The Extraordinary Properties of Water Water A water

































- Slides: 52

The Extraordinary Properties of Water

Water A water molecule (H 2 O), is made up of three atoms --- one oxygen and two hydrogen. H O H

Water is Polar • In each water molecule, the oxygen atom attracts more than its "fair share" of electrons • The oxygen end “acts” negative • The hydrogen end “acts” positive • Causes the molecule to be POLAR • However, Water is neutral (equal number of e- and p+) • Zero Net Charge

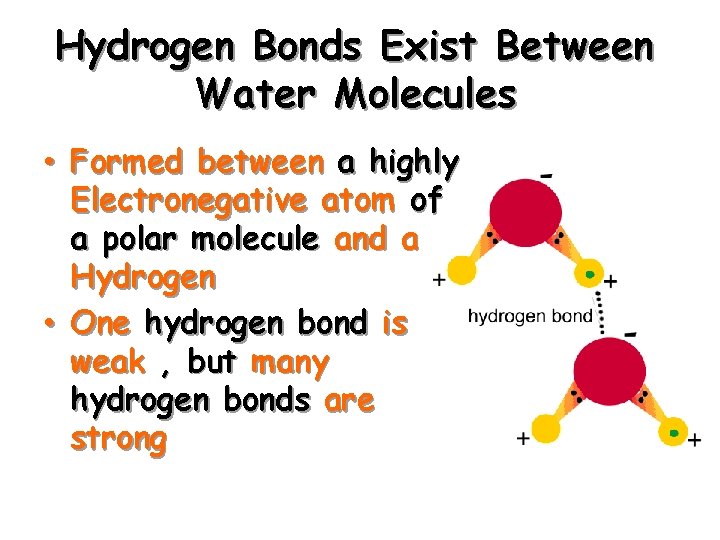
Hydrogen Bonds Exist Between Water Molecules • Formed between a highly Electronegative atom of a polar molecule and a Hydrogen • One hydrogen bond is weak , but many hydrogen bonds are strong

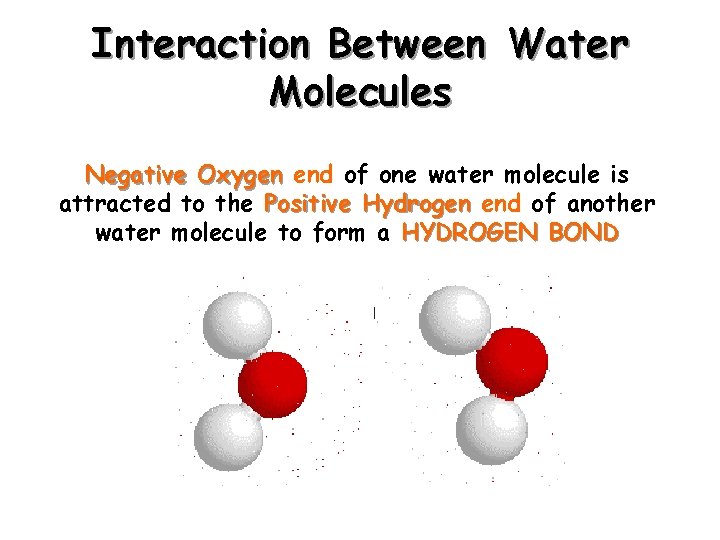
Interaction Between Water Molecules Negative Oxygen end of one water molecule is attracted to the Positive Hydrogen end of another water molecule to form a HYDROGEN BOND

What are the Properties of Water?

Properties of Water • At sea level, pure water boils at 100 °C and freezes at 0 °C. • The boiling temperature of water decreases at higher elevations (lower atmospheric pressure). • For this reason, an egg will take longer to cook at higher altitudes

Properties of Water • Cohesion

Properties of Water • Cohesion • Adhesion

Properties of Water • Cohesion • Adhesion • High Specific Heat

Properties of Water • Cohesion • Adhesion • High Specific Heat • High Heat of Vaporization

Properties of Water • Cohesion • Adhesion • High Specific Heat • High Heat of Vaporization • Less Dense as a Solid

Cohesion • Attraction between particles of the same substance ( why water is attracted to itself) • Results in Surface tension (a measure of the strength of water’s surface) • Produces a surface film on water that allows insects to walk on the surface of water

Surfactants • Surfactants (like detergents) decrease the surface tension of water. • Surfactants interfere with hydrogen bonding. • Makes water more efficient in removing dirt or oil.

