Routine screening programs including pelvic examinations and cervical





























































- Slides: 110

کﺎﻫﺶ ﻣﺮگ ﺩﺭ ﺍﺛﺮ کﺎﻧﺴﺮ ﺳﺮﻭیکﺲ Routine screening programs, including pelvic examinations and cervical cytologic evaluation

ﺍﺗیﻮﻟﻮژی • • • HPV prostitutes first coitus at a young age multiple sexual partners bear children at a young age Promiscuous sexual behavior in male partners

The mean age of women with CIN )کﻨﺴﺮ ﻏیﺮ ﺗﻬﺎﺟﻤی یﺎ پﺮﻩ ( کﻨﺴﺮﺳﺮﻭیکﺲ is about 15 years younger than that of women with invasive cancer, suggesting a slow progression of CIN to invasive carcinoma.

Natural History and Pattern of Spread • exophytic growths • endocervical lesions • Tumor may become fixed to the pelvic wall by direct extension or by coalescence of central tumor with regional adenopathy. • bladder mucosal invasion. • Rectum invasion

: ﺳﺮﻭیکﺲ ﺳﻪ ﻣﺴیﺮ ﺩﺭﻧﺎژ ﻟﻨﻔﺎﻭی ﺩﺍﺭﺩ • The upper branches: follow the uterine artery, and terminate in the uppermost hypogastric nodes. • The middle branches drain to deeper hypogastric (obturator) nodes. • The lowest branches follow a posterior course to the inferior and superior gluteal, common iliac, presacral, and subaortic nodes.

pattern of metastas • Cervical cancer usually follows a relatively orderly pattern of metastatic progression, initially to primary-echelon nodes in the pelvis and then to para-aortic nodes and distant sites. • Even patients with locoregionally advanced disease rarely have detectable hematogenous metastases at initial diagnosis of their cervical cancer. • The most frequent sites of distant recurrence are lung, extrapelvic nodes, liver, and bone

The term cervical intraepithelial neoplasia, refers only to a lesion that may progress to invasive carcinoma. Although CIN 1 and CIN 2 are sometimes referred to as mild-tomoderate dysplasia

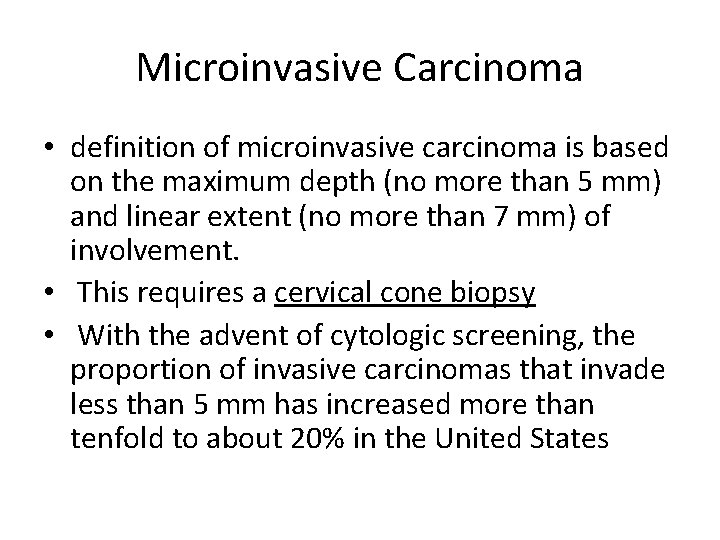
Microinvasive Carcinoma • definition of microinvasive carcinoma is based on the maximum depth (no more than 5 mm) and linear extent (no more than 7 mm) of involvement. • This requires a cervical cone biopsy • With the advent of cytologic screening, the proportion of invasive carcinomas that invade less than 5 mm has increased more than tenfold to about 20% in the United States

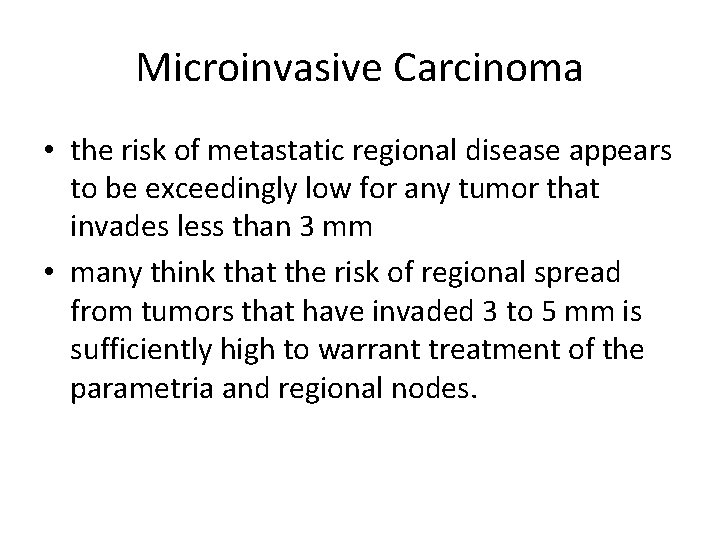
Microinvasive Carcinoma • the risk of metastatic regional disease appears to be exceedingly low for any tumor that invades less than 3 mm • many think that the risk of regional spread from tumors that have invaded 3 to 5 mm is sufficiently high to warrant treatment of the parametria and regional nodes.

Clinical Manifestations • Preinvasive disease during routine cervical cytologic screening. • Early invasive disease usually detected during screening examinations • abnormal vaginal bleeding, often following coitus or vaginal douching. • a clear or foul-smelling vaginal discharge • Pelvic pain • Flank pain (hydronephrosis complicated by pyelonephritis) The triad of sciatic pain, leg edema, and hydronephrosis is almost always associated with extensive pelvic wall involvement by tumor. • hematuria or incontinence from a vesicovaginal fistula • External compression of the rectum by a massive primary tumor may cause constipation

Diagnosis • an ideal target for cancer screening • cervical cytologic examination and pelvic examination has led to a decrease in the mortality rate • Only nations with well-developed screening programs have experienced substantial decreases in cervical cancer death rates

Screening (The American Cancer Society) • 3 years after the onset of vaginal intercourse, but no later than 21 years of age • Annually : cervical cytology smears • women who have had three consecutive, technically satisfactory negative test results may be screened every 2 to 3 years.

Clinical Evaluation of Patients with Invasive Carcinoma 1. detailed history 2. physical examination, 3. complete blood cell count and renal function and liver function tests 4. chest radiography 5. intravenous pyelography (or computed tomography [CT]) 6. Cystoscopy and either a proctoscopy or a barium enema study should be done in patients with bulky tumors. 7. CT S or MRI 8. PET

CT S or MRI to evaluate regional nodes, but these studies have suboptimal accuracy because they fail to detect small metastases and because patients with bulky necrotic tumors often have enlarged reactive lymph nodes that may be free of metastasis. PET appears to be a very sensitive noninvasive method of evaluating the regional nodes of patients with cervical cancer and a useful method for following response to treatment, although its high cost has prevented widespread routine use. MRI can provide useful information about the distribution and depth of invasion of tumors in the cervix but tends to yield less accurate assessments of the parametrium.

