What temperature (in °C) is needed to change the volume to 640 m. L? 2) 170°C T 2 = 291 K x 640 m. L = 443 K 420 m. L = 443 K - 273 K = 170°C
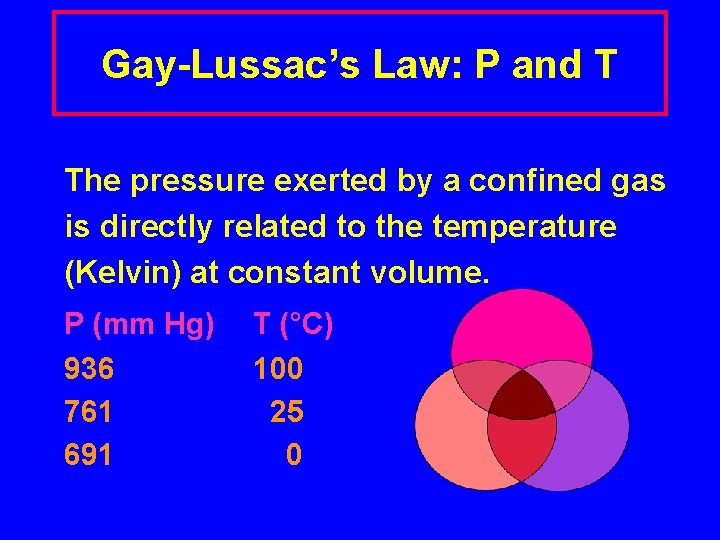
Gay-Lussac’s Law: P and T The pressure exerted by a confined gas is directly related to the temperature (Kelvin) at constant volume. P (mm Hg) 936 761 691 T (°C) 100 25 0

Learning Check GL 5 Use Gay-Lussac’s law to complete the statements below: 1. When temperature decreases, the pressure of a gas (decreases or increases). 2. When temperature increases, the pressure of a gas (decreases or increases).

Solution GL 5 1. When temperature decreases, the pressure of a gas (decreases). 2. When temperature increases, the pressure of a gas (increases).
PT Problem A gas has a pressure at 2. 0 atm at 18°C. What will be the new pressure if the temperature rises to 62°C? (V constant) T = 18°C T = 62°C
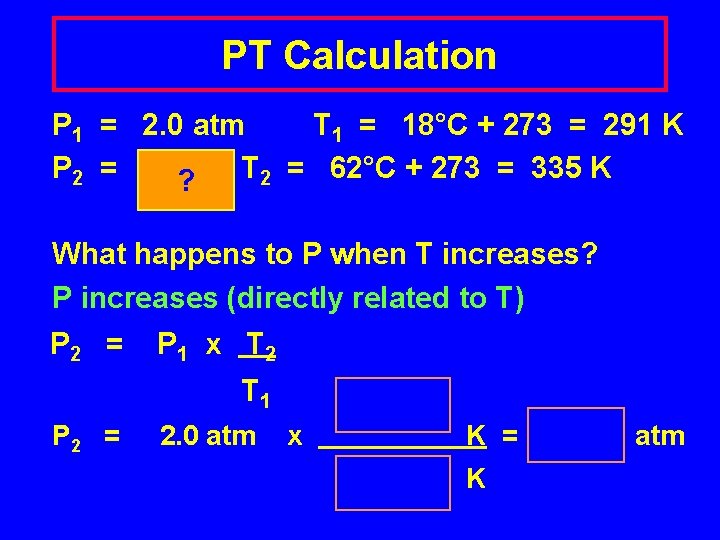
PT Calculation P 1 = 2. 0 atm T 1 = 18°C + 273 = 291 K P 2 = ? ? T 2 = 62°C + 273 = 335 K What happens to P when T increases? P increases (directly related to T) P 2 = P 1 x T 2 T 1 P 2 = 2. 0 atm x K = K atm
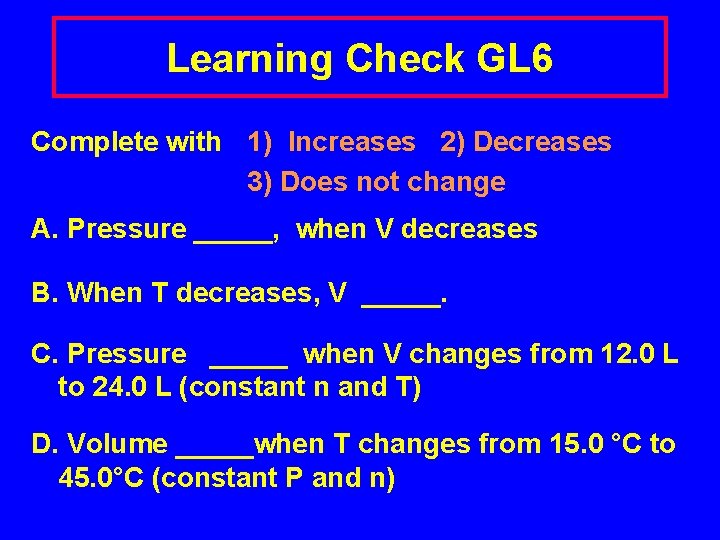
Learning Check GL 6 Complete with 1) Increases 2) Decreases 3) Does not change A. Pressure _____, when V decreases B. When T decreases, V _____. C. Pressure _____ when V changes from 12. 0 L to 24. 0 L (constant n and T) D. Volume _____when T changes from 15. 0 °C to 45. 0°C (constant P and n)

Solution GL 6 A. Pressure 1) Increases, when V decreases B. When T decreases, V 2) Decreases C. Pressure 2) Decreases when V changes from 12. 0 L to 24. 0 L (constant n and T) D. Volume 1) Increases when T changes from 15. 0 °C to 45. 0°C (constant P and n)
- Slides: 26