Cohesion … Helps insects walk across water

Adhesion • Attraction between two different substances. • Water will make hydrogen bonds with other surfaces such as glass, soil, plant tissues, and cotton. • Capillary action-water molecules will “tow” each other along when in a thin glass tube. • Example: transpiration process which plants and trees remove water from the soil, and paper towels soak up water.

Adhesion Causes Capillary Action Capillary action gives water the ability to “climb” structures

Adhesion Also Causes Water to Form spheres & hold onto plant leaves Attach to a silken spider web

High Specific Heat • Amount of heat needed to raise or lower the temperature of 1 g of a substance 1° C. • Water resists temperature change, both for heating and cooling. • Water can absorb or release large amounts of heat energy with little change in actual temperature.

High Heat of Vaporization • Amount of energy to convert 1 g of a substance from a liquid to a gas • In order for water to evaporate, hydrogen bonds must be broken. • As water evaporates, it removes a lot of heat with it.

High Heat of Vaporization • Water's heat of vaporization is 540 cal/g. • In order for water to evaporate, each gram must GAIN 540 calories (temperature doesn’t change stays at 100 o. C). • As water evaporates, it removes a lot of heat with it (cooling effect).

• Water vapor forms a kind of global “blanket” which helps to keep the Earth warm. • Heat from the sun is radiated back from the warmed surface of the earth and is is absorbed and held by the vapor.

Water is Less Dense as a Solid • Ice is less dense as a solid than as a liquid (ice floats) • Liquid water has hydrogen bonds that are constantly being broken and reformed. • Frozen water forms a crystal-like lattice whereby molecules are set at fixed distances.

Water is Less Dense as a Solid • Which is ice and which is water?

Water is Less Dense as a Solid Water Ice

Homeostasis • Ability to maintain a steady state despite changing conditions • Water is important to this process because: a. Makes a good insulator b. Resists temperature change c. Universal solvent d. Coolant e. Ice protects against temperature extremes (insulates frozen lakes)

Solutions

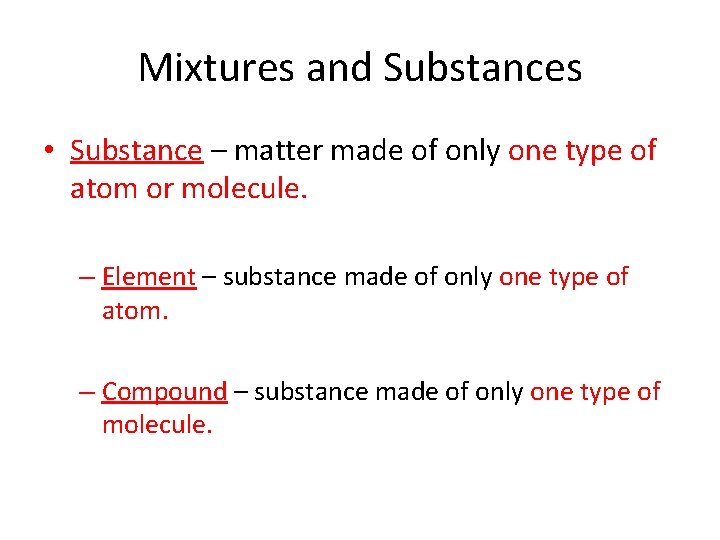
Mixtures and Substances • Substance – matter made of only one type of atom or molecule. – Element – substance made of only one type of atom. – Compound – substance made of only one type of molecule.

