Thermochem Unit 10 Lesson 2 Thermochemistry Study of











- Slides: 47

Thermochem Unit 10 Lesson 2

Thermochemistry Study of energy changes that occur during chemical reactions and changes of state

Endothermic Process that absorbs/gains heat Heat flows into the system (+q) Heat removed from the surroundings (-q) Surroundings feel cold

Exothermic Process that releases/produces heat Heat flows out of the system (-q) Heat added to the surroundings (+q) Surroundings may feel warm/hot

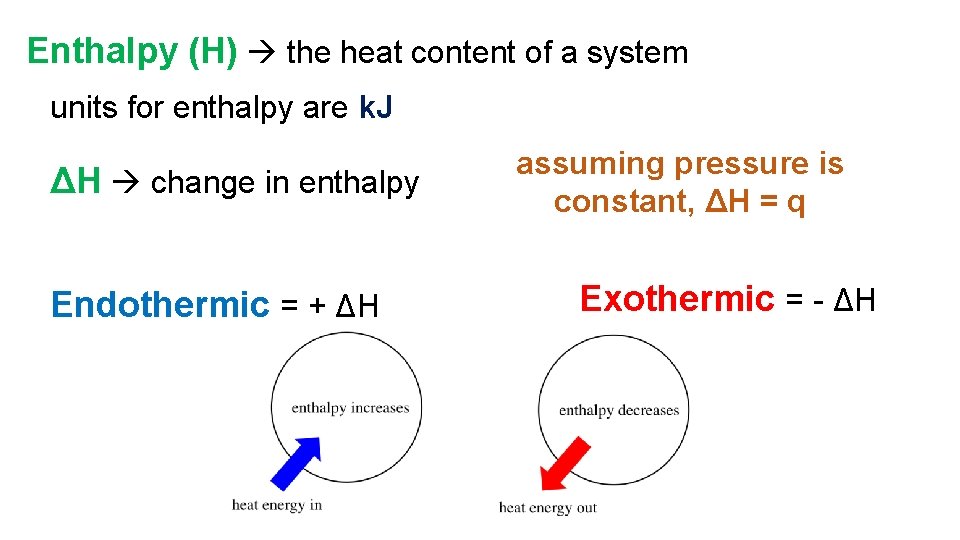
Enthalpy (H) the heat content of a system units for enthalpy are k. J ΔH change in enthalpy Endothermic = + ΔH assuming pressure is constant, ΔH = q Exothermic = - ΔH

ΔH & Changes of State Change in state always involves a change in heat energy

Endothermic changes of state absorb heat energy, increasing in temperature Melting (fusion) Vaporization Sublimation

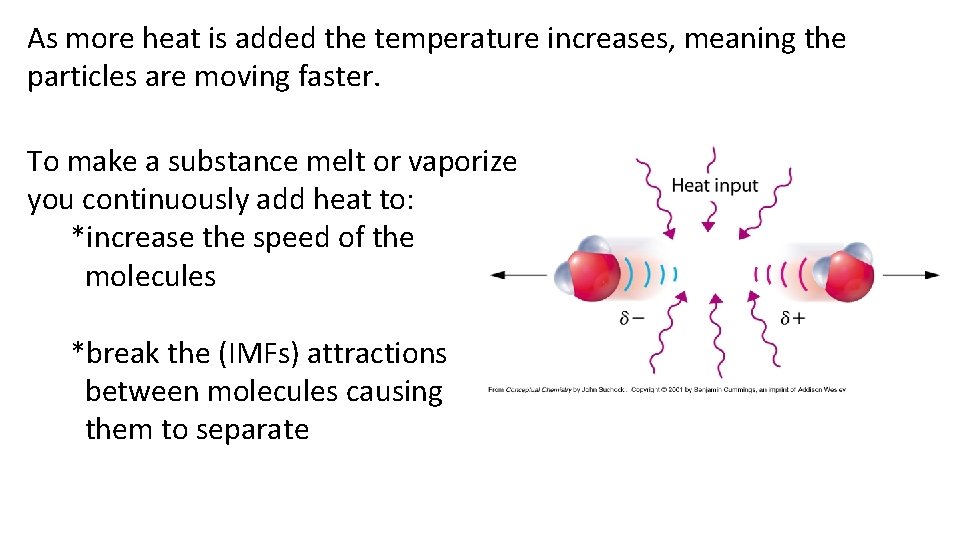
As more heat is added the temperature increases, meaning the particles are moving faster. To make a substance melt or vaporize you continuously add heat to: *increase the speed of the molecules *break the (IMFs) attractions between molecules causing them to separate

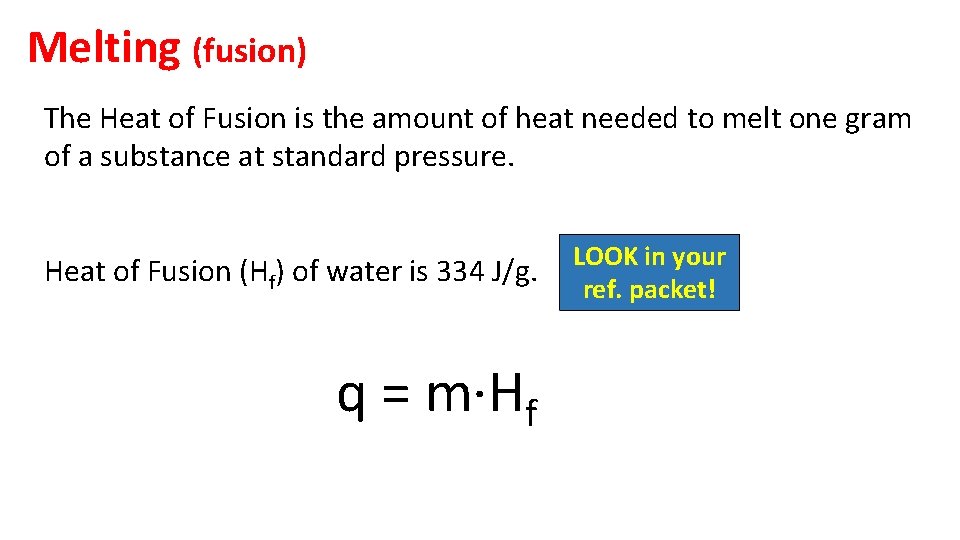
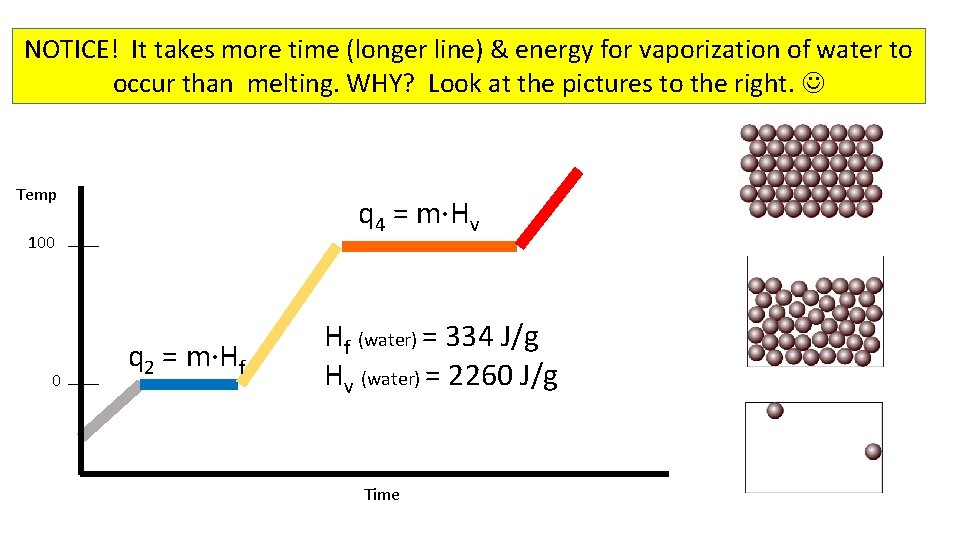
Melting (fusion) The Heat of Fusion is the amount of heat needed to melt one gram of a substance at standard pressure. Heat of Fusion (Hf) of water is 334 J/g. q = m·Hf LOOK in your ref. packet!