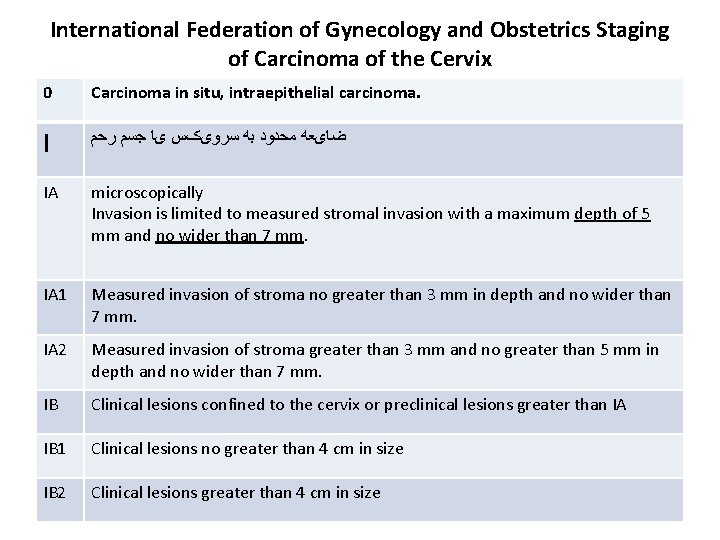
International Federation of Gynecology and Obstetrics Staging of Carcinoma of the Cervix 0 Carcinoma in situ, intraepithelial carcinoma. I ﺿﺎیﻌﻪ ﻣﺤﺪﻭﺩ ﺑﻪ ﺳﺮﻭیکﺲ یﺎ ﺟﺴﻢ ﺭﺣﻢ IA microscopically Invasion is limited to measured stromal invasion with a maximum depth of 5 mm and no wider than 7 mm. IA 1 Measured invasion of stroma no greater than 3 mm in depth and no wider than 7 mm. IA 2 Measured invasion of stroma greater than 3 mm and no greater than 5 mm in depth and no wider than 7 mm. IB Clinical lesions confined to the cervix or preclinical lesions greater than IA IB 1 Clinical lesions no greater than 4 cm in size IB 2 Clinical lesions greater than 4 cm in size



Clinical Staging • FIGO stage is based on careful clinical examination and the results of specific radiologic studies and procedures. • The clinical stage should never be changed on the basis of subsequent findings. • When it is doubtful , case should be assigned to the earlier stage.

Clinical Staging • According to FIGO, growth fixed to the pelvic wall by a short and indurated, but nodular, parametrium should be allotted to stage IIb. • A case should be classified as stage III only if the parametrium is nodular to the pelvic wall or if the growth itself extends to the pelvic wall.

FIGO rules for clinical staging, • • • . Palpation Inspection Colposcopy endocervical curettage hysteroscopy cystoscopy Proctoscopy intravenous urography radiographic examination of the lungs and skeleton

1. lymphangiography 2. Laparoscopy 3. CT, and MRI : are of value for planning therapy but because these are not yet generally available and the interpretation of results is variable should not be the basis for changing the clinical stage

Surgical Evaluation of Regional Spread • • transperitoneal extraperitoneal dissection laparoscopic lymph node dissection ? sentinel node ?

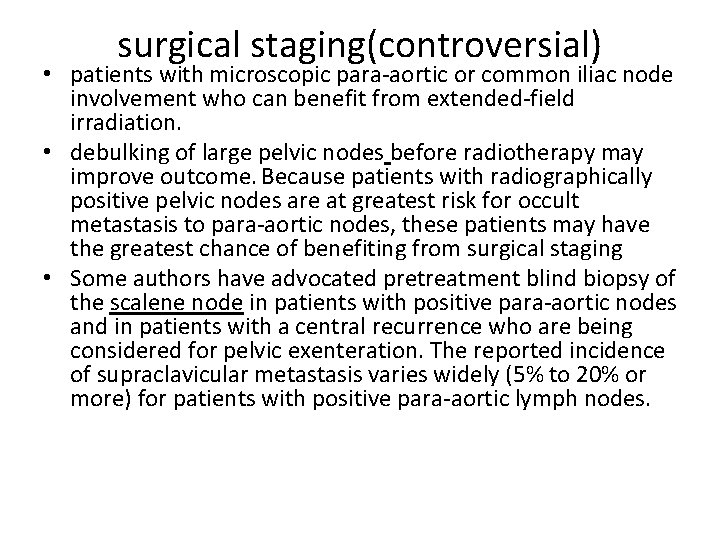
surgical staging(controversial) • patients with microscopic para-aortic or common iliac node involvement who can benefit from extended-field irradiation. • debulking of large pelvic nodes before radiotherapy may improve outcome. Because patients with radiographically positive pelvic nodes are at greatest risk for occult metastasis to para-aortic nodes, these patients may have the greatest chance of benefiting from surgical staging • Some authors have advocated pretreatment blind biopsy of the scalene node in patients with positive para-aortic nodes and in patients with a central recurrence who are being considered for pelvic exenteration. The reported incidence of supraclavicular metastasis varies widely (5% to 20% or more) for patients with positive para-aortic lymph nodes.

Prognostic Factors ● ● ● ● FIGO stage Clinical tumor diameter presence of medial versus lateral parametrial involvement presence of unilateral versus bilateral parametrial or pelvic wall involvement Lymph node metastasis ﺗﻬﺎﺟﻢ ﺑﻪ ﻋﺮﻭﻕ ﻋﺼﺒی ﻭﻟﻨﻔی deep stromal invasion (10 mm or more than 70% invasion) parametrial extension inflammatory response Uterine-body involvement histologic features histologic grade (adenocarcinomas) (ﻫﻤﻮگﻠﻮﺑﻮﻟیﻦ locally advanced )

predictive power: • • Age peritoneal cytology platelet count tumor vascularity DNA ploidy or S phase cyclooxygenase-2 expression growth factor receptors. HPV DNA

Treatment depended on tumor size stage histologic features evidence of lymph node metastasis risk factors for complications of surgery or radiotherapy • patient preference. • • •

Treatment • HSILs : loop electroexcision procedure (LEEP) • Although surgical treatment is standard for in situ and microinvasive cancer, patients with severe medical problems or other contraindications to surgical treatment can be successfully treated with radiotherapy.

• stage IA 1: conservative surgery • stage IA 2 and IB 1 and some small stage IIA tumors: ﺟﺮﺍﺣی یﺎ ﺭﺍﺩیﻮﺗﺮﺍپی • stages IB 2 through IVA: radiotherapy • Selected patients with centrally recurrent disease after RT may be treated with radical exenterative surgery • isolated pelvic recurrence after hysterectomy is treated with irradiation. • the routine addition of concurrent cisplatin-containing chemotherapy to radiotherapy for patients whose cancers have a high risk of locoregional recurrence.

