The Extraordinary Properties of Water What are the

























- Slides: 40

The Extraordinary Properties of Water

What are the Properties of Water?

Properties of Water • Polar molecule • Cohesion and adhesion • High specific heat • Density – greatest at 4 o. C • Universal solvent of life

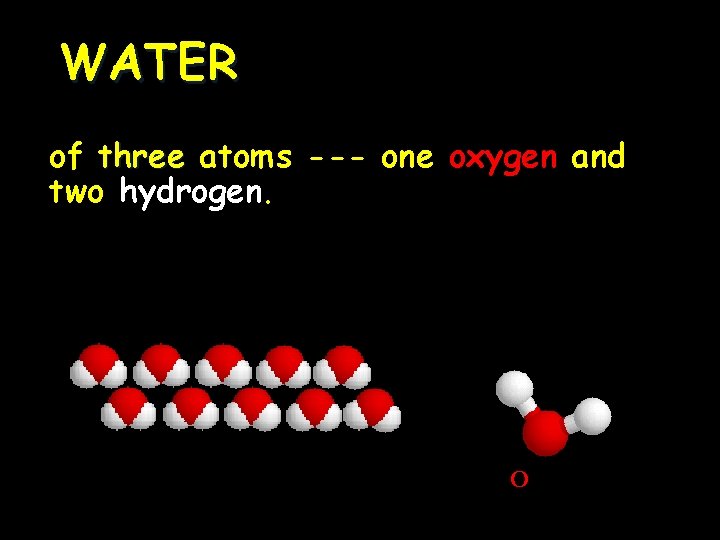
WATER A water molecule (H 2 O), is made up of three atoms --- one oxygen and two hydrogen. H O H

Water is Polar • In each water molecule, the oxygen atom attracts more than its "fair share" of electrons • The oxygen end “acts” negative • The hydrogen end “acts” positive • Causes the water to be POLAR • However, Water is neutral (equal number of e- and p+) --- Zero Net Charge

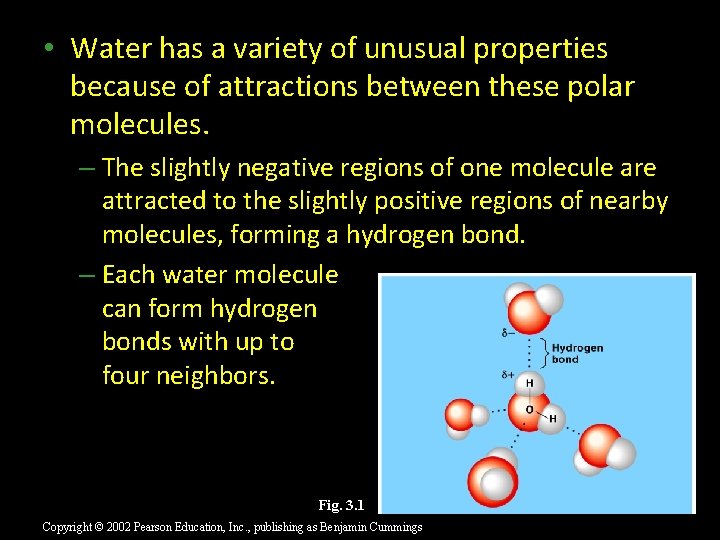
• Water has a variety of unusual properties because of attractions between these polar molecules. – The slightly negative regions of one molecule are attracted to the slightly positive regions of nearby molecules, forming a hydrogen bond. – Each water molecule can form hydrogen bonds with up to four neighbors. Fig. 3. 1 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