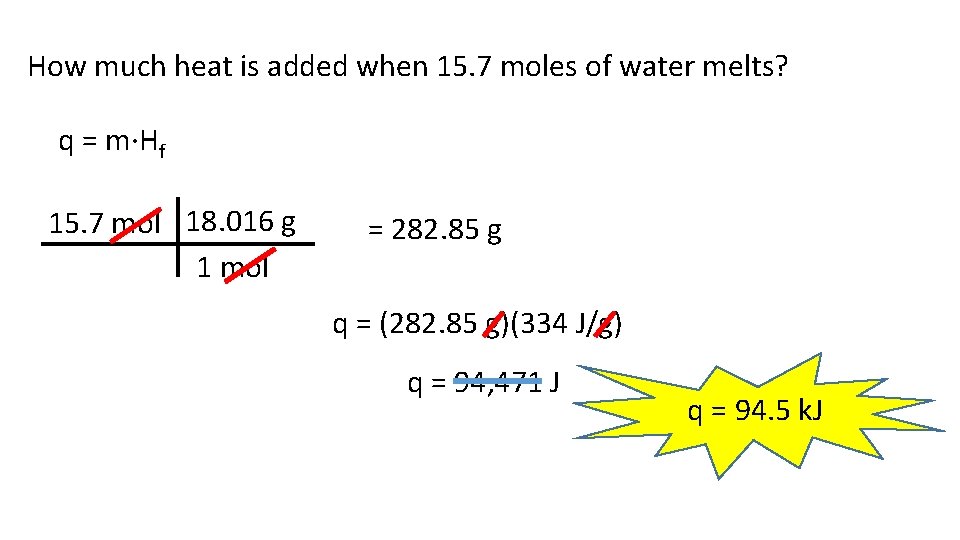
How much heat is added when 15. 7 moles of water melts? q = m·Hf 15. 7 mol 18. 016 g 1 mol = 282. 85 g q = (282. 85 g)(334 J/g) q = 94, 471 J q = 94. 5 k. J

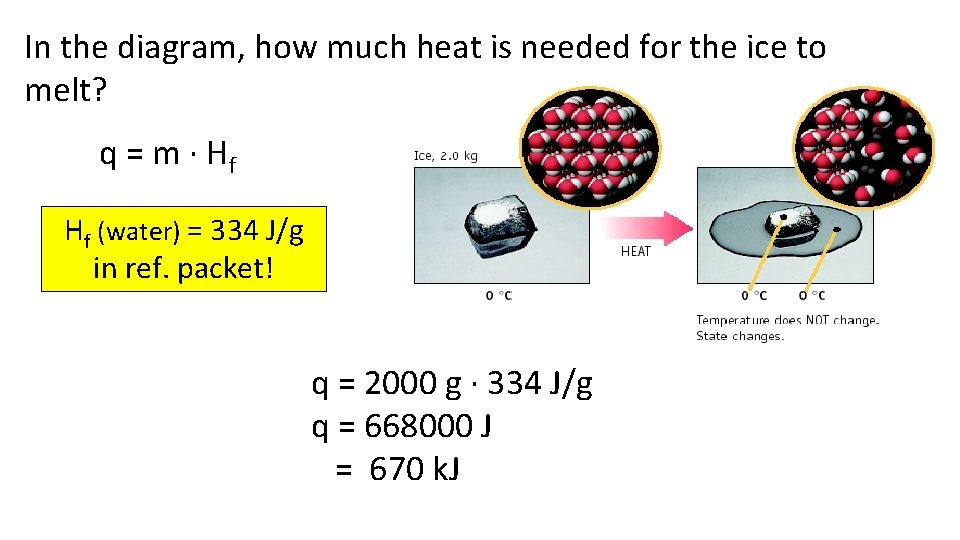
In the diagram, how much heat is needed for the ice to melt? q = m · Hf Hf (water) = 334 J/g in ref. packet! q = 2000 g · 334 J/g q = 668000 J = 670 k. J

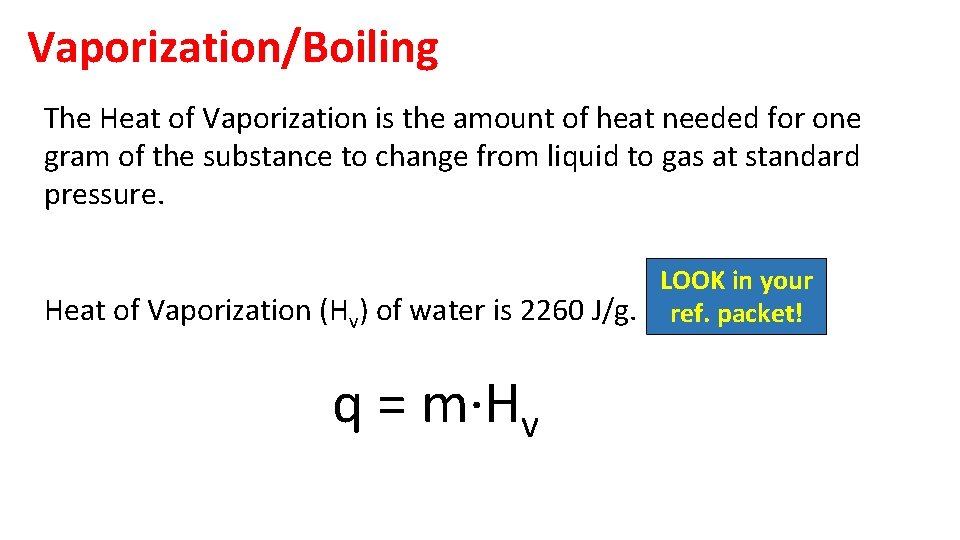
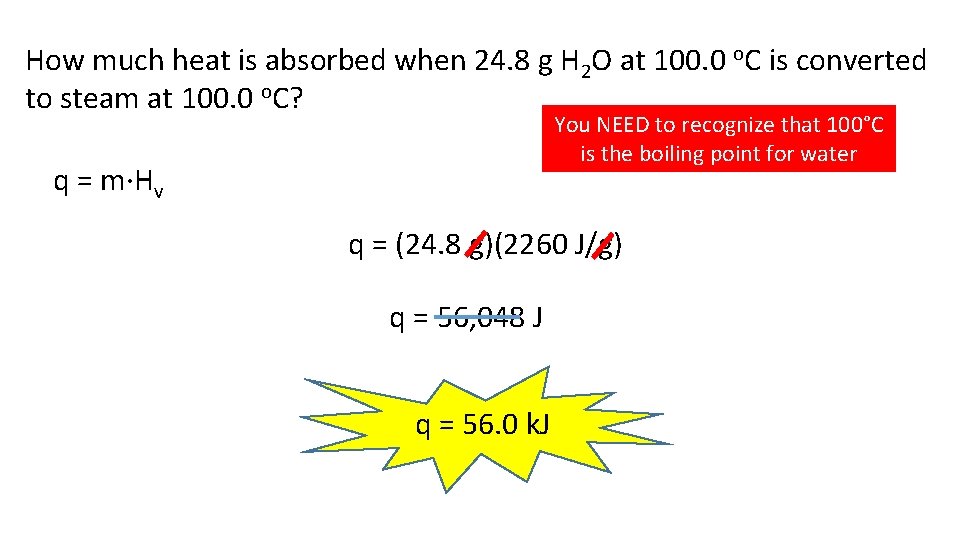
Vaporization/Boiling The Heat of Vaporization is the amount of heat needed for one gram of the substance to change from liquid to gas at standard pressure. LOOK in your Heat of Vaporization (Hv) of water is 2260 J/g. ref. packet! q = m·Hv

How much heat is absorbed when 24. 8 g H 2 O at 100. 0 o. C is converted to steam at 100. 0 o. C? You NEED to recognize that 100°C is the boiling point for water q = m·Hv q = (24. 8 g)(2260 J/g) q = 56, 048 J q = 56. 0 k. J

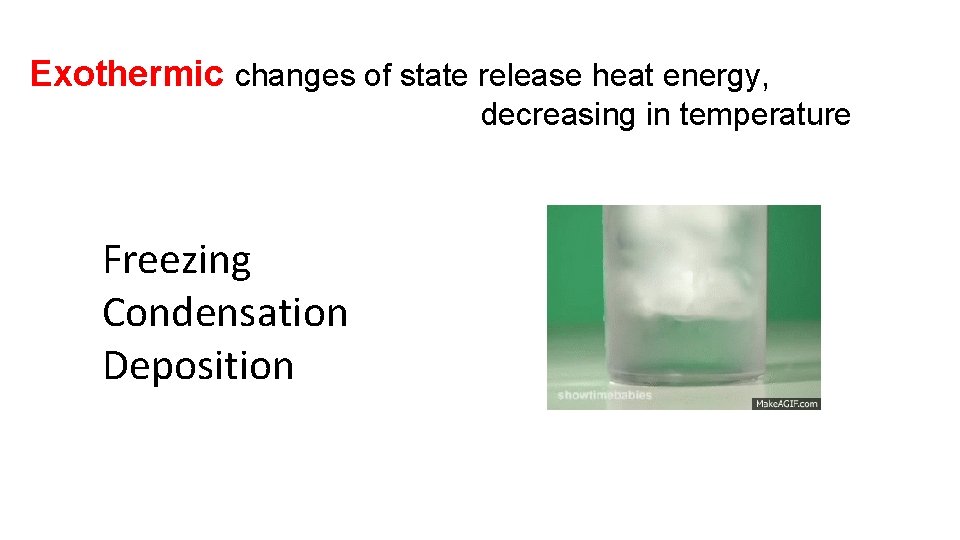
Exothermic changes of state release heat energy, decreasing in temperature Freezing Condensation Deposition