Stage IB and IIA Disease • Early stage IB cervical carcinomas can be treated effectively with combined external-beam irradiation and brachytherapy or with radical hysterectomy and bilateral pelvic lymphadenectomy. The goal of both treatments is to destroy malignant cells in the cervix, paracervical tissues, and regional lymph nodes. Patients who are treated with radical hysterectomy whose tumors are found to have high-risk features may benefit from postoperative radiotherapy or chemoradiation.

stage IB 1 • For patients with similar tumors, the overall rate of major complications is similar with surgery and radiotherapy, although urinary tract complications tend to be more common after surgical treatment and bowel complications are more common after radiotherapy. • Surgical treatment tends to be preferred for young women with small tumors because it permits preservation of ovarian function and may cause less vaginal shortening. Radiotherapy is often selected for older, postmenopausal women to avoid the morbidity of a major surgical procedure.

stage IB 2 (bulky) • radical radiotherapy patients who have tumors measuring more than 4 cm in diameter usually have deep stromal invasion and are at high risk for lymph node involvement and parametrial extension. Because patients with these risk factors have an increased rate of pelvic disease recurrence, surgical treatment is usually followed by postoperative irradiation, which means that the patient is exposed to the risks of both treatments. Consequently, many gynecologic and radiation oncologists believe that patients with stage IB 2 carcinomas are better treated with radical radiotherapy.

concurrent administration of cisplatincontaining chemotherapy • • bulky stage I cancers lymph node metastasis involved surgical margins IB 2<=

Radical Hysterectomy • For premenopausal women : If intraoperative findings suggest a need for postoperative pelvic irradiation, the ovaries may be transposed out of the pelvis. • The risk of complications may be increased in patients who undergo preoperative or postoperative irradiation

Bladder complications 1. decreased bladder sensation 2. chronic bladder hypotonia or atony (3% to 5% ) 3. Bladder dysfunction 4. stress incontinence(influenced by RT) 5. bladder contraction and instability(RT post s) Bowel complications • constipation and, rarely, chronic obstipation • small bowel obstruction(RT post s)

Radical Radiotherapy • excellent survival and pelvic disease control rates in patients with stage IB cervical cancer. • Survival rates for patients with FIGO stage IIA disease treated with radiation alone range between 70% and 85% and are also strongly correlated with tumor size. • for patients with bulky tumors, results may be improved further with concurrent administration of chemotherapy

Radical radiotherapy • goal of radical radiotherapy =cervix, paracervical tissues, and regional LN • ERT+BT • Even small tumors that involve multiple quadrants of the cervix are usually treated with total doses of 80 to 85 Gy to point A. The dose may be reduced by 5% to 10% for very small superficial tumors. Although patients with small tumors may be treated with somewhat smaller fields than patients with more advanced locoregional disease, care must still be taken to adequately cover the obturator, external iliac, low common iliac, and presacral nodes.

Stage IIB, III, and IVA Disease • With appropriate chemoradiotherapy, even patients with massive locoregional disease have a significant chance for cure. • The success of treatment depends on a careful balance between external-beam radiotherapy and brachytherapy that optimizes the dose to tumor and normal tissues and the overall duration of treatment.

Stage IIB, III, and IVA Disease • EBRT shrinking bulky tumor and bringing it within the range of the high-dose portion of the brachytherapy dose distribution. • Subsequent brachytherapy exploits the inverse square law to deliver a high dose to the cervix and paracervical tissues while minimizing the dose to adjacent normal tissues.

Stage IIB, III, and IVA Disease • complete the entire treatment in less than 7 to 8 weeks. • longer treatment courses are associated with decreased pelvic disease control and survival rates.

External-Beam Radiotherapy Technique • High-energy photons • if 4 - to 6 -MV photons, four fields are usually used

External-Beam Radiotherapy Technique • CT simulation (iliac lymph nodes. ) • Information gained from radiologic studies such as MRI, CT, and PET improve estimates of disease extent and assist in localization of regional nodes and paracervical tissues that may contain microscopic disease. • The caudad extent of disease (radiopaque markers )

organ motion • positions of the uterus and cervix can vary by as much as 4 cm from day to day. • it is usually wise to cover the entire presacrococcygeal region when locally advanced cancers are treated. • some clinicians prefer to use the simpler technique for patients with bulky tumors.


• Tumor response should be evaluated with periodic pelvic examinations to determine the best time to deliver brachytherapy. • a central block after 40 Gy?

Central block • it can result in overdoses to medial structures such as the ureters or underdosage of posterior uterosacral disease. For these reasons, other clinicians prefer to give an initial dose of 40 to 45 Gy to the whole pelvis, believing that the ability to deliver a homogeneous distribution to the entire region at risk for microscopic disease and the additional tumor shrinkage achieved before brachytherapy

• more than 40 to 45 Gy to the central pelvis tend to compromise the dose deliverable to paracentral tissues and increase the risk of late complications.

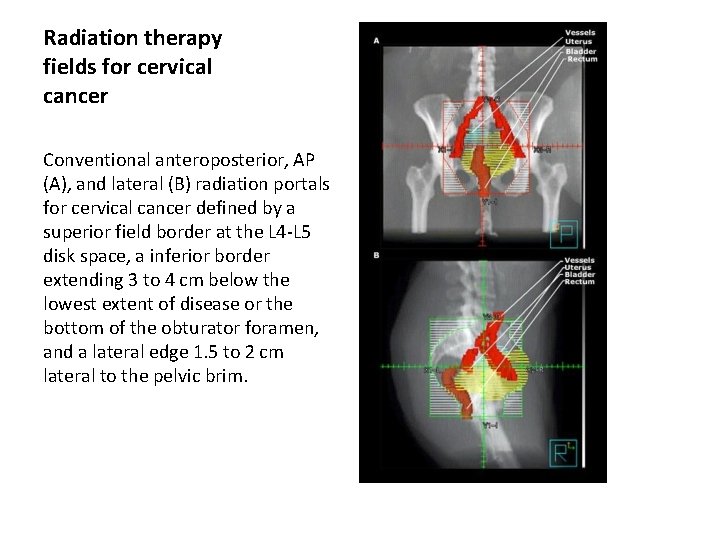
Radiation therapy fields for cervical cancer Conventional anteroposterior, AP (A), and lateral (B) radiation portals for cervical cancer defined by a superior field border at the L 4 -L 5 disk space, a inferior border extending 3 to 4 cm below the lowest extent of disease or the bottom of the obturator foramen, and a lateral edge 1. 5 to 2 cm lateral to the pelvic brim.

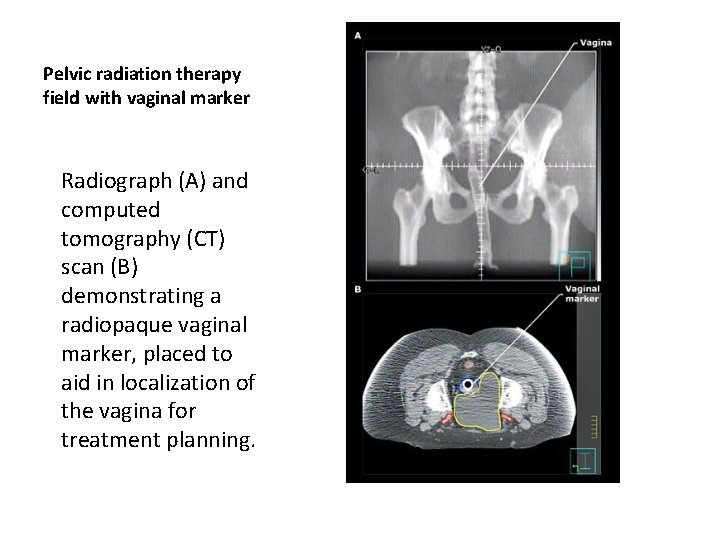
Pelvic radiation therapy field with vaginal marker Radiograph (A) and computed tomography (CT) scan (B) demonstrating a radiopaque vaginal marker, placed to aid in localization of the vagina for treatment planning.