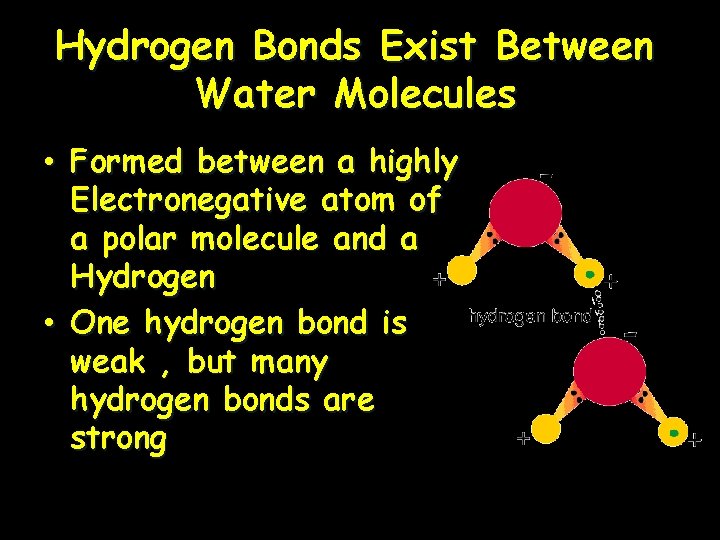
Hydrogen Bonds Exist Between Water Molecules • Formed between a highly Electronegative atom of a polar molecule and a Hydrogen • One hydrogen bond is weak , but many hydrogen bonds are strong

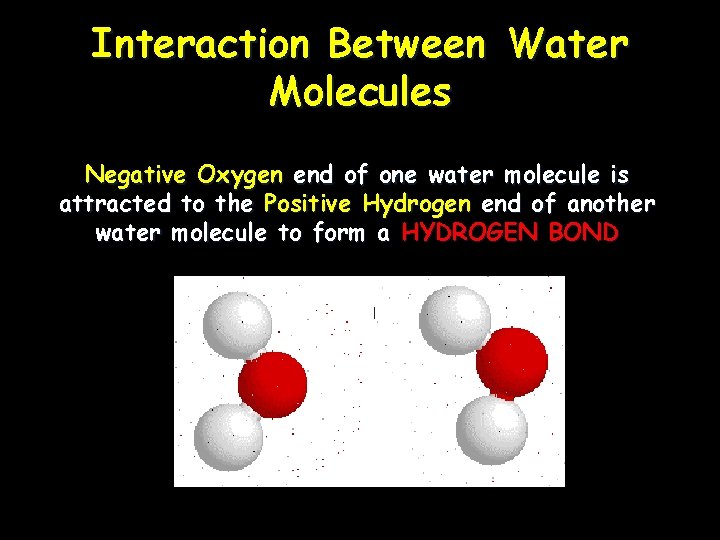
Interaction Between Water Molecules Negative Oxygen end of one water molecule is attracted to the Positive Hydrogen end of another water molecule to form a HYDROGEN BOND

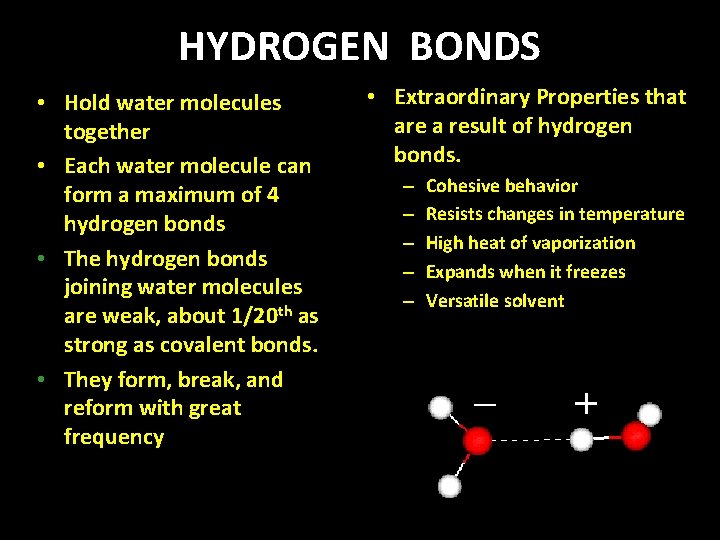
HYDROGEN BONDS • Hold water molecules together • Each water molecule can form a maximum of 4 hydrogen bonds • The hydrogen bonds joining water molecules are weak, about 1/20 th as strong as covalent bonds. • They form, break, and reform with great frequency • Extraordinary Properties that are a result of hydrogen bonds. – – – Cohesive behavior Resists changes in temperature High heat of vaporization Expands when it freezes Versatile solvent