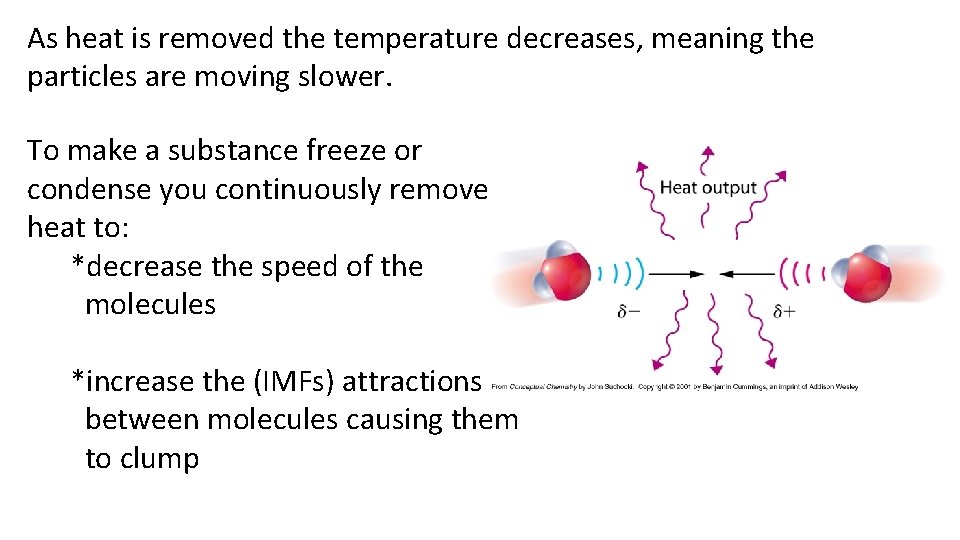
As heat is removed the temperature decreases, meaning the particles are moving slower. To make a substance freeze or condense you continuously remove heat to: *decrease the speed of the molecules *increase the (IMFs) attractions between molecules causing them to clump


Heating/C ooling

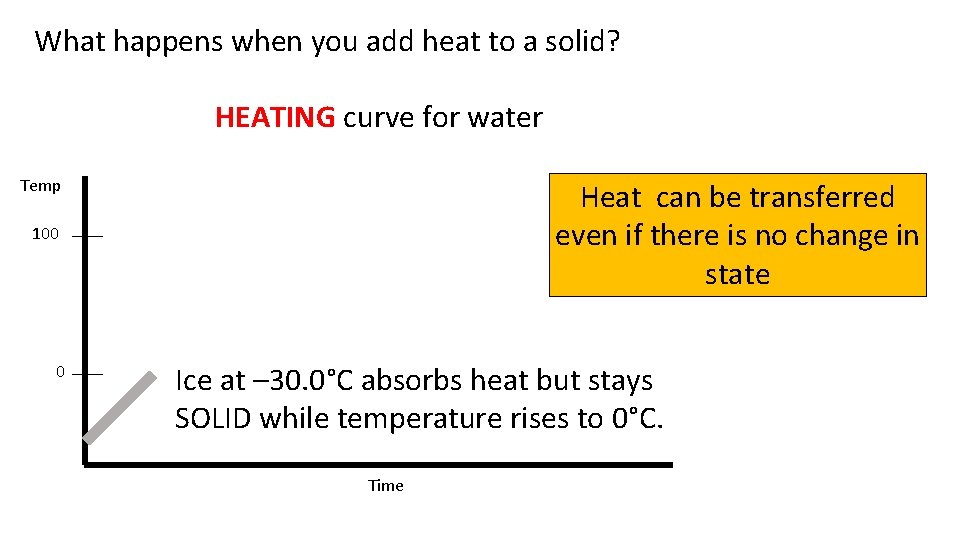
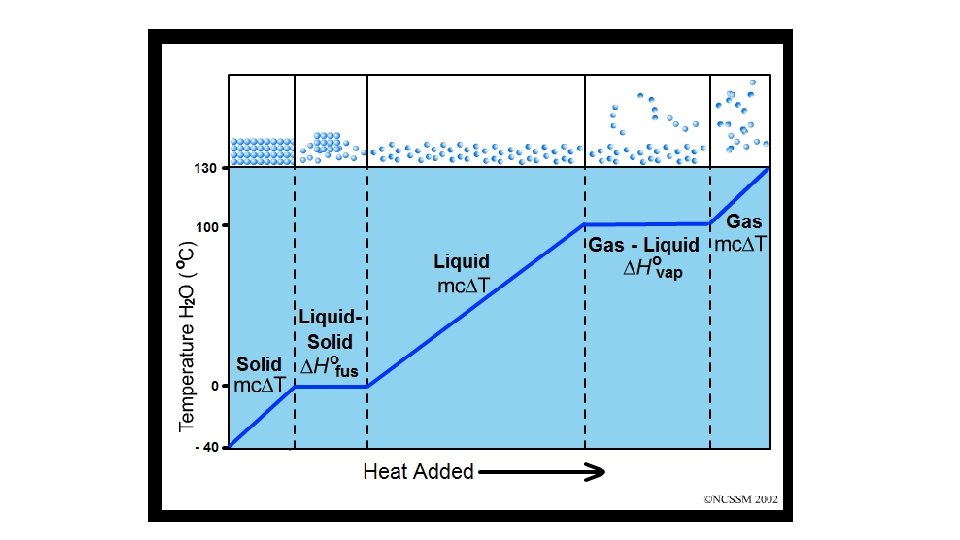
What happens when you add heat to a solid? HEATING curve for water Temp Heat can be transferred even if there is no change in state 100 0 Ice at – 30. 0°C absorbs heat but stays SOLID while temperature rises to 0°C. Time

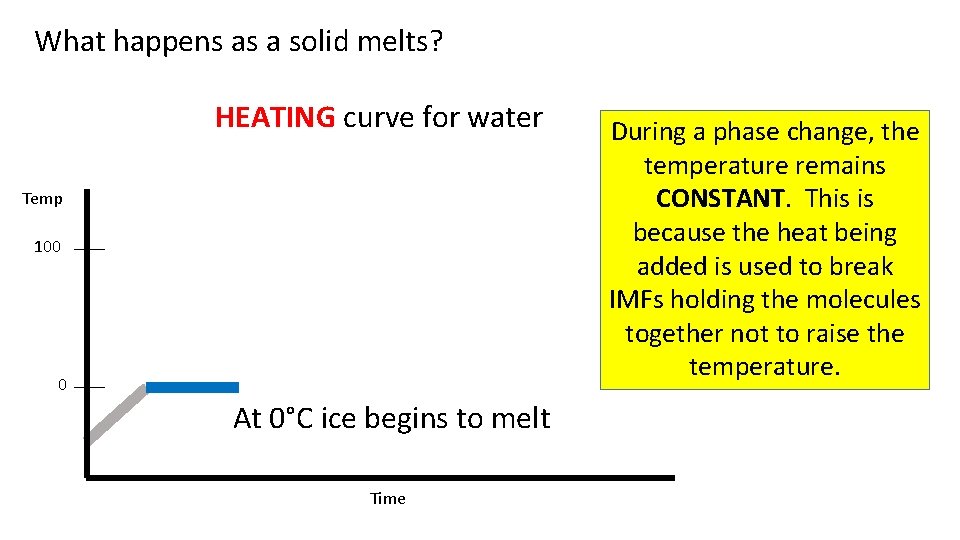
What happens as a solid melts? HEATING curve for water Temp 100 0 At 0°C ice begins to melt Time During a phase change, the temperature remains CONSTANT. This is because the heat being added is used to break IMFs holding the molecules together not to raise the temperature.

