Water Concept Map Liquid water forms hydrogen bonds

Water Concept Map
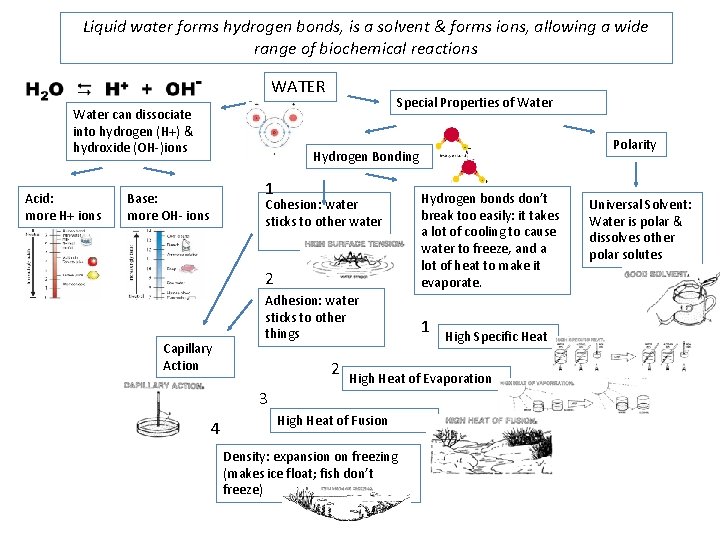
Liquid water forms hydrogen bonds, is a solvent & forms ions, allowing a wide range of biochemical reactions WATER Water can dissociate into hydrogen (H+) & hydroxide (OH-)ions Acid: more H+ ions Special Properties of Water Polarity Hydrogen Bonding 1 Base: more OH- ions 2 Hydrogen bonds don’t break too easily: it takes a lot of cooling to cause water to freeze, and a lot of heat to make it evaporate. Adhesion: water sticks to other things 1 Cohesion: water sticks to other water Capillary Action 2 High Heat of Evaporation 3 4 High Specific Heat High Heat of Fusion Density: expansion on freezing (makes ice float; fish don’t freeze) Universal Solvent: Water is polar & dissolves other polar solutes
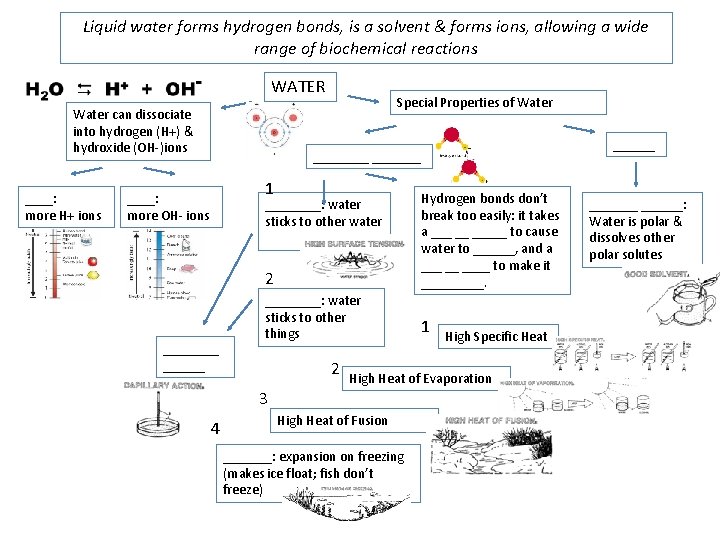
Liquid water forms hydrogen bonds, is a solvent & forms ions, allowing a wide range of biochemical reactions WATER Water can dissociate into hydrogen (H+) & hydroxide (OH-)ions ____: more H+ ions Special Properties of Water ________ 1 ____: more OH- ions 2 Hydrogen bonds don’t break too easily: it takes a ___ __ _____ to cause water to ______, and a ___ __ ____ to make it _____. ____: water sticks to other things 1 ____: water sticks to other water ______ 2 High Heat of Evaporation 3 4 High Specific Heat High Heat of Fusion _______: expansion on freezing (makes ice float; fish don’t freeze) _______: Water is polar & dissolves other polar solutes
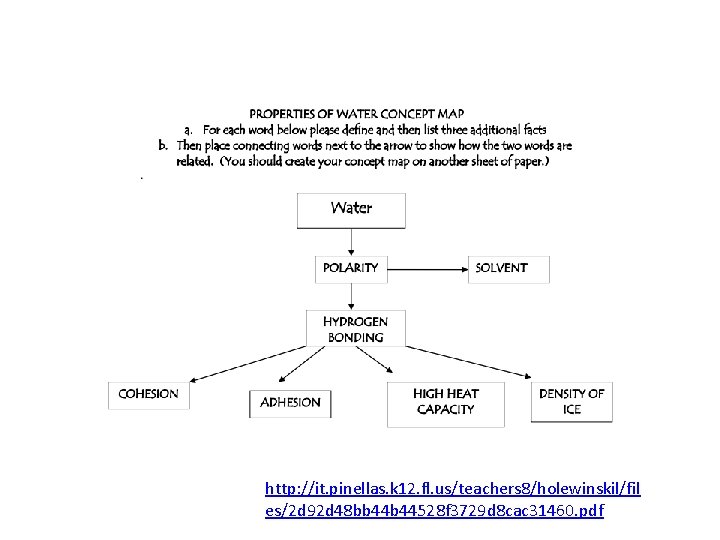
http: //it. pinellas. k 12. fl. us/teachers 8/holewinskil/fil es/2 d 92 d 48 bb 44528 f 3729 d 8 cac 31460. pdf
- Slides: 4