Spotlight on Cervical Cancer Screening Cervical Cancer Screening




















- Slides: 35

Spotlight on Cervical Cancer Screening

Cervical Cancer Screening • https: //www. cancercare. on. ca/pcs/screening/cervscr eening/

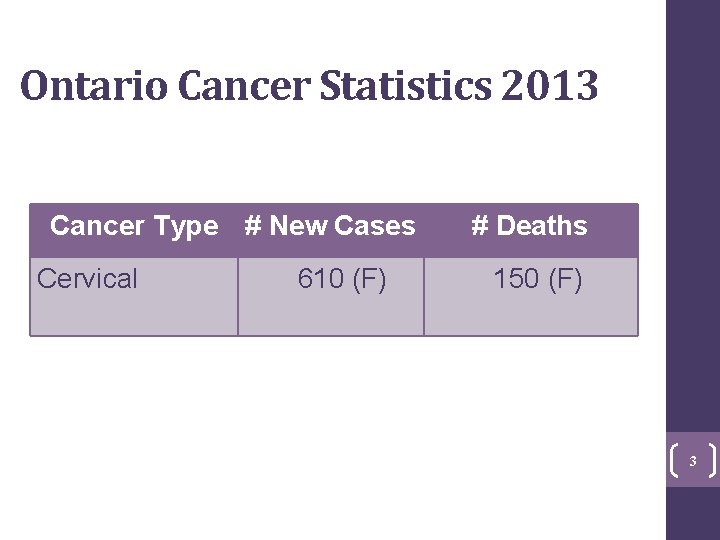
Ontario Cancer Statistics 2013 Cancer Type # New Cases Cervical 3 610 (F) # Deaths 150 (F) 3

Burden of Disease in Ontario • Estimated 610 women will be diagnosed and 150 will die of cervical cancer in 2013 • Up to 80, 000 abnormal Pap tests require assessment each year • 4 th most common cancer among women under age 50 4

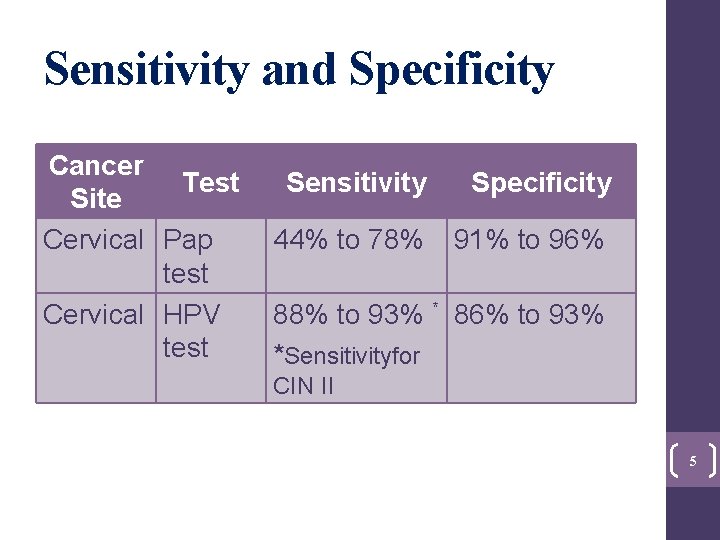
Sensitivity and Specificity Cancer Test Site Cervical Pap test Cervical HPV test Sensitivity Specificity 44% to 78% 91% to 96% 88% to 93% * 86% to 93% *Sensitivityfor CIN II 5