What happens when you add heat to a liquid? HEATING curve for water Temp 100 0 Water remains a LIQUID as it absorbs heat and goes from 0°C to 100°C. Time

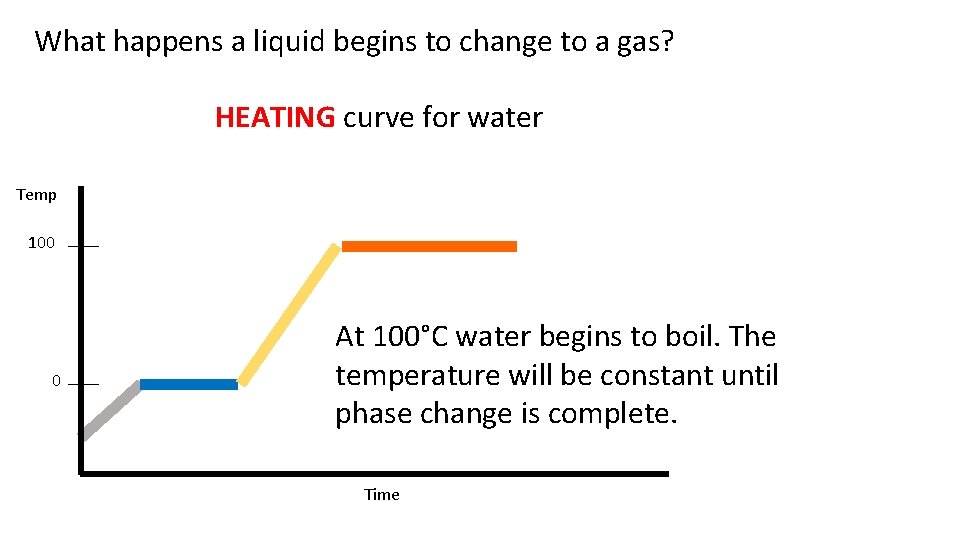
What happens a liquid begins to change to a gas? HEATING curve for water Temp 100 0 At 100°C water begins to boil. The temperature will be constant until phase change is complete. Time

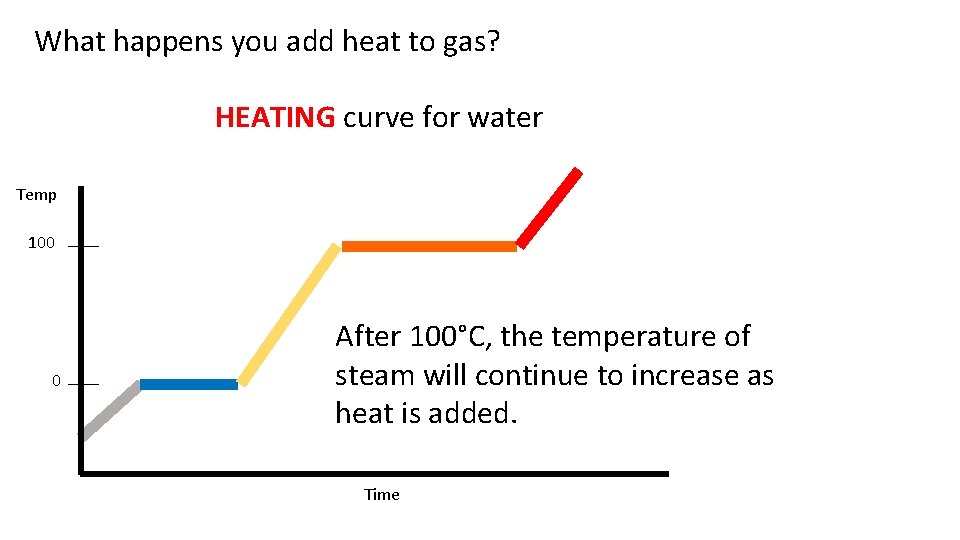
What happens you add heat to gas? HEATING curve for water Temp 100 0 After 100°C, the temperature of steam will continue to increase as heat is added. Time

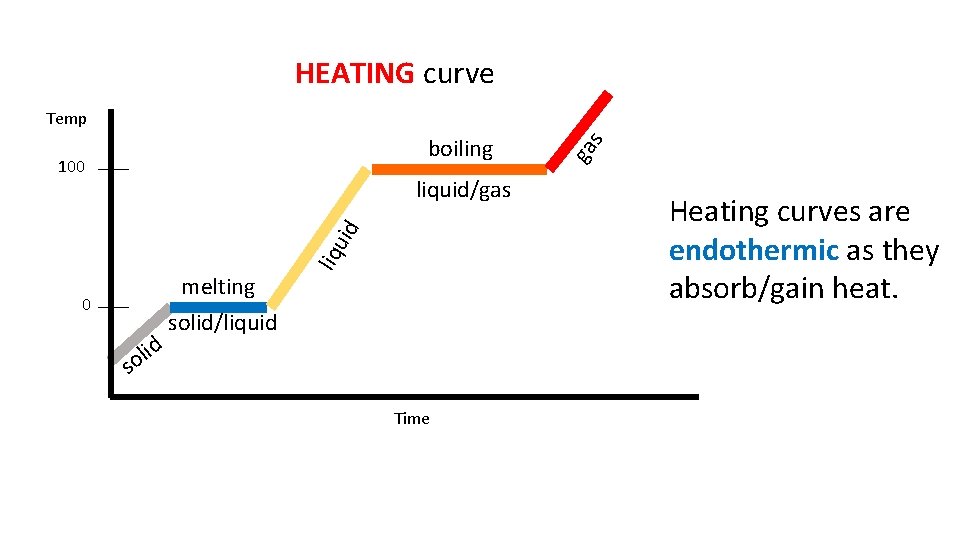
HEATING curve 100 0 d i l so melting solid/liquid liquid/gas Time ga boiling s Temp Heating curves are endothermic as they absorb/gain heat.

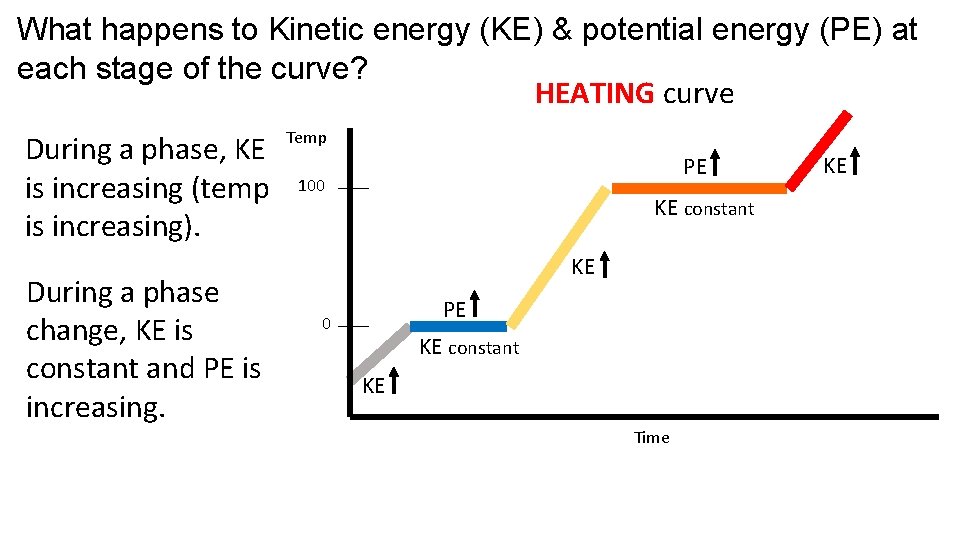
What happens to Kinetic energy (KE) & potential energy (PE) at each stage of the curve? HEATING curve During a phase, KE is increasing (temp is increasing). During a phase change, KE is constant and PE is increasing. Temp PE 100 KE constant KE PE 0 KE constant KE Time KE