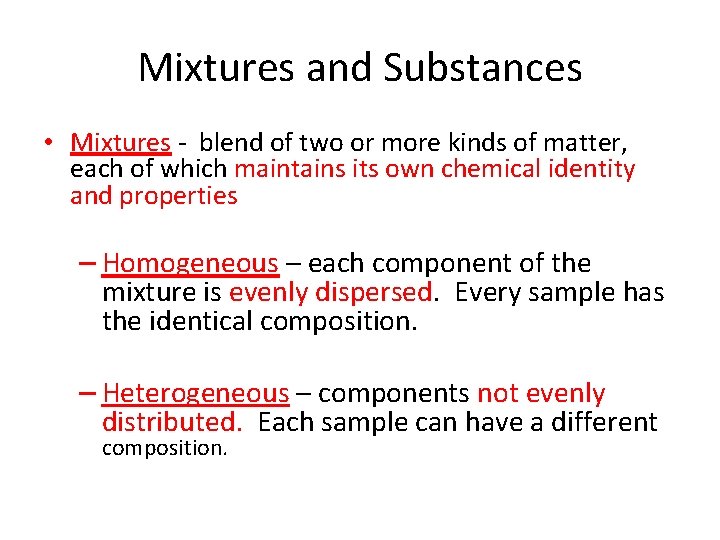
Mixtures and Substances • Mixtures - blend of two or more kinds of matter, each of which maintains its own chemical identity and properties – Homogeneous – each component of the mixture is evenly dispersed. Every sample has the identical composition. – Heterogeneous – components not evenly distributed. Each sample can have a different composition.

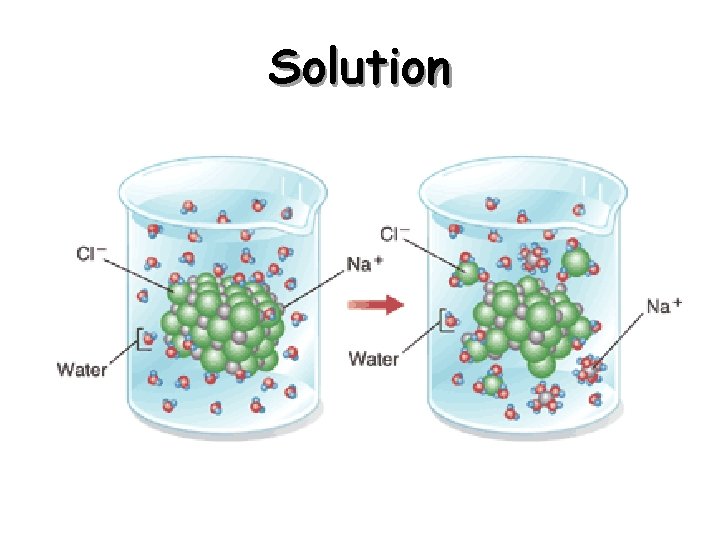
Solutions • Solution - homogeneous mixture of two or more substances in a single phase – Soluble – capable of being dissolved. – Solute - substance dissolved in a solution – Solvent – dissolving medium in a solution.

Solution

Solutions Types of Solutions • Gas Mixtures – Gas/gas – oxygen in nitrogen (air) – Gas/liquid – carbon dioxide in water (soda) • Solid Mixtures – Solid/liquid – sugar in water – Solid/solid – alloy (silver in gold – white gold) • Liquid Mixtures – Liquid/liquid – alcohol in water (rubbing alcohol) – Liquid/solid – mercury in silver and tin (dental amalgam)

Suspensions • Suspension – if the particles in a mixture are so large that they settle out unless being constantly stirred or agitated. • Particles in a suspension can be separated from heterogeneous mixtures by passing the mixture through a filter.

Suspensions • Substances that don’t dissolve but separate into tiny pieces. • Water keeps the pieces suspended so they don’t settle out.