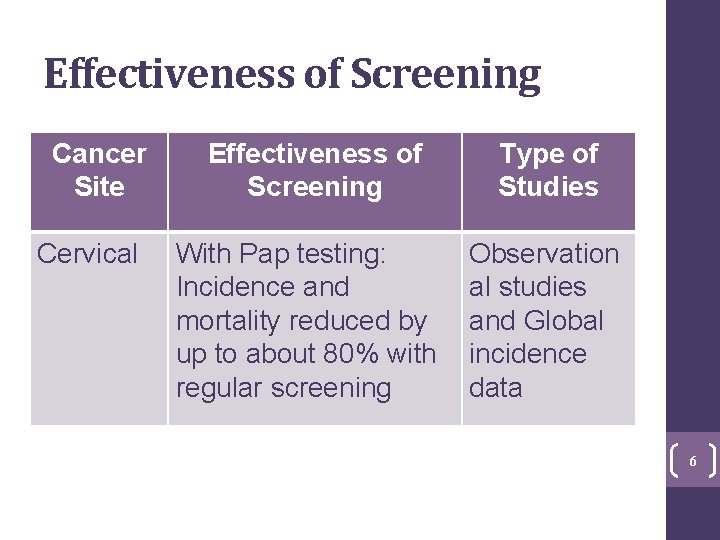
Effectiveness of Screening Cancer Site Cervical Effectiveness of Screening With Pap testing: Incidence and mortality reduced by up to about 80% with regular screening Type of Studies Observation al studies and Global incidence data 6

Cervical Abnormalities Cancer (0. 015%) Atypical Glandular Cells (0. 1%) Atypical Squamous Cells: HSIL Cannot be Excluded (0. 1%) High-Grade Squamous Intraepithelial Lesion (HSIL) (0. 3%) Pre-cancer lesions/ Pap abnormalities: 80, 000 Low-Grade Squamous Intraepithelial Lesion (2. 1%) Atypical Squamous Cells of Undetermined Significance (2. 3%) Negative for Intraepithelial Lesion or Malignancy (95. 0%) Women (aged 20– 69) Eligible for Cervical Cancer Screening 7 7

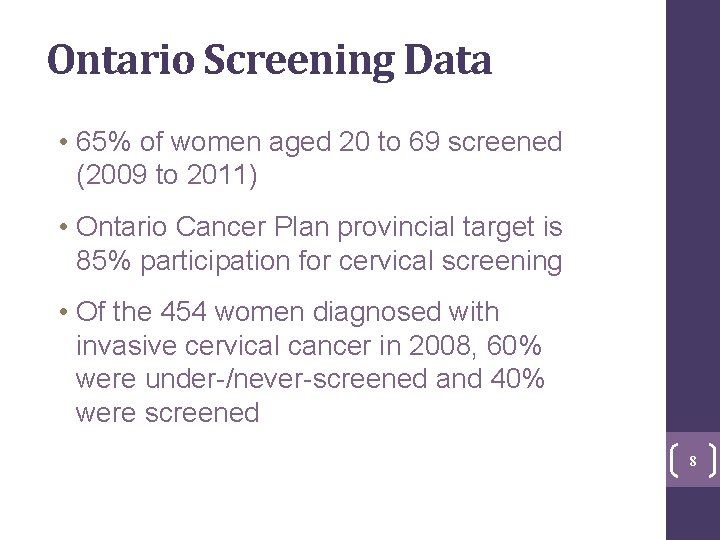
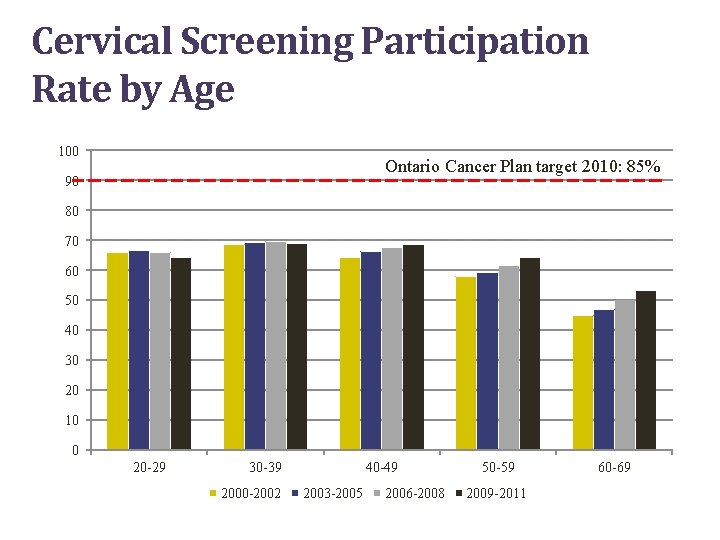
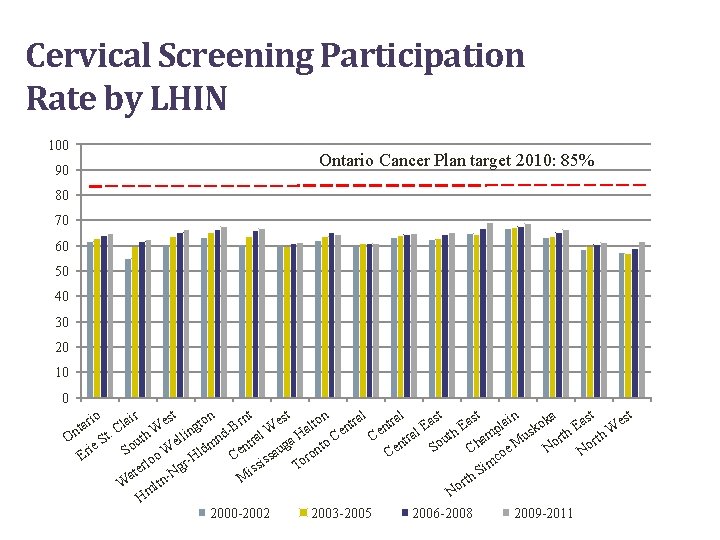
Ontario Screening Data • 65% of women aged 20 to 69 screened (2009 to 2011) • Ontario Cancer Plan provincial target is 85% participation for cervical screening • Of the 454 women diagnosed with invasive cervical cancer in 2008, 60% were under-/never-screened and 40% were screened 8