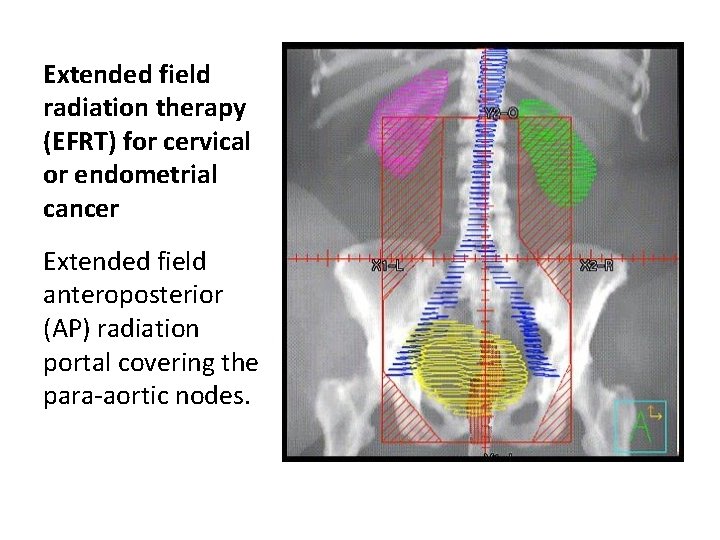
Extended field radiation therapy (EFRT) for cervical or endometrial cancer Extended field anteroposterior (AP) radiation portal covering the para-aortic nodes.

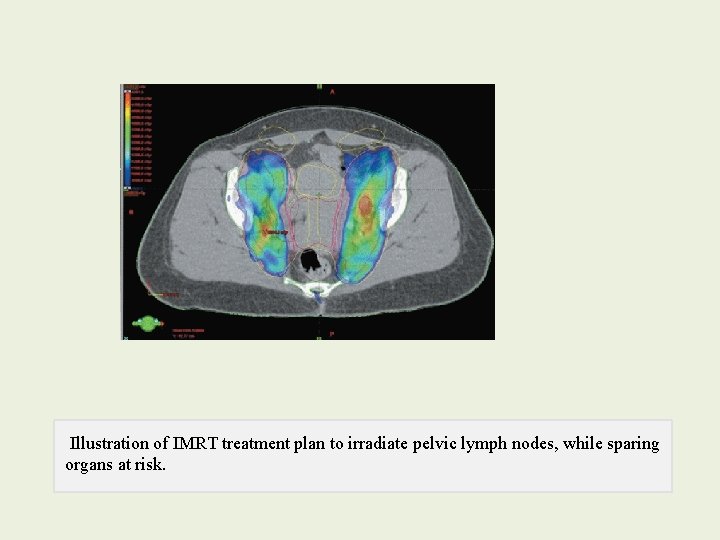
IMRT • Unlike standard two-field and four-field techniques, IMRT makes it possible to deliver a lower daily dose to the intrapelvic contents than to surrounding pelvic lymph nodes. • reduced bone marrow toxicity and acute gastrointestinal side

IMRT • IMRT is less sparing of bowel. • IMRT allows delivery of doses exceeding 60 Gy with relative sparing of adjacent critical structures. • increase error • influence of internal organ motion and intratreatment tumor response on the doses to tumor and critical structures.

• There is no evidence that IMRT can safely be used as an alternative to brachytherapy for routine treatment of intact cervical cancer. • IMRT cannot accurately reproduce the highdose gradients produced with intracavitary therapy. • unpredictable variations mandate the use of large treatment margins

External-beam pelvic irradiation is delivered before intracavitary insertions in patients with: • Bulky cervical lesions or tumors beyond stage IIA to improve the geometry of the intracavitary application • Exophytic, easily bleeding tumors; • Tumors with necrosis or infection • Parametrial involvement.

high-risk features need ERT • parametrial involvement, deep stromal invasion, or positive nodes, positive or close operative margins,

Volume Treated • the primary tumor and the pelvic lymph nodes • superior border at the L 4 -5 (external iliac and hypogastric lymph node) • inferior border at the inferior edge of the ischium • This margin must be extended to the L 3 -4 interspace if common iliac nodal coverage is indicated. • 1. 5 -cm -2. 5 cm margin on the pelvic rim;

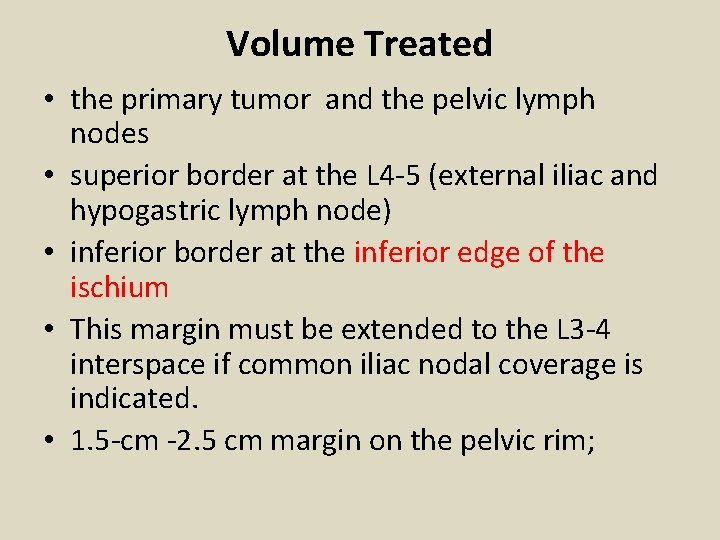
• A: Ap simulation film. The 15 by 15 cm portals at SSD are used for stage IB (broken line), and 18 by 15 cm portals are used for more advanced disease (solid line). This allows better coverage of the common iliac lymph nodes. The distal margin is usually placed at the bottom of the obturator foramina. B: Standard portal for stage IB tumors is outlined (solid line indicated as A). . If there is vaginal tumor extension, the lower margin of the field is drawn at the introitus (indicated in section C).


lateral field • the anterior border of the lateral fields over the anterior edge of the pubic symphysis • the posterior margin usually is designed to cover at least 50% of the rectum in stage IB tumors, and it should extend to the sacral hollow in patients with more advanced tumors • Three-dimensional treatment planning for pelvic irradiation of cervical carcinoma may reduce the treated volume, but further research must be done to determine whether the complication rate can be decreased as well.

• For stage IB disease, : 15 by 15 cm at the surface • For patients with stage IIA, IIB, III, and IVA carcinoma, (18 by 15 cm at surface) are required to cover all of the common iliac nodes in addition to the cephalad half of the vagina • If there is no vaginal extension, the lower margin of the portal is at the inferior border of the obturator foramen. • When there is vaginal involvement, the entire length of this organ should be treated down to the introitus

• In patients with tumor involving the distal half of the vagina, the portals should be modified to cover the inguinal lymph nodes because of the increased probability of metastases

Midline Shielding in Ap–PA Portals • Depending on the institution and brachytherapy dose administered, midline shielding with rectangular or specially designed blocks are used for a portion of the external beam dose delivered with the Ap–PA ports

Parametrial Boost • When parametrial tumor persists after 50 to 60 Gy is delivered to the parametria, an additional 10 Gy in five or six fractions may be delivered with reduced AP-PA portals (8 by 12 cm for unilateral and 12 by 12 cm portals for bilateral parametrial coverage). The central shield should be in place to protect the bladder and rectum.