Colloids • Colloid – particles intermediate in size between those in solution and suspensions form colloidal dispersions or colloids. – Particles make up dispersed phase – Solvent is dispersing phase – Examples – • Sol – solid in liquid – paint • Gel – solid network in liquid – gelatin • Liquid emulsion – milk, mayonnaise • Foam – shaving cream, whipped cream • Solid emulsion – cheese, butter

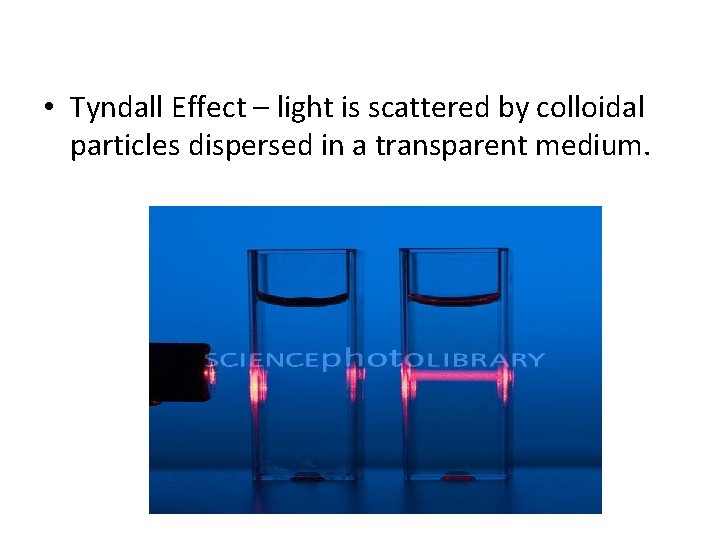
• Tyndall Effect – light is scattered by colloidal particles dispersed in a transparent medium.

Solutes – Electrolytes vs. Non. Electrolytes • Electrolyte – substance that dissolves in water to give a solution that conducts electric current. e. g. Na. Cl in water • To conduct electricity, the solute must separate into IONS when dissolved. • Non-electrolyte – substance that dissolves in water to give a solution that does NOT conduct an electric current e. g. sugar in water

Factors Affecting Solubility – Increasing the surface area of the solute. Crushing the solute increases the surface area in contact with the solvent increasing the rate of dissolution.

Factors Affecting Solubility – Agitating a solution. Agitating (stirring) the solution brings fresh solvent into contact with the solute.

Factors Affecting Solubility Heating a solvent. As the temperature increases, the solvent molecules move faster and collisions between the solvent molecules and the solute are more frequent.

Solution Equilibrium – physical state in which the opposing processes of dissolution and crystallization of a solute occur at equal rates. Saturated Solution that contains the maximum amount of dissolved solute under the existing conditions. Unsaturated Solution that contains less solute than a saturated solution under the existing conditions. Supersaturated Solution that contains more dissolved solute than a saturated solution contains under the existing conditions.

Solubility Values • The solubility of a substance is the amount of that substance required to form a saturated solution with a specific amount of solvent at a specified temperature. E. g. solubility of sugar is 204 g per 100 g water at 20 o. C • Solubility varies with temperature. • Generally, solubility increases with temperature, but there are exceptions.

Solubility

Solute/Solvent Interactions • Like dissolves like is a general rule. – What makes substances similar depends on type of bonding, polarity or non-polarity of molecules, and the intermolecular forces between the solute and solvent. • Aqueous solutions/ionic compounds – The solution process with water as the solvent is referred to as hydration. – Ions are said to be hydrated. • Non polar solutions/molecular compounds – Ionic compounds are generally NOT soluble in non-polar solvents such as CCl 4 and toluene (C 6 H 5 CH 3)

Immiscible/Miscible • Liquids that are not soluble in each other are immiscible. • Oil and water are not miscible in each other.

Pressure and Solubility • Increase in pressure increases the solubility of a gas in a liquid. • Henry’s Law (English chemist William Henry) – The solubility of a gas in a liquid is directly proportional to the partial pressure of that gas on the surface of the liquid. • Effervescence The rapid escape of a gas from a liquid in which it is dissolved is called effervescence.

