The Extraordinary Properties of Water Properties of Water







- Slides: 11

The Extraordinary Properties of Water

Properties of Water 1. 2. 3. 4. 5. 6. Polar molecule Cohesion and Adhesion Surface Tension High Specific Heat Density & Solid Floats Solvent for Life

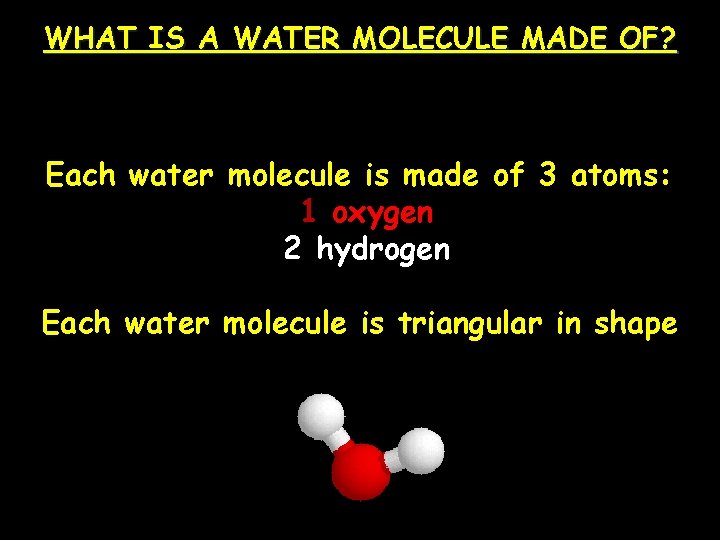
WHAT IS A WATER MOLECULE MADE OF? A water molecule (H 2 O), is made up Each water molecule is made of 3 atoms: 1 oxygen 2 hydrogen Each water molecule is triangular in shape

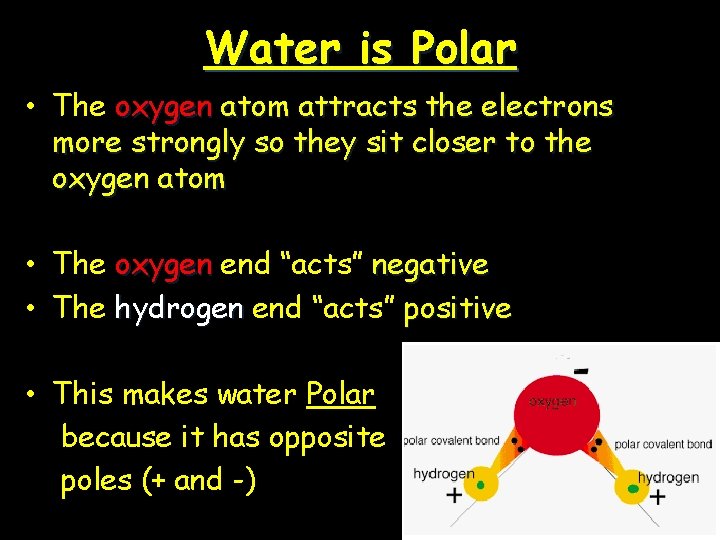
Water is Polar • The oxygen atom attracts the electrons more strongly so they sit closer to the oxygen atom • The oxygen end “acts” negative • The hydrogen end “acts” positive • This makes water Polar because it has opposite poles (+ and -)

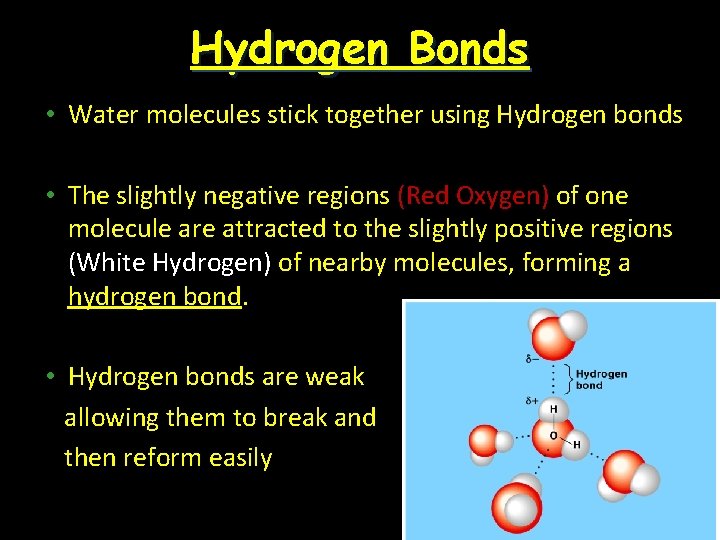
Hydrogen Bonds • Water molecules stick together using Hydrogen bonds • The slightly negative regions (Red Oxygen) of one molecule are attracted to the slightly positive regions (White Hydrogen) of nearby molecules, forming a hydrogen bond. • Hydrogen bonds are weak allowing them to break and then reform easily