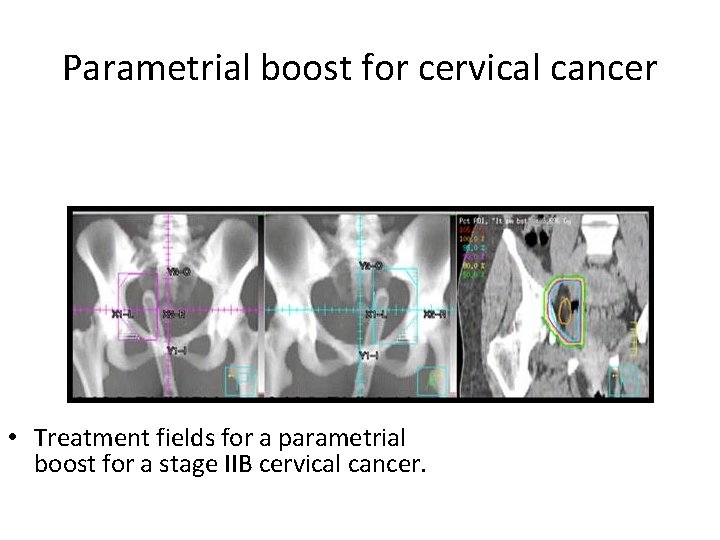
Parametrial boost for cervical cancer • Treatment fields for a parametrial boost for a stage IIB cervical cancer.

Para-Aortic Lymph Node Irradiation • an extended field or through a separate portal • 45 to 50 Gy to the para-aortic area plus a 5 to 10 Gy boost to enlarged lymph nodes

separate portal • a “gap calculation( excessive dose to the small intestines) • The upper margin = T 12 -L 1 • lower margin at L 5 -S 1 • The width of the para-aortic portals can be determined by CT scans, MRI, lymphangiography, FDG-PET scans, or IV pyelography outlining the ureters.

• The spinal cord dose (T 12 to L 2 -3) should be kept below 45 Gy by interposing a 2 -cm wide 5 –half-value layer (HVL) shield on the posterior portal (usually after 40 -Gy tumor dose) or using lateral ports and the kidneys below 1, 800 c. Gy

Beam Energies • 10 MV or higher • They decrease the dose of radiation delivered to the peripheral normal tissues (particularly bladder and rectum) and provide a more homogeneous dose distribution in the central pelvis. • With lower-energy photons (Cobalt-60 or 4 - to 6 -MV x -rays), higher maximum doses must be given, and more complicated field arrangements should be used to achieve the same midplane tumor dose (3 or 4 field pelvic box or rotational techniques) while minimizing the dose to the bladder and rectum and to avoid subcutaneous fibrosis

• a metallic prosthesis when using lateral fields or a box pelvic irradiation technique may result in a dose decrease of approximately 2% for 25 -MV x-rays and average increases of 2% for 10 -MV x-rays and 5% for 60 Co.

Hyperfractionated Radiation Therapy for Locally Advanced Cervix Cancer 1. 2 Gy to the whole pelvis twice daily at 4 - to 6 hour intervals, 5 days per weeka total pelvic dose of 57. 5 Gy. A boost dose with brachytherapy

Concomitant Boost • On Monday, Wednesday, and Friday of the last 3 weeks, an additional 1. 6 -Gy boost was given 6 hours after the whole pelvis treatment (14. 4 Gy) through lateral fields encompassing the parametria and primary tumor, for a total tumor dose of 59. 4 Gy.

Three-Dimensional or IMRT • patients with stage IIB or IVA cervical cancer with medical illness or severe tumor-related anatomic distortion that limited delivery of brachytherapy. • The toxicity of IMRT was acceptable, and early tumor responses were encouraging.

IMRT • Although not as critical in older patients, it is important to keep in mind that while IMRT has dosimetric advantages over conventional RT, IMRT exposes a greater amount of normal tissues to lower irradiation levels, which has the potential to increase the incidence of radiation-induced second cancers.

Brachytherapy Ø intracavitary techniques • (137 Cs) • (192 Ir)


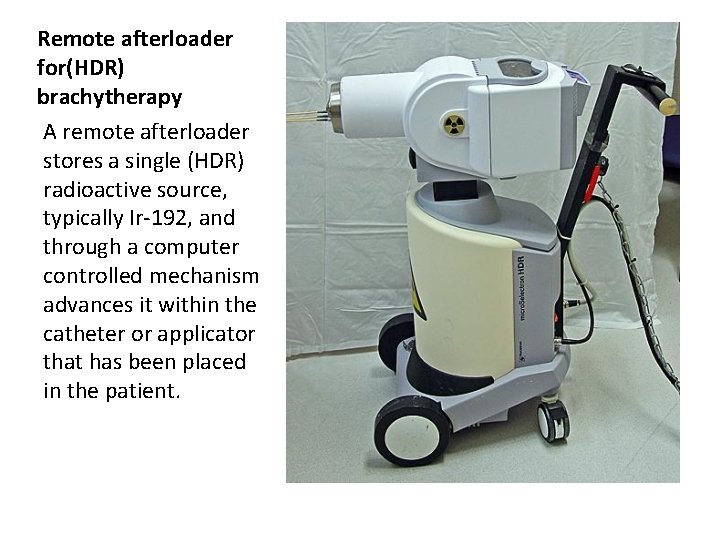
Remote afterloader for(HDR) brachytherapy A remote afterloader stores a single (HDR) radioactive source, typically Ir-192, and through a computer controlled mechanism advances it within the catheter or applicator that has been placed in the patient.

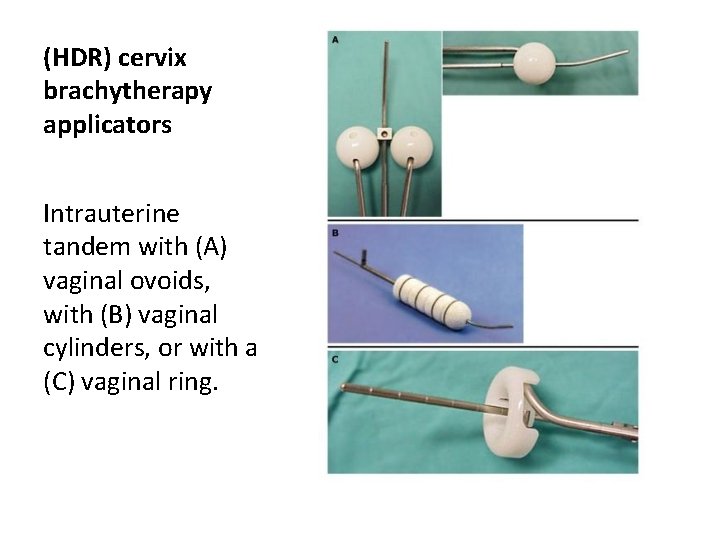
(HDR) cervix brachytherapy applicators Intrauterine tandem with (A) vaginal ovoids, with (B) vaginal cylinders, or with a (C) vaginal ring.