Properties of Water • At sea level, pure water boils at 100 °C and freezes at 0 °C. • The boiling temperature of water decreases at higher elevations (lower atmospheric pressure). • For this reason, an egg will take longer to boil at higher altitudes

Cohesion • Attraction between particles of the same substance ( why water is attracted to itself) • Results in Surface tension (a measure of the strength of water’s surface) • Produces a surface film on water that allows insects to walk on the surface of water

Cohesion … Helps insects walk across water

• Surface tension, a measure of the force necessary to stretch or break the surface of a liquid, is related to cohesion. – Water has a greater surface tension than most other liquids because hydrogen bonds among surface water molecules resist stretching or breaking the surface. – Water behaves as if covered by an invisible film. – Some animals can stand, walk, or run on water without breaking the Fig. 3. 3 surface. Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

Adhesion • Attraction between two different substances. • Water will make hydrogen bonds with other surfaces such as glass, soil, plant tissues, and cotton. • Capillary action-water molecules will “tow” each other along when in a thin glass tube. • Example: transpiration process which plants and trees remove water from the soil, and paper towels soak up water.

Adhesion Causes Capillary Action Which gives water the ability to “climb” structures

Organisms Depend on Cohesion Hydrogen bonds hold the substance together, a phenomenon called cohesion • Cohesion is responsible for the transport of the water column in plants • Cohesion among water molecules plays a key role in the transport of water against gravity in plants • Adhesion, clinging of one substance to another, contributes too, as water adheres to the wall of the vessels.

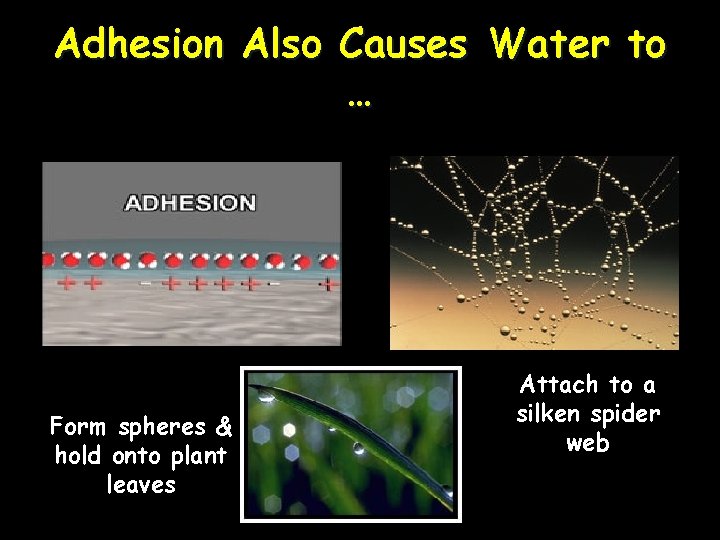
Adhesion Also Causes Water to … Form spheres & hold onto plant leaves Attach to a silken spider web

High Specific Heat • Amount of heat needed to raise or lower 1 g of a substance 1° C. • Water resists temperature change, both for heating and cooling. • Water can absorb or release large amounts of heat energy with little change in actual temperature.

Specific Heat is the amount of heat that must be absorbed or lost for one gram of a substance to change its temperature by 1 o. C. Three-fourths of the earth is covered by water. The water serves as a large heat sink responsible for: 1. Prevention of temperature fluctuations that are outside the range suitable for life. 2. Coastal areas having a mild climate 3. A stable marine environment