Changing the Temperature of Water • Water has a HIGH specific heat – Specific Heat – Amount of heat needed to change the temperature of water by just 1 o – It takes A LOT of heat to change the temperature of water! • In fall as temperatures become cool lakes are still warm • In spring as temperatures become warm lakes are still cool Boils at 100 °C (212 o. F) Freezes at 0 °C (32 o. F)

Cohesion • Cohesion – Ability to stick to itself – Water molecules stick to other water molecules • Surface tension – The strength of the surface of water due to cohesion – Since water molecules stick strongly to one another the surface becomes very strong • Ex. Insects can “walk on water” • Ex. If a plane crashes into the ocean it is like it crashed into concrete!!! Yikes!!!

Adhesion • Adhesion – The ability for water molecules to stick to other substances – Water will stick to glass – Water will stick to metals, such as copper pennies – Important in plants • Plants suck water from the soil and the ability of water to stick to itself and other substances allows it to rise up though the plant to the top so that the leaves get water and nutrients – This is known as capillary action

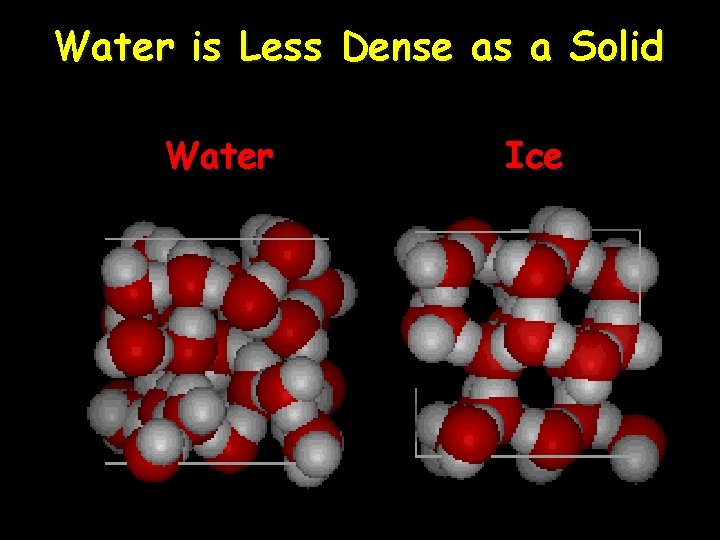
Water Density • Density – A measure of how tightly packed together molecules are • Water is weird because… – The solid form (ice) floats, which does not happen in any other substance • This is because solid ice is less dense than liquid water • The density of water is 1 g/m. L and is used as a reference point for other substances – If the density is more than 1 it sinks. » Ex. Density of lead is 11. 34 g/ml so it sinks in water. – If the density is less than 1 is floats. » Ex. Density of gasoline is 0. 75 g/m. L so it floats on water. (Think of oil spills. )

Water is Less Dense as a Solid Water Ice

Water is the Solvent for Life • Solution – A liquid where one substance has been dissolved in another • Solute – The molecule that is dissolved • Solvent – The substance that does the dissolving – Ex. Salt water • Solute = Salt • Solvent = Water • Solution = Salt Water • This is super important for life because our bodies and cells are NOT full of pure water, but a salty water (saline. ) – Did you know that if a person were given an IV of pure water it would be deadly because the body needs salt water not pure water!
 The extraordinary properties of water
The extraordinary properties of water The extraordinary properties of water
The extraordinary properties of water Water and water and water water
Water and water and water water Extraordinary country
Extraordinary country Extraordinary life quotes
Extraordinary life quotes Obsessions of an extraordinary executive
Obsessions of an extraordinary executive Extraordinary assumptions and hypothetical conditions
Extraordinary assumptions and hypothetical conditions Extraordinary team
Extraordinary team Violent connotation
Violent connotation Inactive favorable connotation
Inactive favorable connotation Intinction communion
Intinction communion Characteristics of extraordinary teachers
Characteristics of extraordinary teachers




