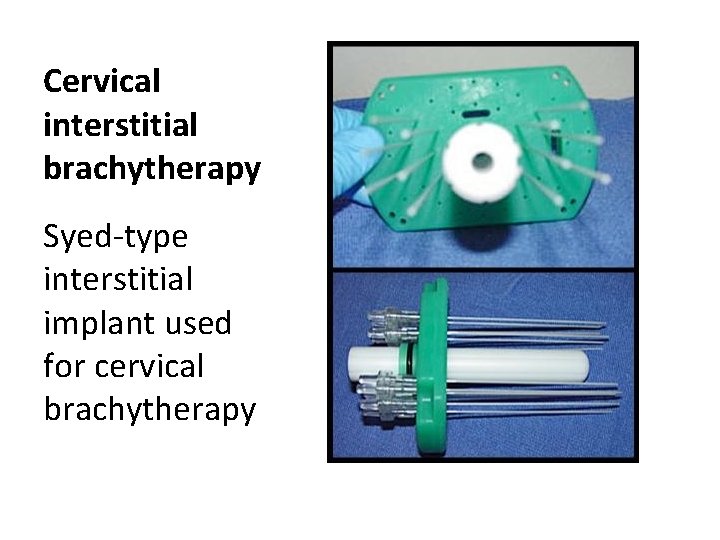
Cervical interstitial brachytherapy Syed-type interstitial implant used for cervical brachytherapy

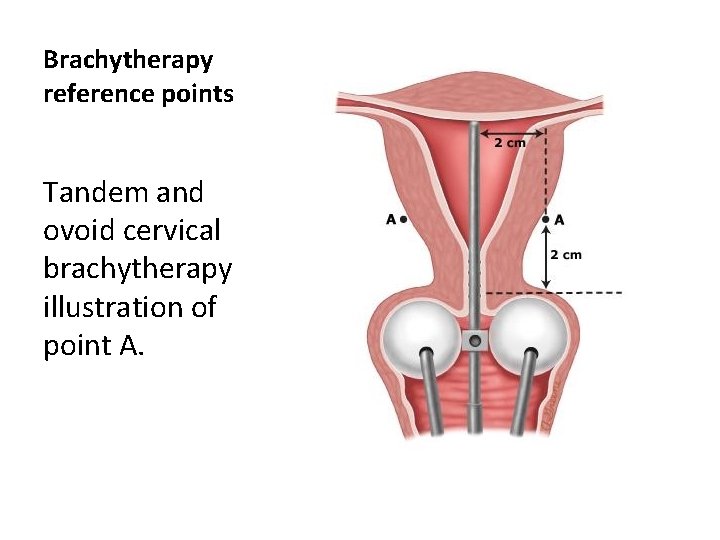
Brachytherapy reference points Tandem and ovoid cervical brachytherapy illustration of point A.

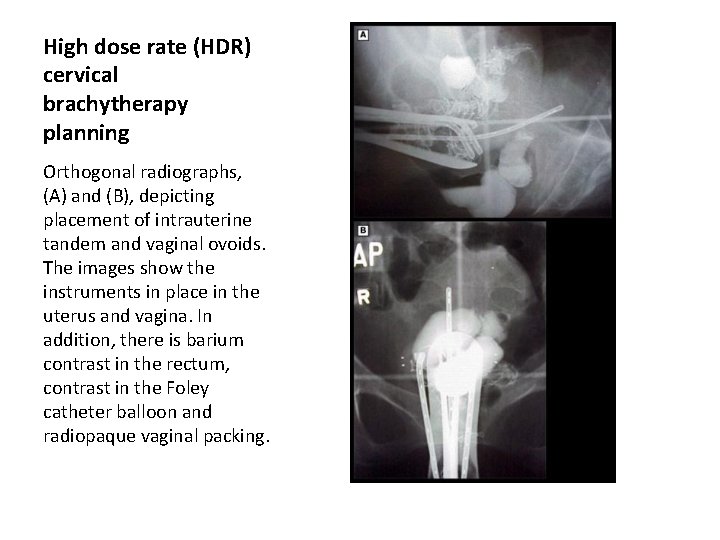
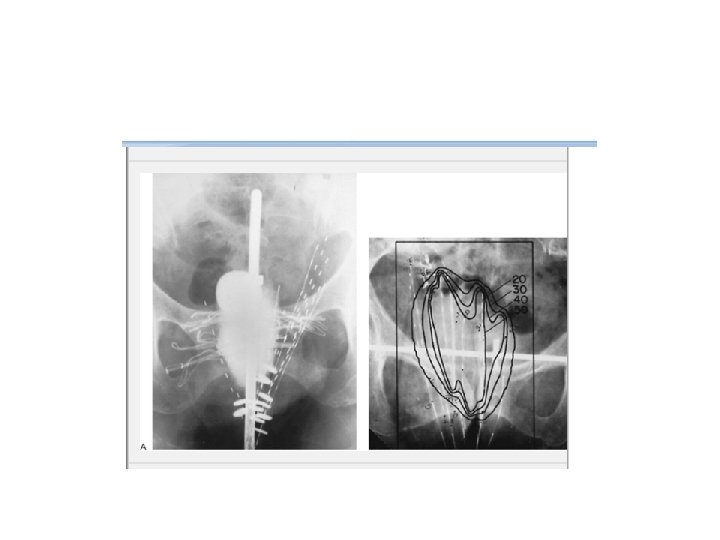
High dose rate (HDR) cervical brachytherapy planning Orthogonal radiographs, (A) and (B), depicting placement of intrauterine tandem and vaginal ovoids. The images show the instruments in place in the uterus and vagina. In addition, there is barium contrast in the rectum, contrast in the Foley catheter balloon and radiopaque vaginal packing.

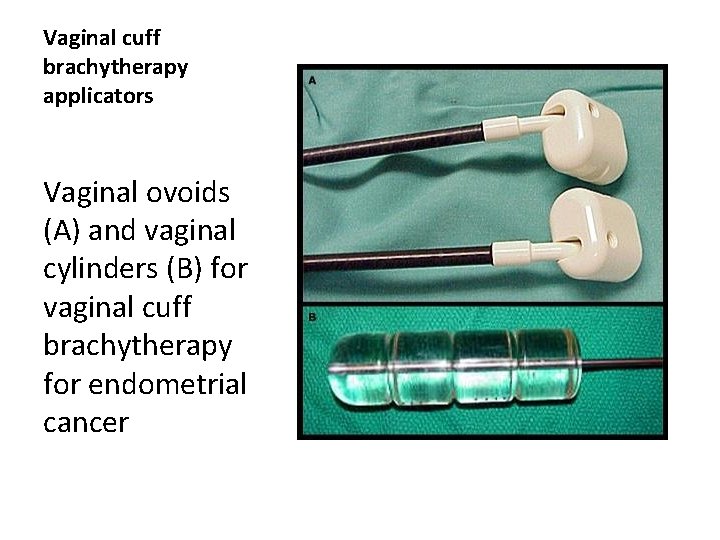
Vaginal cuff brachytherapy applicators Vaginal ovoids (A) and vaginal cylinders (B) for vaginal cuff brachytherapy for endometrial cancer

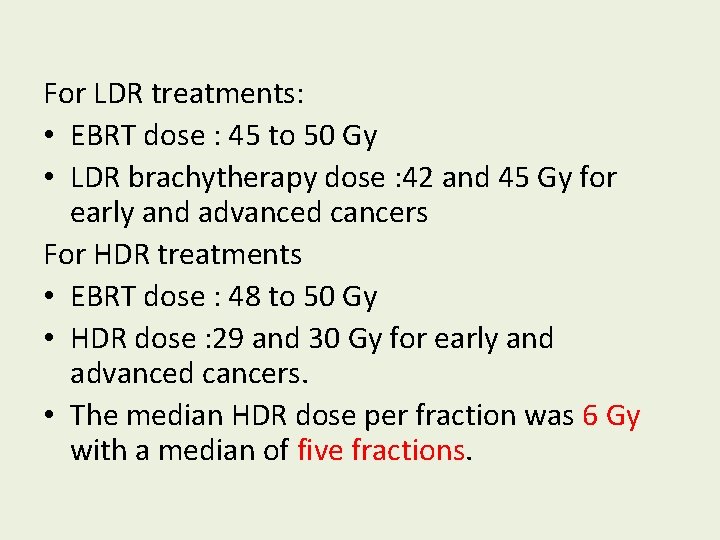
For LDR treatments: • EBRT dose : 45 to 50 Gy • LDR brachytherapy dose : 42 and 45 Gy for early and advanced cancers For HDR treatments • EBRT dose : 48 to 50 Gy • HDR dose : 29 and 30 Gy for early and advanced cancers. • The median HDR dose per fraction was 6 Gy with a median of five fractions.