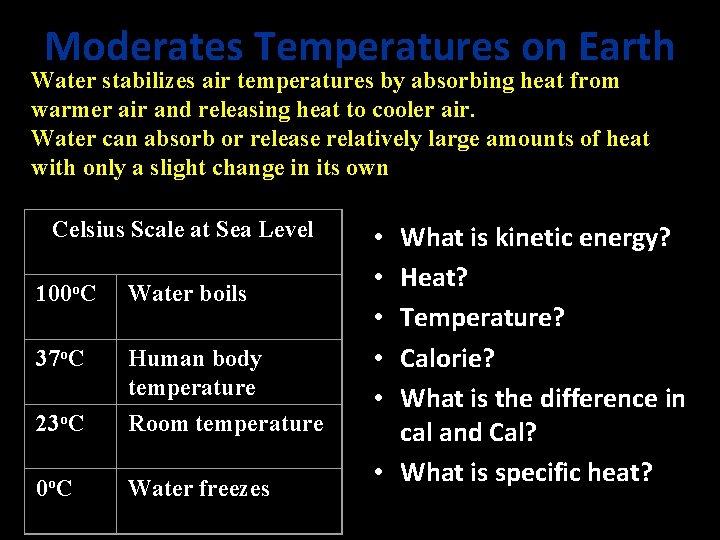
Moderates Temperatures on Earth Water stabilizes air temperatures by absorbing heat from warmer air and releasing heat to cooler air. Water can absorb or release relatively large amounts of heat with only a slight change in its own temperature. Celsius Scale at Sea Level 100 o. C Water boils 37 o. C 23 o. C Human body temperature Room temperature 0 o. C Water freezes What is kinetic energy? Heat? Temperature? Calorie? What is the difference in cal and Cal? • What is specific heat? • • •

High Heat of Vaporization • Amount of energy to convert 1 g or a substance from a liquid to a gas • In order for water to evaporate, hydrogen bonds must be broken. • As water evaporates, it removes a lot of heat with it.

High Heat of Vaporization • Water's heat of vaporization is 540 cal/g. • In order for water to evaporate, each gram must GAIN 540 calories (temperature doesn’t change --100 o. C). • As water evaporates, it removes a lot of heat with it (cooling effect).

Evaporative Cooling • The cooling of a surface occurs when the liquid evaporates • This is responsible for: – Moderating earth’s climate – Stabilizes temperature in aquatic ecosystems – Preventing organisms from overheating

• Water vapor forms a kind of global ‘‘blanket” which helps to keep the Earth warm. • Heat radiated from the sun warmed surface of the earth is absorbed and held by the vapor

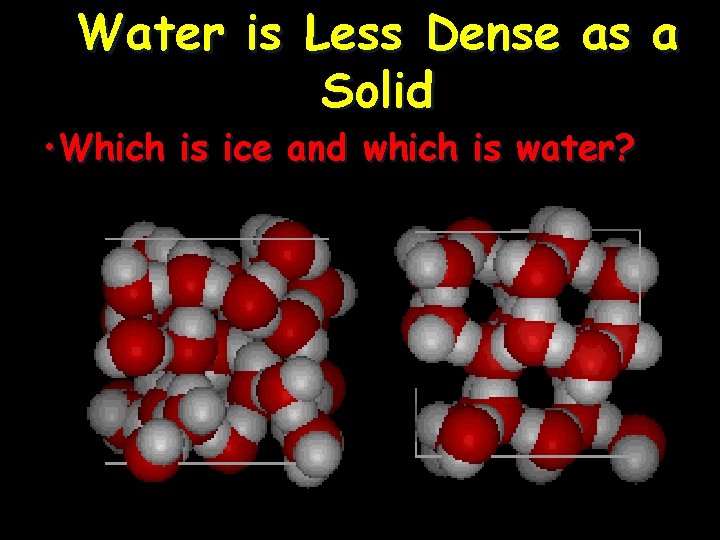
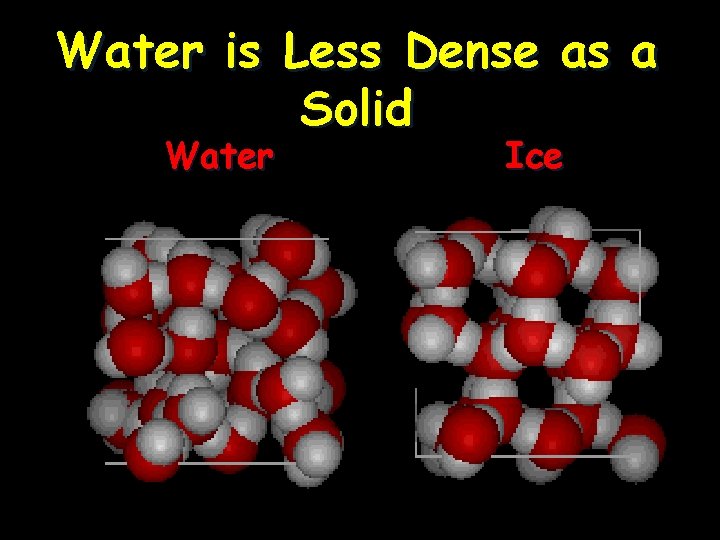
Water is Less Dense as a Solid • Ice is less dense as a solid than as a liquid (ice floats) • Liquid water has hydrogen bonds that are constantly being broken and reformed. • Frozen water forms a crystal-like lattice whereby molecules are set at fixed distances.