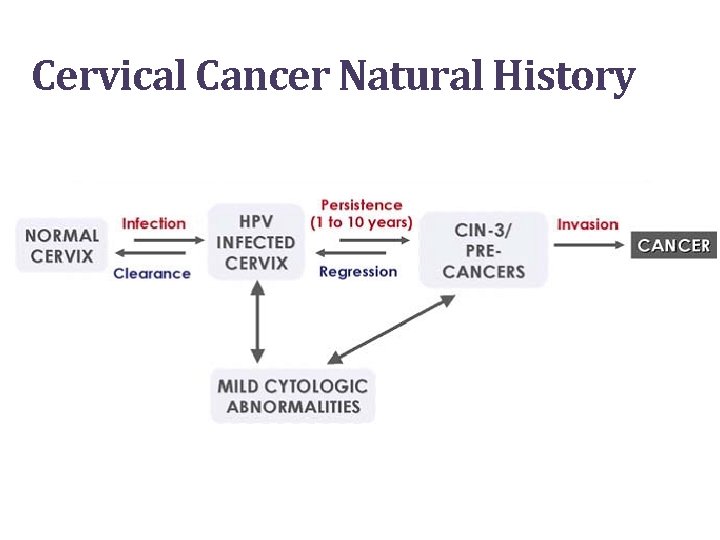
Cervical Cancer Causes • Persistent infection with high risk (oncogenic) types of HPV (human papillomavirus) • HPV is commonly found in sexually active men and women and transmitted through any skin to skin sexual contact • Most HPV infections are transient; about 90% will clear within 2 years 9

Cervical Cancer Causes • Pap tests detect cervical cell changes that are a result of HPV infections • Some abnormal Pap tests are also a reflection of premalignant change • Other co-factors (like smoking), that are not well-understood, are also involved in oncogenesis 10

Cervical Cancer Natural History

HPV Vaccine • Two vaccines—bivalent (Cervarix®) and quadrivalent (Gardasil®)—prevent 2 high risk HPV types that cause 70% of cervical cancers • Injected in 3 doses over 6 months 12

HPV Vaccine • Provides best protection if received prior to HPV exposure • Natural infection does not reliably result in immunity • Does not replace regular cervical cancer screening 13

Ontario HPV Vaccination Program • Publicly funded school-based immunization program for grade 8 girls • New catch-up program since September 2012 for girls in grades 9 -12 • 59% uptake in grade 8 girls (2009/2010) • More vaccine program information at www. hpvontario. ca 14