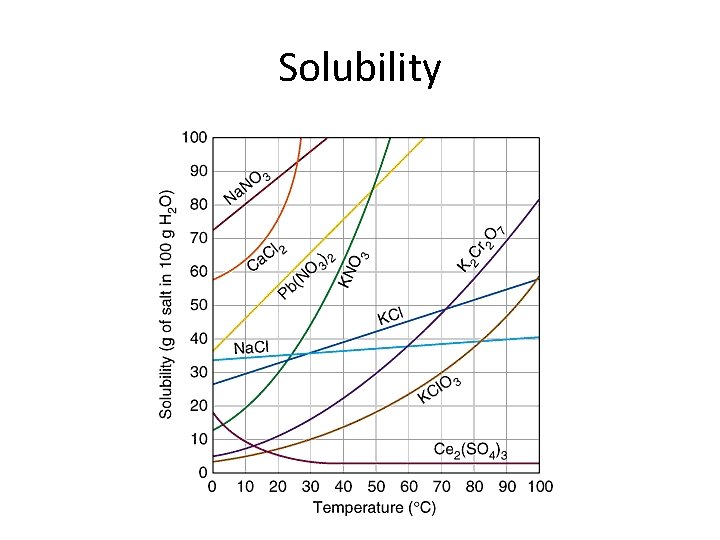
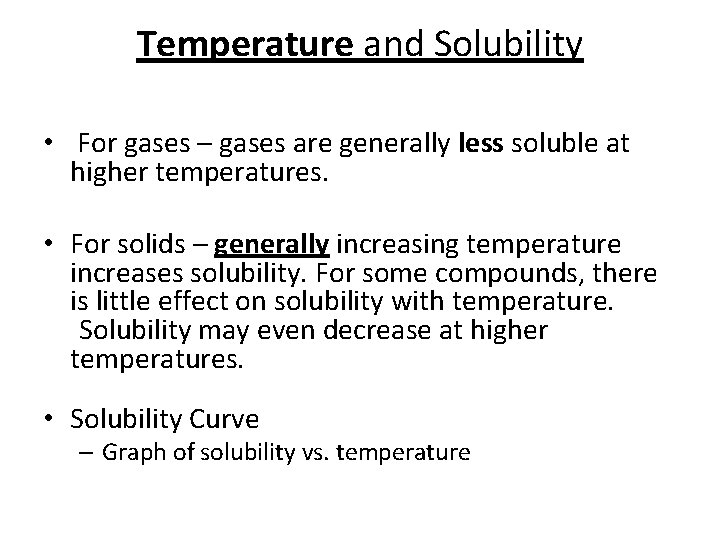
Temperature and Solubility • For gases – gases are generally less soluble at higher temperatures. • For solids – generally increasing temperature increases solubility. For some compounds, there is little effect on solubility with temperature. Solubility may even decrease at higher temperatures. • Solubility Curve – Graph of solubility vs. temperature

Heat of Solution • Heat of Solution (enthalpy of solution) • Net amount of energy absorbed as heat by the solution when a specific amount of solute dissolves in a solvent is the enthalpy of solution.

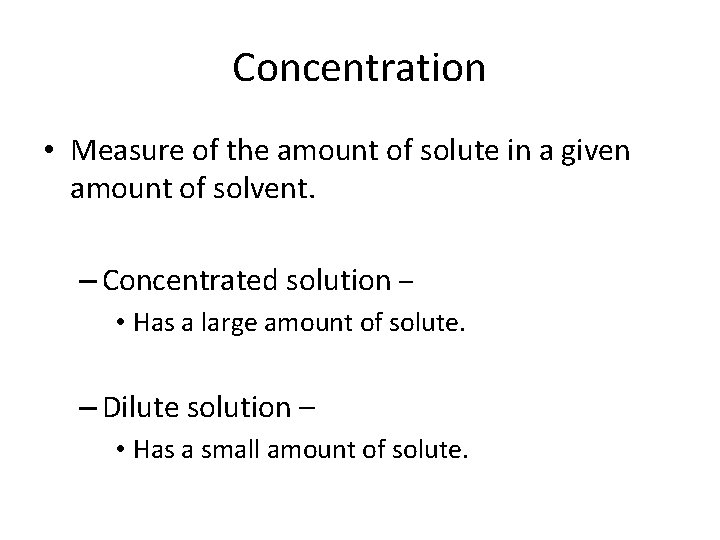
Concentration • Measure of the amount of solute in a given amount of solvent. – Concentrated solution – • Has a large amount of solute. – Dilute solution – • Has a small amount of solute.