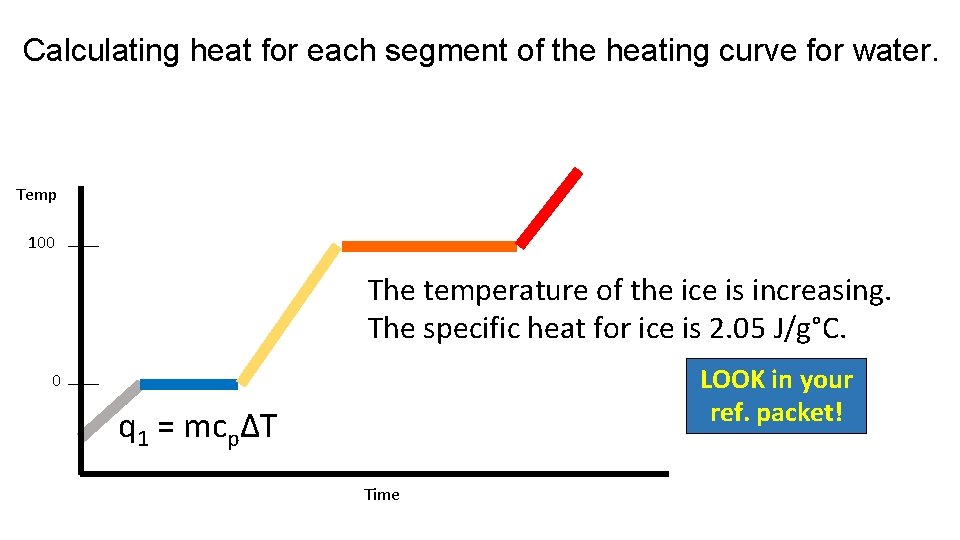
Calculating heat for each segment of the heating curve for water. Temp 100 The temperature of the ice is increasing. The specific heat for ice is 2. 05 J/g°C. LOOK in your ref. packet! 0 q 1 = mcpΔT Time

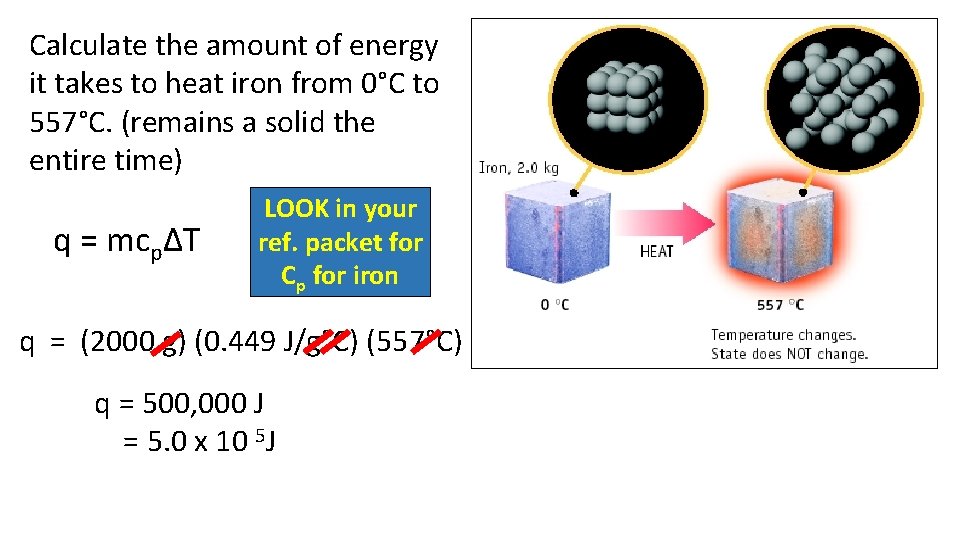
Calculate the amount of energy it takes to heat iron from 0°C to 557°C. (remains a solid the entire time) q = mcpΔT LOOK in your ref. packet for Cp for iron q = (2000 g) (0. 449 J/g°C) (557°C) q = 500, 000 J = 5. 0 x 10 5 J

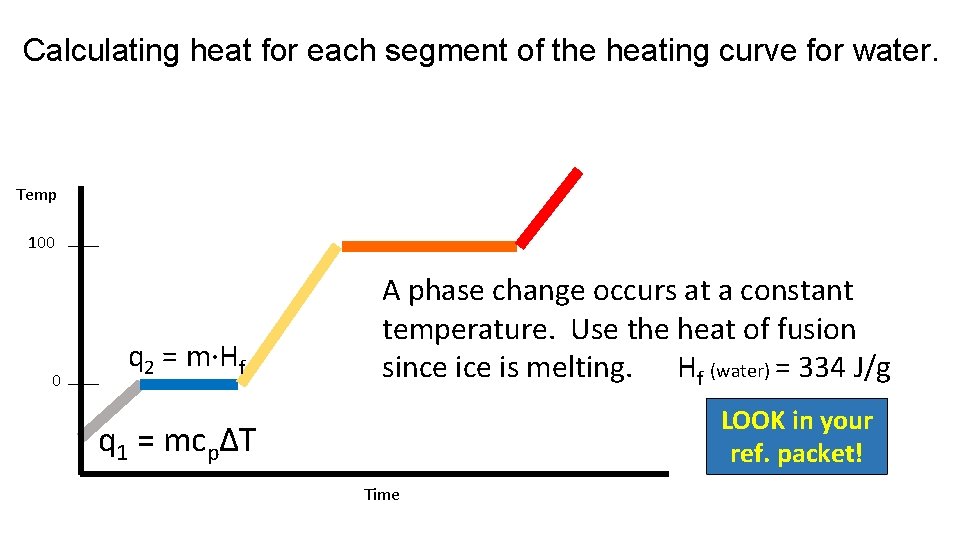
Calculating heat for each segment of the heating curve for water. Temp 100 0 q 2 = m·Hf A phase change occurs at a constant temperature. Use the heat of fusion since is melting. Hf (water) = 334 J/g LOOK in your ref. packet! q 1 = mcpΔT Time

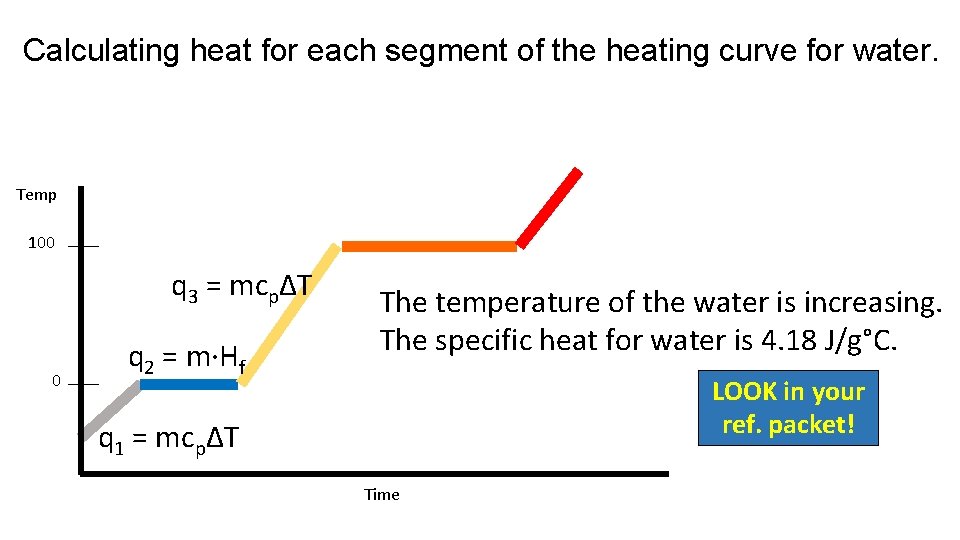
Calculating heat for each segment of the heating curve for water. Temp 100 q 3 = mcpΔT 0 q 2 = m·Hf The temperature of the water is increasing. The specific heat for water is 4. 18 J/g°C. LOOK in your ref. packet! q 1 = mcpΔT Time

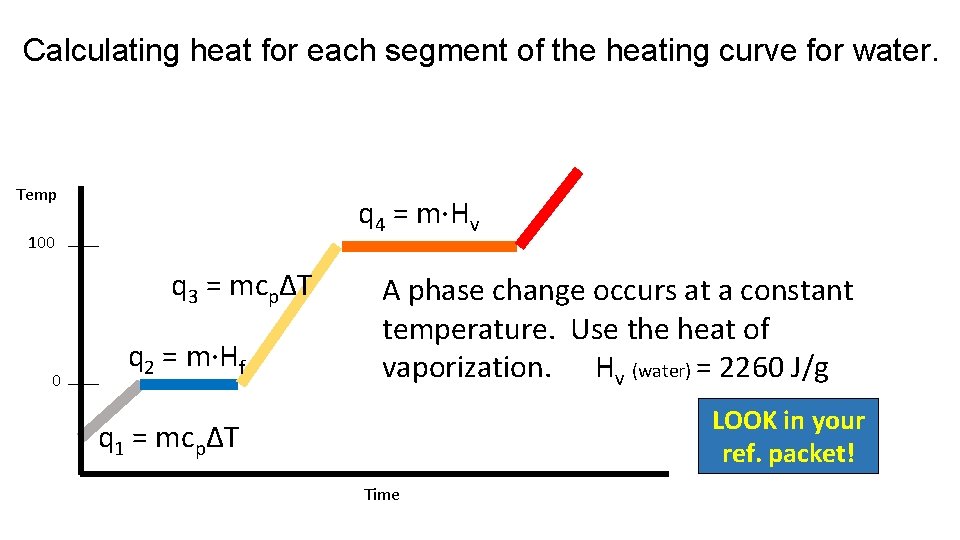
Calculating heat for each segment of the heating curve for water. Temp q 4 = m·Hv 100 q 3 = mcpΔT 0 q 2 = m·Hf A phase change occurs at a constant temperature. Use the heat of vaporization. Hv (water) = 2260 J/g LOOK in your ref. packet! q 1 = mcpΔT Time