Current Guidelines • Clear evidence for primary HPV screening with cytology triage, starting at age 30, every 5 years • Must implement within organized program • Must be publicly funded • Follow cytology-based guidelines during transition to funded HPV screening 15

Comparison of 2005 and 2011 Guidelines Question Initiation Interval after Negative Test Cessation 2005 Guidelines 2011 Guidelines Within 3 years of first vaginal sexual activity with cytology (Pap test) Age 21 Annual until 3 consecutive negative cytology tests, then every 2 to 3 years Every 3 years Age 70 if adequate and negative screening history in previous 10 years (≥ 3 negative tests) No change Management guidelines for follow-up of abnormal cytology did not change Guidelines summary: www. cancercare. on. ca/screenforlife

Screening Initiation Start at age 21 in sexually active women (includes intercourse as well as digital and/or oral sexual activity involving the genital area with a partner of either gender) • Cervical cancer rare < 25 years and extremely rare < 21 years • 10 to 15 years to develop cervical cancer 17

STI Screening • The new guidelines for cervical screening do no affect the guidelines for STI screening • Asymptomatic sexually active females under age 25 should be screened annually for chlamydia, or more often if there are multiple partners 18

Harms of Screening Adolescents • 90% will clear infection within 2 years • High rates of low-grade mostly transient and clinically inconsequential abnormalities • Unnecessary anxiety from detection, biopsies and treatment • Treatment linked to possibility of adverse future pregnancy outcomes • No protective effect with screening 19

Screening Interval • Cytology screening every 3 years unless immunocompromised or previously treated for dysplasia • No incremental benefit of screening more frequently than every 3 years • Aligns with other jurisdictions 20

Screening Cessation Stop screening at age 70 if adequate and negative screening history • Low incidence of cancer in women who have been adequately screened • Potential discomfort of procedure • Difficulties visualizing squamocolumnar junction 21

Cervical Screening Participation Rate 100 Ontario Cancer Plan target 2010: 85% 95 90 85 80 75 70 65 60 55 2000 -2002 2003 -2005 2006 -2008 2009 -2011

Cervical Screening Participation Rate by Age 100 Ontario Cancer Plan target 2010: 85% 90 80 70 60 50 40 30 20 10 0 20 -29 30 -39 2000 -2002 40 -49 2003 -2005 2006 -2008 50 -59 2009 -2011 60 -69

Cervical Screening Participation Rate by LHIN 100 Ontario Cancer Plan target 2010: 85% 90 80 70 60 50 40 30 20 10 0 n n ir st st st al al io in nt ka East o o r r a a e r e a e r a t t l o l a l E E B g t n n k d- ral W a Ha al th mp t. C uth W ellin h. W us orth Ce Ce r On t n a u t S r t o en N So ie Ch oe M No So o W Hldm Cen saug ront C Er c s m rlo To gr ssi e i Si t N a h M t W ltn or m N H 2000 -2002 2003 -2005 2006 -2008 2009 -2011

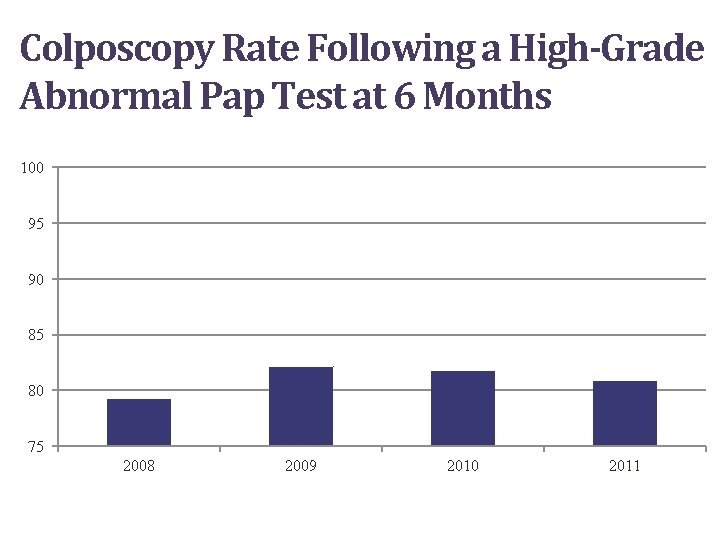
Colposcopy Rate Following a High-Grade Abnormal Pap Test at 6 Months 100 95 90 85 80 75 2008 2009 2010 2011