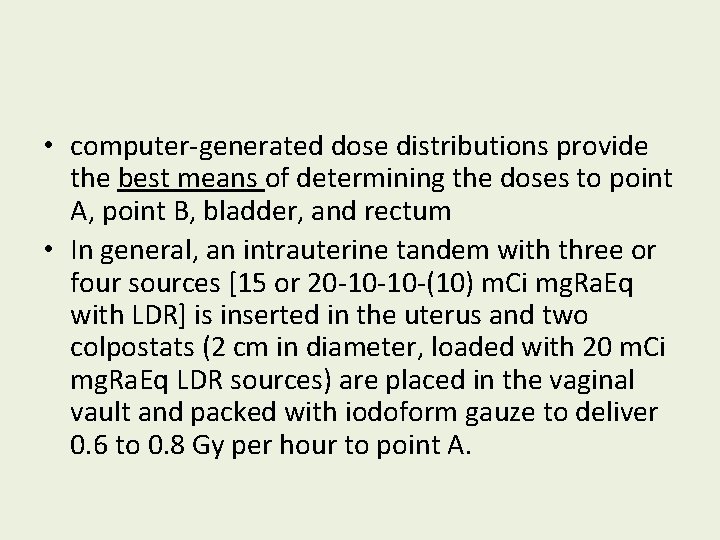
• computer-generated dose distributions provide the best means of determining the doses to point A, point B, bladder, and rectum • In general, an intrauterine tandem with three or four sources [15 or 20 -10 -10 -(10) m. Ci mg. Ra. Eq with LDR] is inserted in the uterus and two colpostats (2 cm in diameter, loaded with 20 m. Ci mg. Ra. Eq LDR sources) are placed in the vaginal vault and packed with iodoform gauze to deliver 0. 6 to 0. 8 Gy per hour to point A.

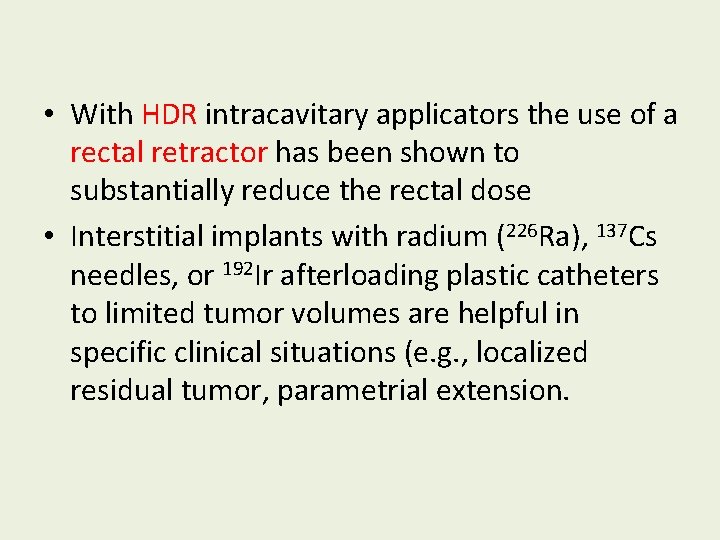
• With HDR intracavitary applicators the use of a rectal retractor has been shown to substantially reduce the rectal dose • Interstitial implants with radium (226 Ra), 137 Cs needles, or 192 Ir afterloading plastic catheters to limited tumor volumes are helpful in specific clinical situations (e. g. , localized residual tumor, parametrial extension.



• As Fletcher emphasized, conditions for an adequate intracavitary insertion include the following 1. The geometry of the insertion must prevent underdosing around the cervix; 2. Sufficient dose must be delivered to the paracervical areas 3. Vaginal mucosal (and, we add, bladder and rectal) tolerance doses must be respected.

Biology of High–Dose-Rate Brachytherapy for Cervical Carcinoma • Late damage rises sharply as the number of HDR fractions is decreased. • Displacing the bladder and rectum away from the HDR sources for the short duration of therapy may offset the radiobiologic disadvantage of using a few brachytherapy fractions

At Washington University • HDR brachytherapy with sedation and without anesthesia. • bladder catheter and gentle packing of the vagina with iodoform gauze • anteroposterior and lateral pelvic radiographs • The usual dose per fraction prescribed at 0. 5 cm depth is 3 to 6 Gy, and three to six fractions are given once or twice weekly

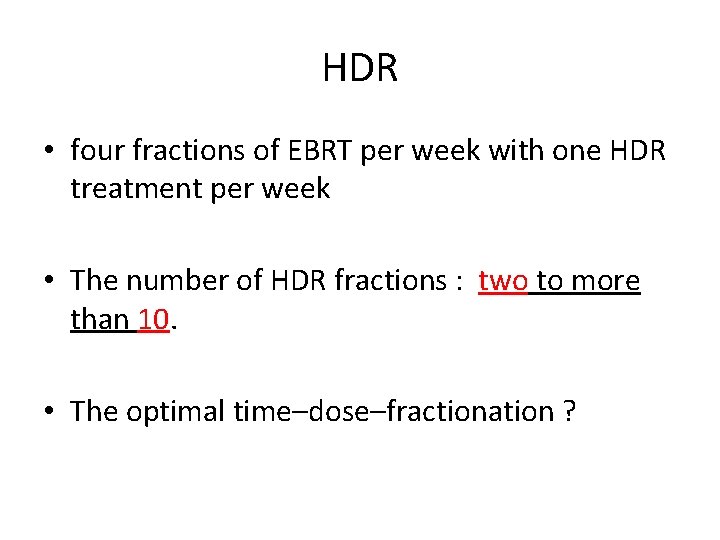
HDR • four fractions of EBRT per week with one HDR treatment per week • The number of HDR fractions : two to more than 10. • The optimal time–dose–fractionation ?

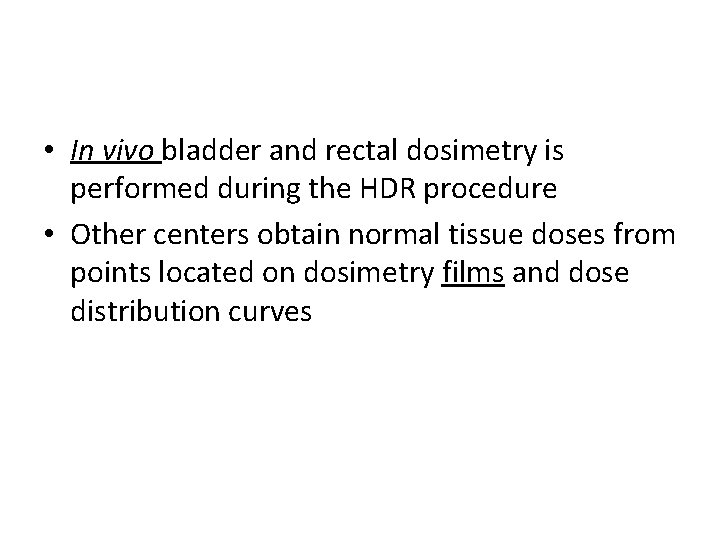
• In vivo bladder and rectal dosimetry is performed during the HDR procedure • Other centers obtain normal tissue doses from points located on dosimetry films and dose distribution curves