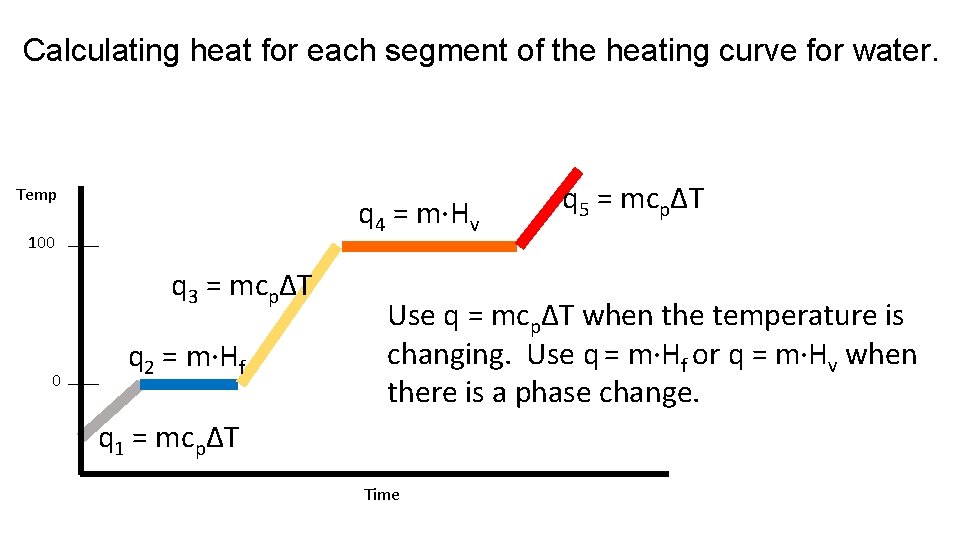
Calculating heat for each segment of the heating curve for water. Temp q 4 = m·Hv 100 q 3 = mcpΔT 0 q 2 = m·Hf q 5 = mcpΔT The temperature of the steam is increasing. The specific heat for steam is 2. 02 J/g°C. LOOK in your ref. packet! q 1 = mcpΔT Time

Calculating heat for each segment of the heating curve for water. Temp q 4 = m·Hv 100 q 3 = mcpΔT 0 q 2 = m·Hf q 5 = mcpΔT Use q = mcpΔT when the temperature is changing. Use q = m·Hf or q = m·Hv when there is a phase change. q 1 = mcpΔT Time

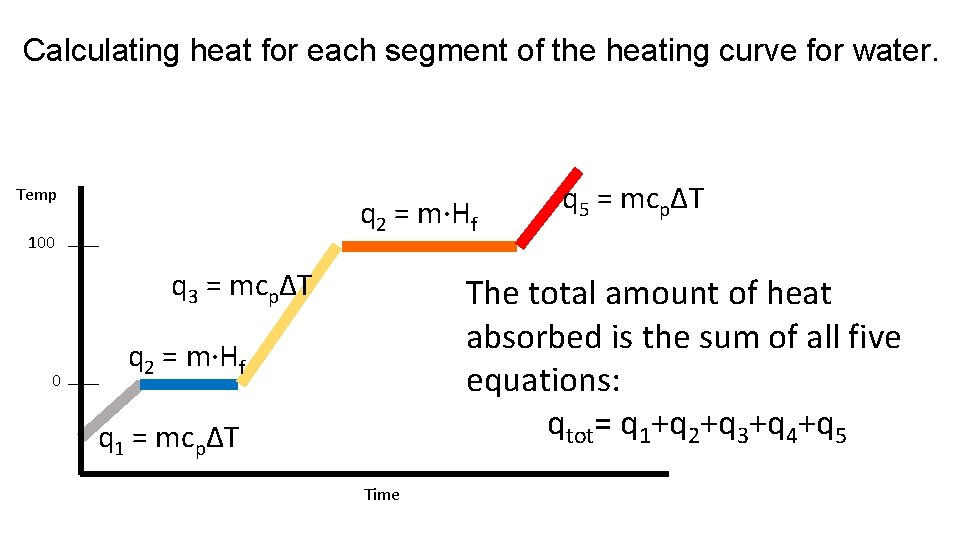
Calculating heat for each segment of the heating curve for water. Temp q 2 = m·Hf 100 q 3 = mcpΔT 0 q 5 = mcpΔT The total amount of heat absorbed is the sum of all five equations: qtot= q 1+q 2+q 3+q 4+q 5 q 2 = m·Hf q 1 = mcpΔT Time


NOTICE! It takes more time (longer line) & energy for vaporization of water to occur than melting. WHY? Look at the pictures to the right. Temp q 4 = m·Hv 100 0 q 2 = m·Hf Hf (water) = 334 J/g Hv (water) = 2260 J/g Time

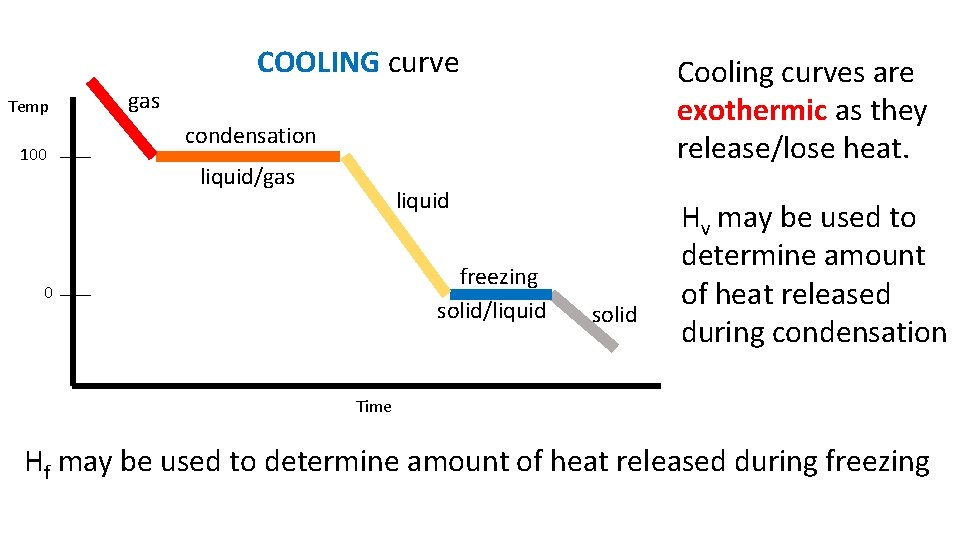
COOLING curve Temp 100 Cooling curves are exothermic as they release/lose heat. gas condensation liquid/gas liquid freezing solid/liquid 0 solid Hv may be used to determine amount of heat released during condensation Time Hf may be used to determine amount of heat released during freezing

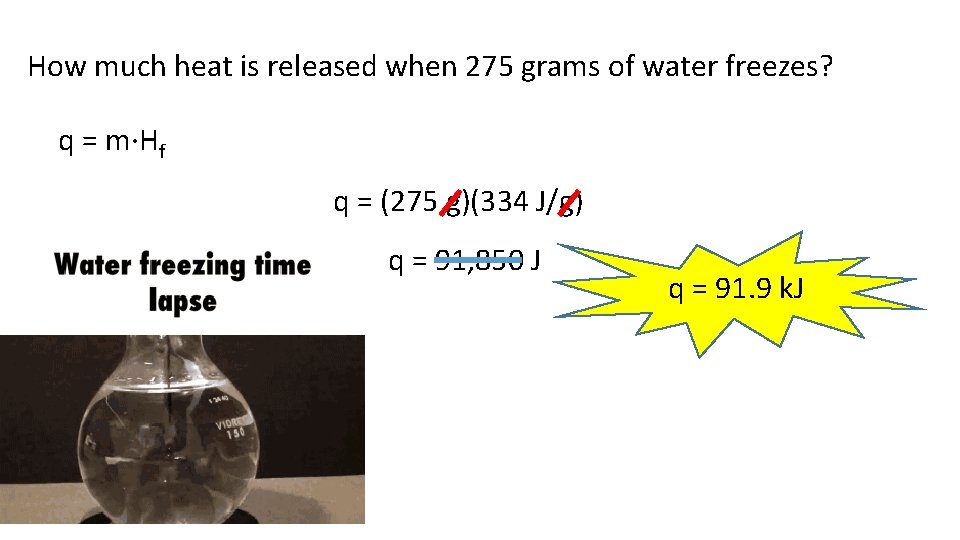
How much heat is released when 275 grams of water freezes? q = m·Hf q = (275 g)(334 J/g) q = 91, 850 J q = 91. 9 k. J