Challenges • Cervical cancer screening often linked to periodic health exam, hormonal contraception and bimanual exam • Difficult for physicians/providers to track 3 year screening interval • Roll-out of program correspondence started in 2013 26

CCO Initiatives Underway Phased correspondence to women started in Fall of 2013 • Privacy notification • Result (normal, abnormal, unsatisfactory) letters • Followed by recalls and invitations 27

Opportunities • Updated guidelines reflect new evidence • Increase awareness of balance between benefits and potential harms of screening • Reduce interventions in young women whose abnormal Pap tests are due to transient and inconsequential HPV infections • Increase screening rates for under-/neverscreened groups 28

Opportunities • Improve appropriate follow-up after abnormal Pap test result • Continue to encourage primary prevention through HPV immunization • CCO and Public Health Ontario evaluating impact of primary and secondary prevention of HPV-related disease 29

Screening: Future State • Clear evidence for primary HPV screening • Must be implemented within an organized program • HPV test must be publicly funded • Updated cytology guidelines to bridge transition 30

Future Considerations CCO working with ministry regarding implementation of primary HPV screening • Public funding of HPV test • Family physician/primary care provider education/information • Laboratories • Organization of colposcopy services 31

Clinical Case Study 1 • A 17 -year-old female sees you to initiate birth control pill • She started having unprotected intercourse 2 months ago Do you screen her for cervical cancer? 32

Clinical Case Study 2 • A 69 -year-old female had a normal Pap test when she was 59 years old, an abnormal test when she was 63 years old and a normal Pap test most recently when she was 66 At what age can she safely stop screening? 33

Cervical Cancer Resources For more information: https: //www. cancercare. on. ca/pcs/screening/cervscreening/hcpresources/ https: //www. publications. serviceontario. ca/pubont/servlet/ecom / https: //www. cancercare. on. ca/pcs/screening/cervscreening/patient_education/

Questions? Thank You 35
 Cervical cancer hcp
Cervical cancer hcp Stage 4 cervical cancer
Stage 4 cervical cancer Hpv cervical cancer
Hpv cervical cancer National breast and cervical cancer early detection program
National breast and cervical cancer early detection program Hpv cervical cancer
Hpv cervical cancer National breast and cervical cancer early detection program
National breast and cervical cancer early detection program Spotlight 10
Spotlight 10 Readworks paired texts
Readworks paired texts Spotlight 6 module 3 test
Spotlight 6 module 3 test Abs spotlight
Abs spotlight Spotlight
Spotlight Self serving bias definition psychology
Self serving bias definition psychology Test module 1 spotlight 10
Test module 1 spotlight 10 Hydringes
Hydringes Spotlight 3 school again
Spotlight 3 school again 5-1 reteach perpendicular and angle bisectors
5-1 reteach perpendicular and angle bisectors Road safety spotlight 6
Road safety spotlight 6 Spotlight computer graphics
Spotlight computer graphics Lung cancer screening shared decision making tool
Lung cancer screening shared decision making tool Inferior gluteal nerve
Inferior gluteal nerve Cervical burnout
Cervical burnout Método del moco cervical o billings
Método del moco cervical o billings Posterior triangle of neck contents mnemonic
Posterior triangle of neck contents mnemonic Cstd gateshead
Cstd gateshead Protusion cervical
Protusion cervical Platysma
Platysma Manusi examinare
Manusi examinare Cervical stenosis cervix
Cervical stenosis cervix Brilliantly translucent swelling
Brilliantly translucent swelling Cervical ilesi
Cervical ilesi Parts of abdomen
Parts of abdomen What is spleenomegaly
What is spleenomegaly Cervical facet referral patterns
Cervical facet referral patterns Salpingitits
Salpingitits Cervical sympathetic
Cervical sympathetic Cervical fascia
Cervical fascia