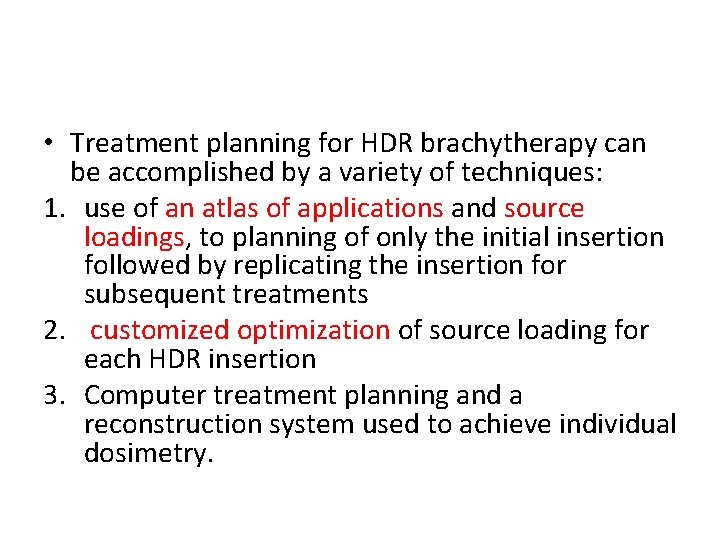
• Treatment planning for HDR brachytherapy can be accomplished by a variety of techniques: 1. use of an atlas of applications and source loadings, to planning of only the initial insertion followed by replicating the insertion for subsequent treatments 2. customized optimization of source loading for each HDR insertion 3. Computer treatment planning and a reconstruction system used to achieve individual dosimetry.

Three-Dimensional Brachytherapy Treatment Planning • The advantages of in vivo dosimetry are easy practicability and the possibility to determine rectal dose during radiation. • The advantages of computer-aided planning at ICRU reference points are that calculations are available before radiation and they can be taken into account for treatment planning.

• The CT-based planning provides information on target and organ volumes and dose– volume histograms. • The radiography-based planning provides dimensions and doses only at selected points.

• They predicted that, using therapeutic gain ratio, similar results would be obtained with either brachytherapy modality with two to four fractions of LDR and four to seven fractions of HDR.

The American Brachytherapy Society published recommendations for HDR brachytherapy for carcinoma of the cervix 1. point A to at least a total LDR equivalent of 80 to 85 Gy for early stage disease and 85 to 90 Gy for advanced-stage disease. 2. The pelvic sidewall : 50 to 55 Gy for early lesions and 55 to 65 Gy for advanced ones. 3. As with LDR BT, every attempt should be made to keep the bladder and rectal doses below 80 Gy and 75 Gy LDR-equivalent doses, respectively.

4. Interstitial brachytherapy should be considered when the tumor cannot be optimally encompassed by intracavitary brachytherapy. 5. It was emphasized that the responsibility for the medical decisions ultimately rests with the treating radiation oncologist.

Doses of Radiation • Stage IA (microinvasive) tumors are treated with intracavitary therapy only (LDR 60 Gy in one insertion or 75 to 80 Gy in two insertions to point A, or HDR 35 to 42 Gy in five to six insertions of 7 Gy to point A, one or two fractions per week). • The optimal dose for invasive carcinoma of the cervix is delivered with a combination of EBRT whole pelvis, intracavitary, and, at times, interstitial therapy.

Illustration of IMRT treatment plan to irradiate pelvic lymph nodes, while sparing organs at risk.

Brachytherapy D • Fletcher described three conditions that should be met for successful cervical brachytherapy: • (1) the geometry of the radioactive sources must prevent underdosed regions on and around the cervix • (2) an adequate dose must be delivered to the paracervical areas • (3) mucosal tolerance must be respected.

verify accurate placement • Radiographs should be obtained at the time of insertion to verify accurate placement, and the system should be repositioned if positioning can be improved.

Brachytherapy Dose • Paracentral doses are most frequently expressed at a single point, usually designated Point A. This reference point has been calculated in a number of different ways. • Optimized source placement can rarely correct for a poorly positioned applicator

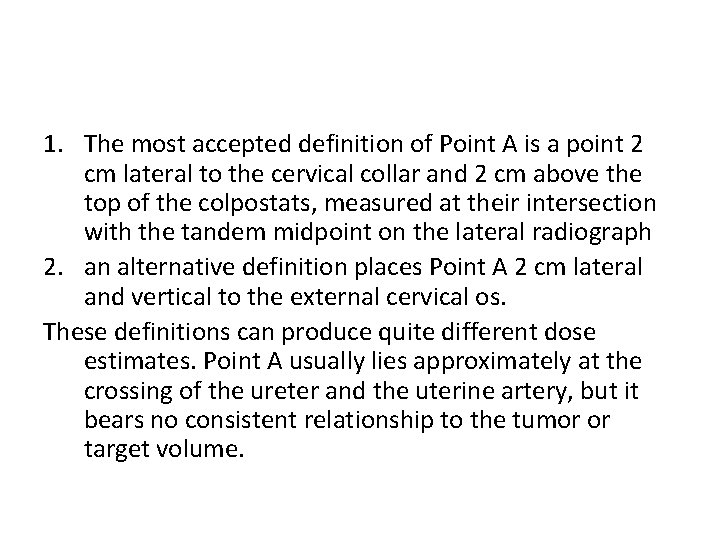
1. The most accepted definition of Point A is a point 2 cm lateral to the cervical collar and 2 cm above the top of the colpostats, measured at their intersection with the tandem midpoint on the lateral radiograph 2. an alternative definition places Point A 2 cm lateral and vertical to the external cervical os. These definitions can produce quite different dose estimates. Point A usually lies approximately at the crossing of the ureter and the uterine artery, but it bears no consistent relationship to the tumor or target volume.

• an effort should always be made to deliver at least 85 Gy (with LDR brachytherapy) to Point A for patients with bulky central disease. • without exceeding a dose of 75 Gy to the bladder reference point or 70 Gy to the rectal reference point, • The dose to the surface of the lateral wall of the apical vagina should not usually exceed 120 to 140 Gy.

• A total dose (external-beam and intracavitary) of 50 to 55 Gy appears to be sufficient to sterilize microscopic disease in the pelvic nodes in most patients. • Gross disease : a total dose of 60 to 65 Gy (including the contribution from brachytherapy treatments).

Palliative Radiotherapy • Localized radiotherapy can provide effective relief of pain caused by metastases in bone, brain, lymph nodes, or other sites. A rapid course of pelvic radiotherapy can also provide excellent relief of pain and bleeding for patients who present with incurable disseminated disease

• TX for patients with an isolated vaginal recurrence is similar to that for patients with a primary carcinoma of the vagina. • Most patients are treated with external-beam radiotherapy with or without brachytherapy. Implants may need to be inserted under laparoscopic or laparotomy guidance.

After Radical Surgery • TX Pelvic wall recurrences are often treated with external-beam irradiation alone, although surgery and intraoperative therapy may contribute to local control in selected patients. • Reported survival rates usually range between 20% and 40% for patients treated with radical radiotherapy.

After Definitive Irradiation • an isolated central recurrence can be cured with surgical treatment. • Less extensive operations, such as radical hysterectomy or anterior exenteration, are reserved for selected patients with small tumors confined to the cervix or lesions that do not encroach on the rectum, respectively.

Unresectable disease • Intraoperative irradiation ( involves the pelvic wall) • chemotherapy alone (previously discussed); response rates and prognosis are generally poor.

• When brachytherapy is used, most investigators have reported survival rates similar to those for patients with carcinomas of the intact cervix.