Water is Less Dense as a Solid • Which is ice and which is water?

Water is Less Dense as a Solid Water Ice

Density of Water • Most dense at 4 o. C • Contracts until 4 o. C • Expands from 4 o. C to 0 o. C The density of water: 1. Prevents water from freezing from the bottom up. 2. Ice forms on the surface first—the freezing of the water releases heat to the water below creating insulation. 3. Makes transition between season less abrupt.

Homeostasis • Ability to maintain a steady state despite changing conditions • Water is important to this process because: a. Makes a good insulator b. Resists temperature change c. Universal solvent d. Coolant e. Ice protects against temperature extremes (insulates frozen lakes)

Solvent for Life • Solution – Solute – solvent • Aqueous solution • Hydrophilic – Ionic compounds dissolve in water – Polar molecules (generally) are water soluble • Hydrophobic – Nonpolar compounds

Solutions & Suspensions • Water is usually part of a mixture. • There are two types of mixtures: – Solutions – Suspensions

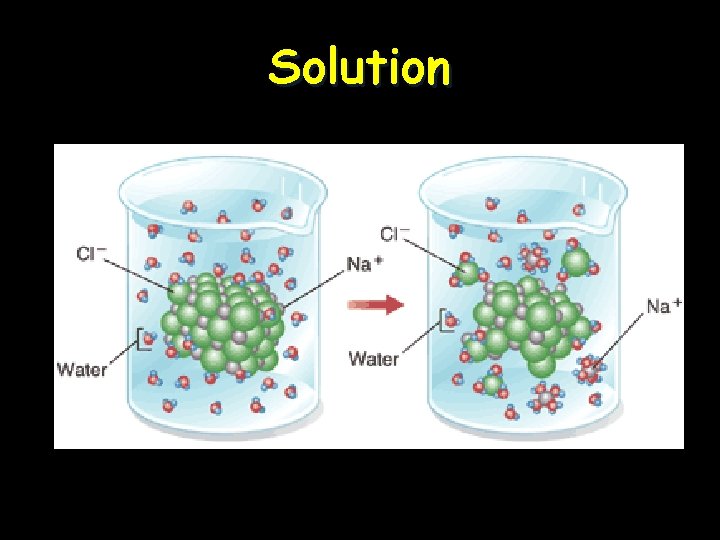
Solution • Ionic compounds disperse as ions in water • Evenly distributed • SOLUTE – Substance that is being dissolved • SOLVENT – Substance into which the solute dissolves

Solution

Suspensions • Substances that don’t dissolve but separate into tiny pieces. • Water keeps the pieces suspended so they don’t settle out.