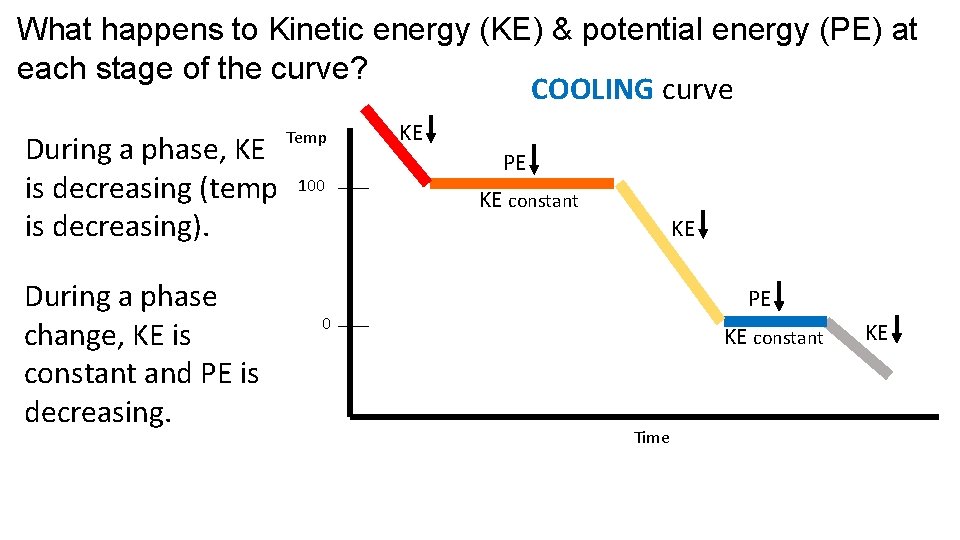
What happens to Kinetic energy (KE) & potential energy (PE) at each stage of the curve? COOLING curve During a phase, KE is decreasing (temp is decreasing). During a phase change, KE is constant and PE is decreasing. Temp 100 KE PE KE constant KE PE 0 KE constant Time KE

ΔH & Chemical Reactions Chemical reactions always involves a change in heat energy

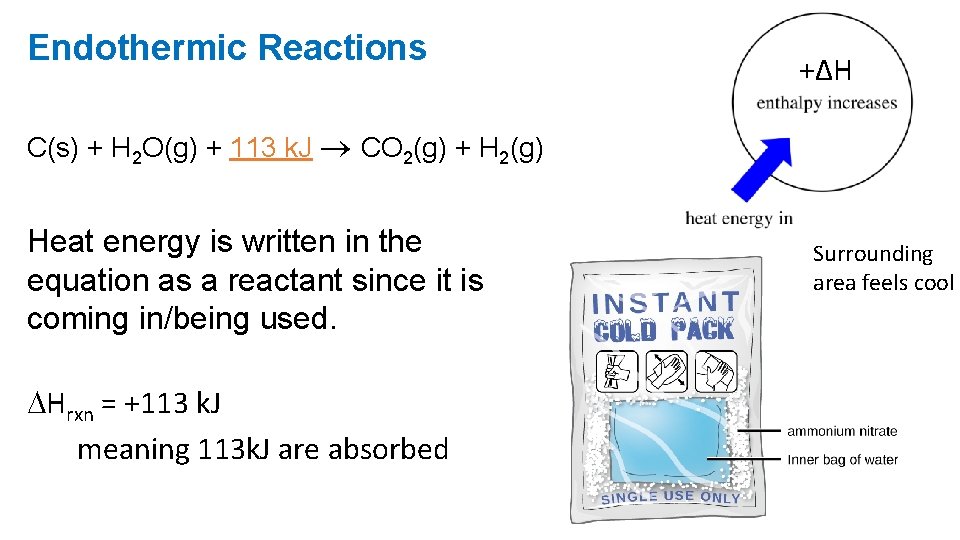
Endothermic Reactions +ΔH C(s) + H 2 O(g) + 113 k. J CO 2(g) + H 2(g) Heat energy is written in the equation as a reactant since it is coming in/being used. Hrxn = +113 k. J meaning 113 k. J are absorbed Surrounding area feels cool

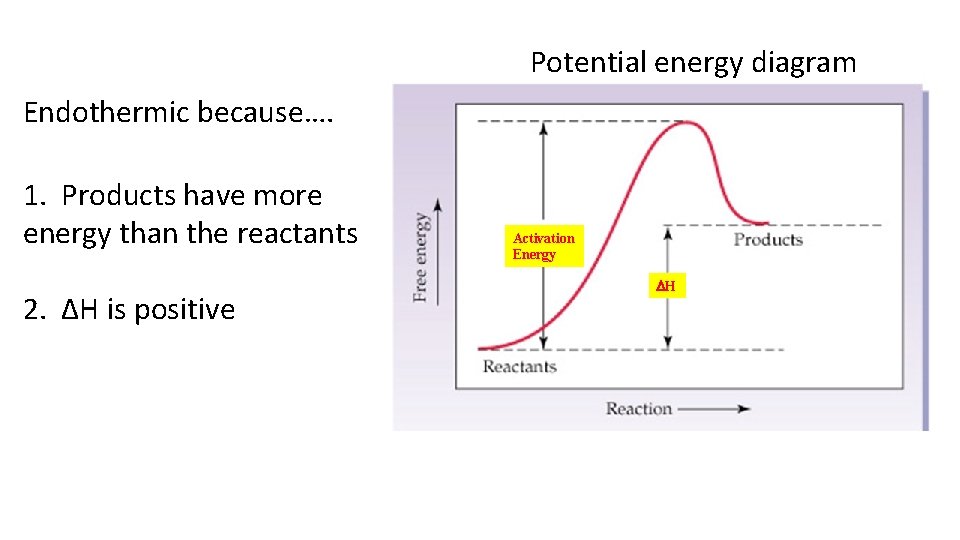
Potential energy diagram Endothermic because…. 1. Products have more energy than the reactants 2. ΔH is positive Activation Energy DH

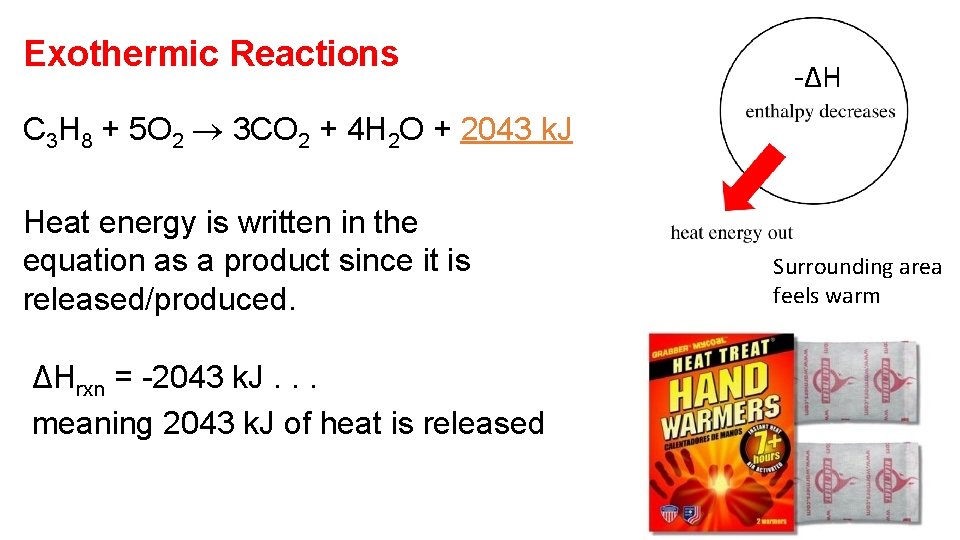
Exothermic Reactions -ΔH C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O + 2043 k. J Heat energy is written in the equation as a product since it is released/produced. ΔHrxn = -2043 k. J. . . meaning 2043 k. J of heat is released Surrounding area feels warm