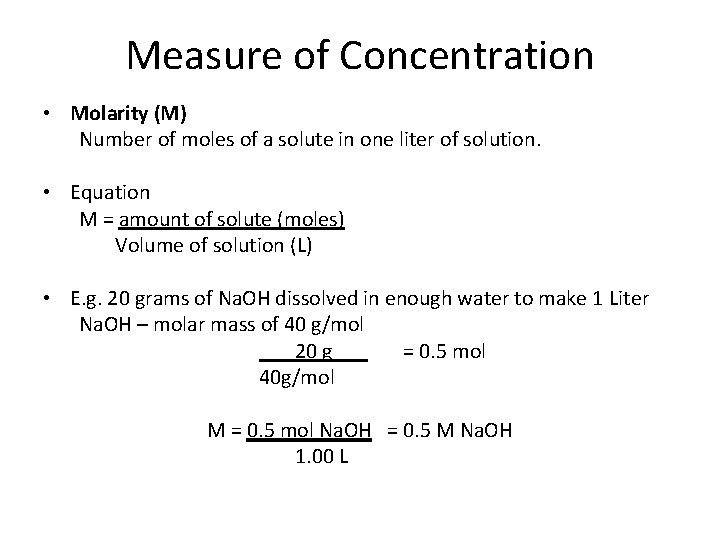
Measure of Concentration • Molarity (M) Number of moles of a solute in one liter of solution. • Equation M = amount of solute (moles) Volume of solution (L) • E. g. 20 grams of Na. OH dissolved in enough water to make 1 Liter Na. OH – molar mass of 40 g/mol 20 g = 0. 5 mol 40 g/mol M = 0. 5 mol Na. OH = 0. 5 M Na. OH 1. 00 L

Dilution – adding more solvent to a solution. The number of moles of solute doesn’t change if you add more solvent. moles before = moles after M = molarity, V = volume M 1 x V 1 = M 2 x V 2 – M 1 and V 1 are the starting concentration and volume – M 2 and V 2 are the ending concentration and volume – Stock solutions are pre-made to known Molarity

Dilution Calculations • 2. 0 L of a 0. 88 M solution are diluted to 3. 8 L. What is the new molarity? • You have 150 m. L of 6. 0 M HCl. What volume of 1. 3 M HCl can you make? • You need 450 m. L of a 0. 15 M Na. OH. All you have available is a 2. 0 M stock solution of Na. OH. How do you make the required solution?
 The extraordinary properties of water
The extraordinary properties of water The extraordinary properties of water
The extraordinary properties of water Water and water and water water
Water and water and water water Are kangaroos dangerous
Are kangaroos dangerous Extraordinary life quotes
Extraordinary life quotes 4 obsessions of an extraordinary executive summary
4 obsessions of an extraordinary executive summary Hypothetical condition vs extraordinary assumption
Hypothetical condition vs extraordinary assumption Personal growth definition
Personal growth definition Tight denotation and connotation
Tight denotation and connotation Relaxed connotation
Relaxed connotation Closing prayer for class
Closing prayer for class Clearvoiceresearch
Clearvoiceresearch Extensive examples
Extensive examples Chemical property of water
Chemical property of water Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Tư thế worm breton là gì
Tư thế worm breton là gì Chúa yêu trần thế
Chúa yêu trần thế Các môn thể thao bắt đầu bằng tiếng đua
Các môn thể thao bắt đầu bằng tiếng đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Phép trừ bù
Phép trừ bù độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Ví dụ giọng cùng tên
Ví dụ giọng cùng tên Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phối cảnh
Phối cảnh Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dot
Dot Số.nguyên tố
Số.nguyên tố Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Hươu thường đẻ mỗi lứa mấy con
Hươu thường đẻ mỗi lứa mấy con Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu











































