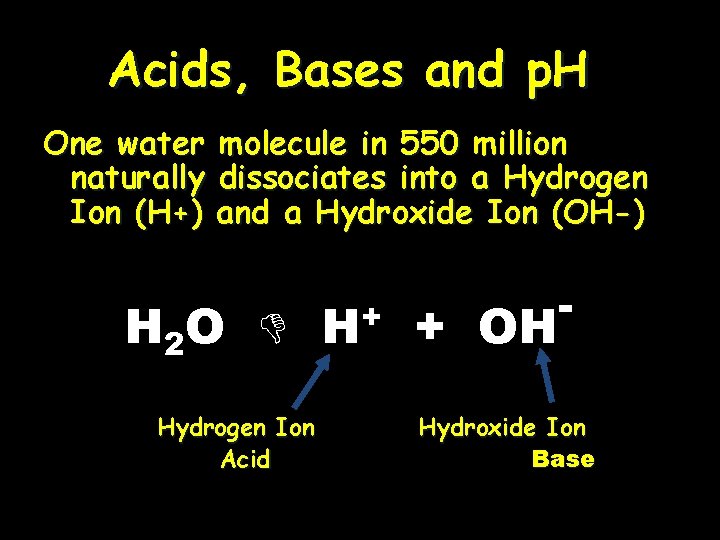
Acids, Bases and p. H One water naturally Ion (H+) molecule in 550 million dissociates into a Hydrogen and a Hydroxide Ion (OH-) H 2 O Hydrogen Ion Acid H+ + OH - Hydroxide Ion Base

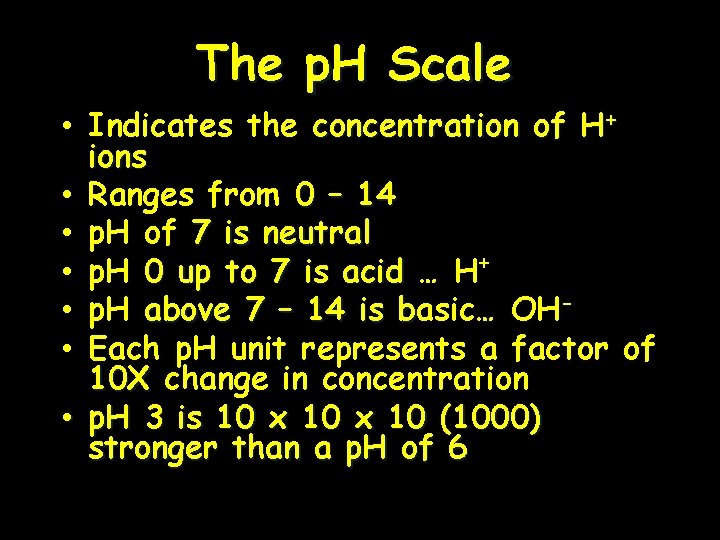
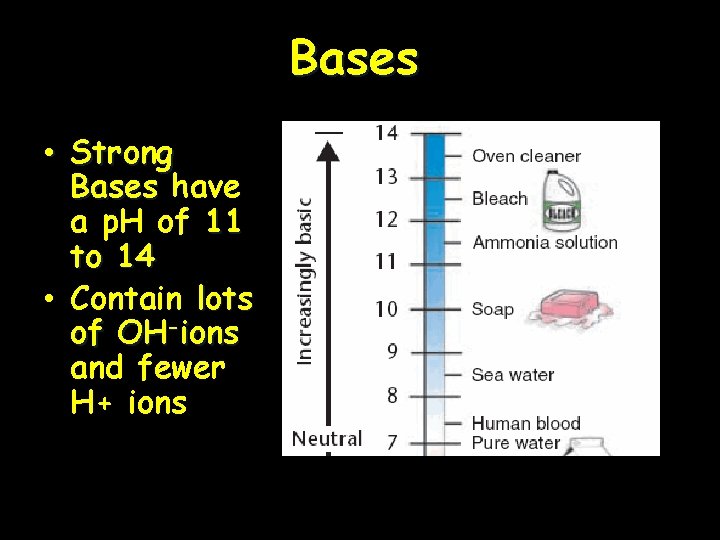
The p. H Scale • Indicates the concentration of H+ ions • Ranges from 0 – 14 • p. H of 7 is neutral • p. H 0 up to 7 is acid … H+ • p. H above 7 – 14 is basic… OHb • Each p. H unit represents a factor of 10 X change in concentration • p. H 3 is 10 x 10 (1000) stronger than a p. H of 6