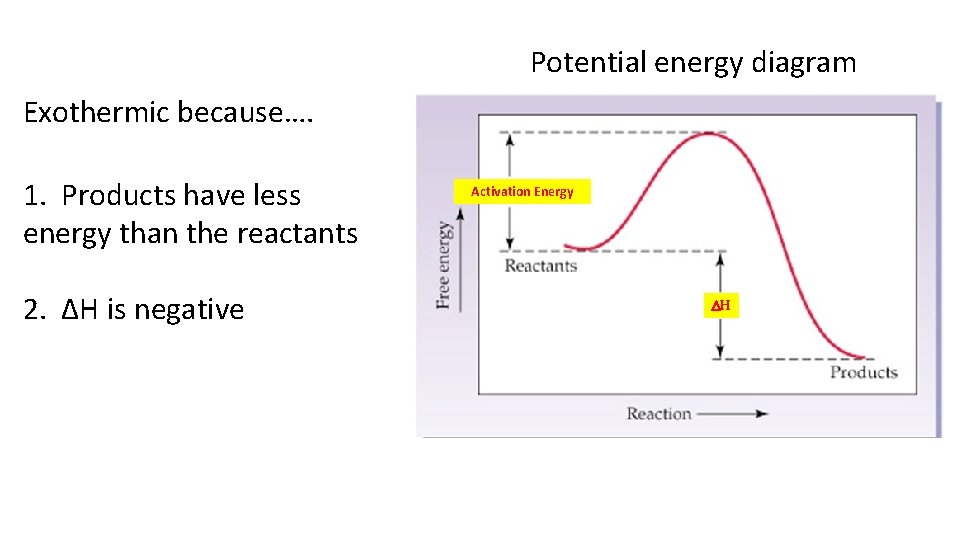
Potential energy diagram Exothermic because…. 1. Products have less energy than the reactants 2. ΔH is negative Activation Energy DH

Thermochemistry & Stoichiometry If you know the ΔH for a balanced equation, you may determine the amount of energy used or released by a reaction.

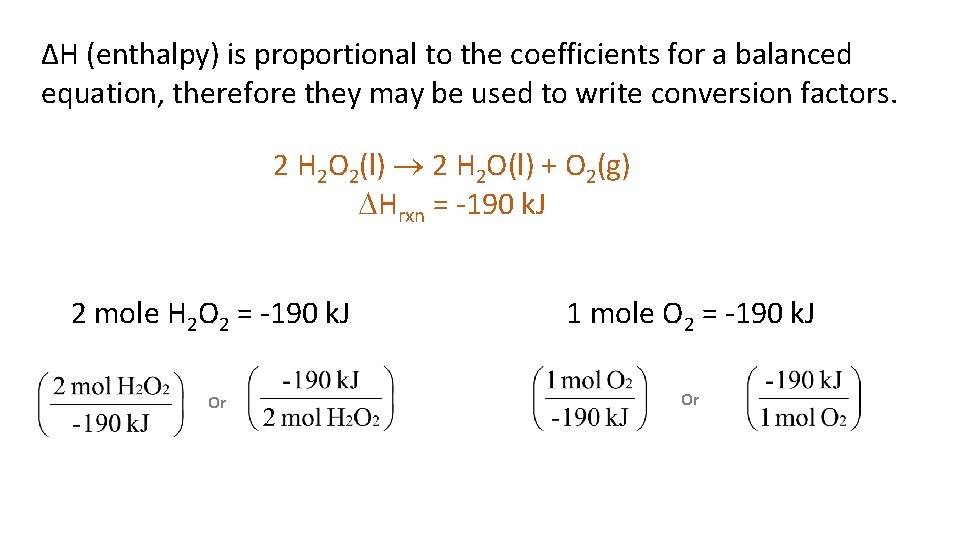
ΔH (enthalpy) is proportional to the coefficients for a balanced equation, therefore they may be used to write conversion factors. 2 H 2 O 2(l) 2 H 2 O(l) + O 2(g) Hrxn = -190 k. J 2 mole H 2 O 2 = -190 k. J Or 1 mole O 2 = -190 k. J Or

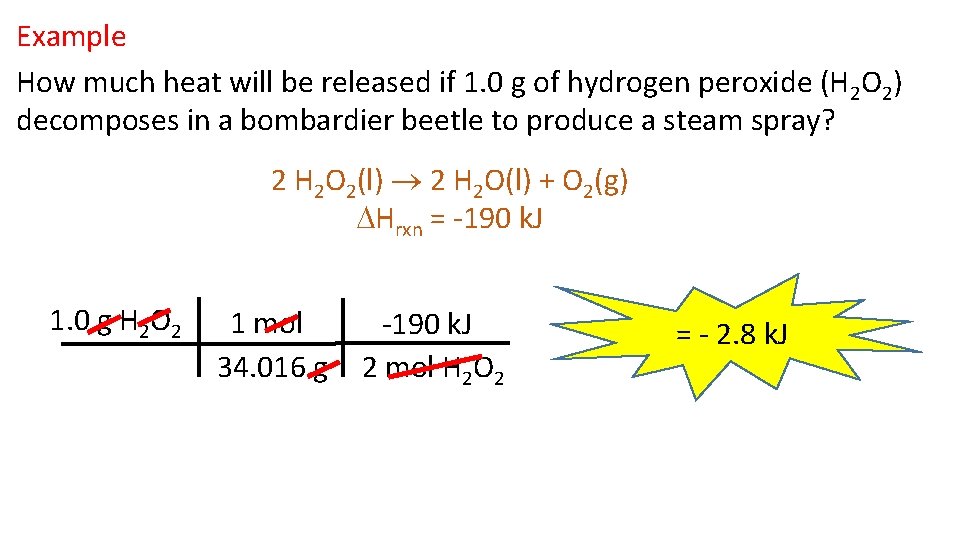
Example How much heat will be released if 1. 0 g of hydrogen peroxide (H 2 O 2) decomposes in a bombardier beetle to produce a steam spray? 2 H 2 O 2(l) 2 H 2 O(l) + O 2(g) Hrxn = -190 k. J 1. 0 g H 2 O 2 1 mol 34. 016 g -190 k. J 2 mol H 2 O 2 = - 2. 8 k. J

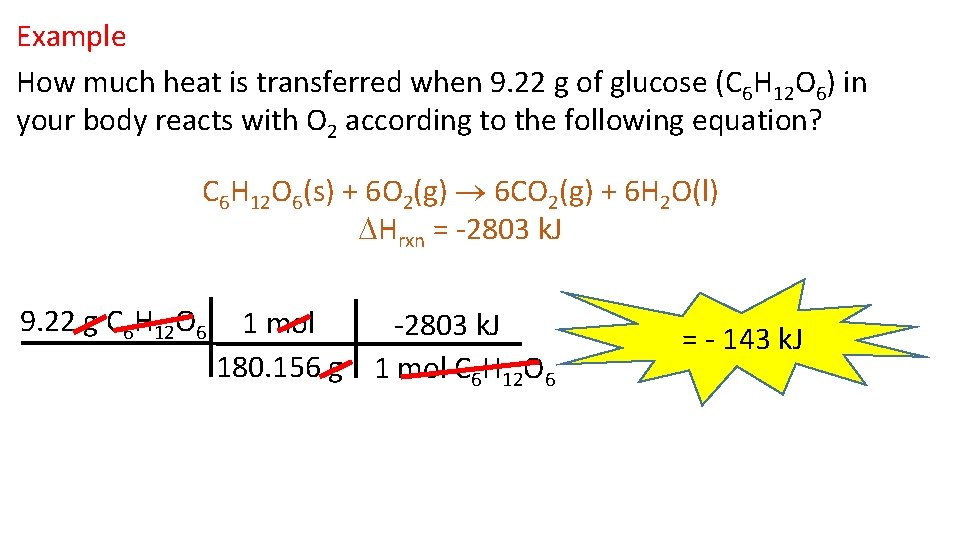
Example How much heat is transferred when 9. 22 g of glucose (C 6 H 12 O 6) in your body reacts with O 2 according to the following equation? C 6 H 12 O 6(s) + 6 O 2(g) 6 CO 2(g) + 6 H 2 O(l) Hrxn = -2803 k. J 9. 22 g C 6 H 12 O 6 1 mol -2803 k. J 180. 156 g 1 mol C 6 H 12 O 6 = - 143 k. J

Example How much energy will be required to extract 59. 5 grams of tin? Sn. O 2(s) + 4 NO 2(g) + 2 H 2 O(l) + 192 k. J Sn(s) + 4 HNO 3(aq) Hrxn = +192 k. J 59. 5 g Sn 1 mol 118. 71 g 192 k. J 1 mol Sn = + 96. 2 k. J