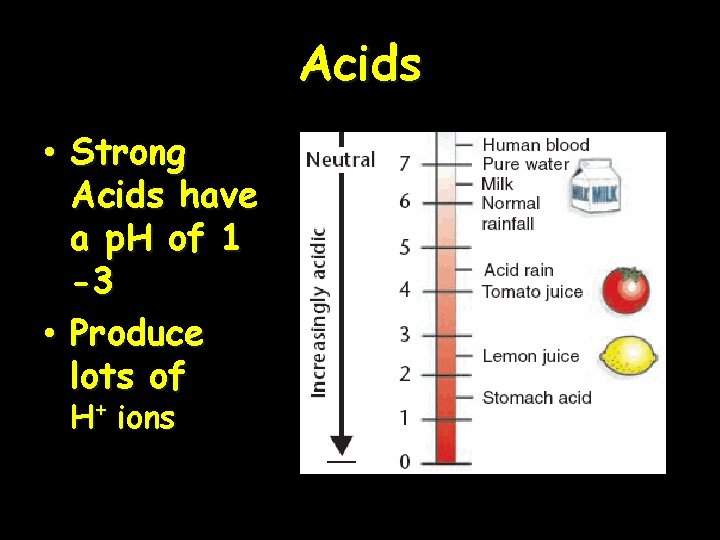
Acids • Strong Acids have a p. H of 1 -3 • Produce lots of H+ ions

Bases • Strong Bases have a p. H of 11 to 14 • Contain lots of OH-ions and fewer H+ ions

Human biology and p. H • Your blood maintains a p. H of 7. 35 – 7. 45 (slightly basic) – Balance is maintained by buffers in blood, breathing, and urinating

Quick Quiz 1. Draw a water molecule with labeled atoms and charges. 2. What is the property called that describes water sticking to itself? 3. What makes a solution acidic? 4. What has a higher p. H, bleach or lemon juice? 5. Why do we sweat?
 Mikael ferm
Mikael ferm The extraordinary properties of water
The extraordinary properties of water The extraordinary properties of water
The extraordinary properties of water Water and water and water water
Water and water and water water How tall are kangaroos
How tall are kangaroos Extraordinary life quotes
Extraordinary life quotes 4 obsessions of an extraordinary executive summary
4 obsessions of an extraordinary executive summary Extraordinary assumption definition
Extraordinary assumption definition Creating extraordinary teams
Creating extraordinary teams Denotations examples
Denotations examples Neutral denotation
Neutral denotation Closing prayer for class
Closing prayer for class Clearvoiceresearch
Clearvoiceresearch Extensive properties and intensive properties
Extensive properties and intensive properties Physical property and chemical property
Physical property and chemical property Concept map properties of water
Concept map properties of water Properties of water ap biology
Properties of water ap biology Physical properties of sea water
Physical properties of sea water Properties of water clipart
Properties of water clipart Water properties lab
Water properties lab Ocean water properties
Ocean water properties Propeties of water
Propeties of water Bill nye surface tension
Bill nye surface tension Honors biology properties of water lab
Honors biology properties of water lab Properties of water foldable
Properties of water foldable Unique facts about water
Unique facts about water High specific heat water property
High specific heat water property Properties of water polarity
Properties of water polarity Properties of water summary
Properties of water summary Properties of water polarity
Properties of water polarity Water molecules
Water molecules Water chemical and physical properties
Water chemical and physical properties What are the life supporting properties of water
What are the life supporting properties of water Water consumption water meter reading worksheet
Water consumption water meter reading worksheet Tpc kapal adalah
Tpc kapal adalah Water o water
Water o water 5 divided by 1/4
5 divided by 1/4 Class 8 english chapter 7 water water everywhere
Class 8 english chapter 7 water water everywhere Water to water heat exchanger
Water to water heat exchanger Fresh water meets salt water
Fresh water meets salt water Warm water rises in a lake. cold water descends.
Warm water rises in a lake. cold water descends.