OxidationReduction Reactions LEO SAYS GER Oxidation and Reduction









































- Slides: 83

Oxidation-Reduction Reactions LEO SAYS GER

Oxidation and Reduction (Redox) Electrons are transferred Spontaneous redox rxns can transfer energy Electrons (electricity) Heat Non-spontaneous redox rxns can be made to happen with electricity

Redox • • Oxidation- Reduction Reactions Transfer of electrons Competition for e. Redox Reaction Tarnishing of Silverware • Redox Chemistry of Tin and Zinc

What Are Oxidation and Reduction? Oxidation – a substance reacting with oxygen – LEO – Lose electrons oxidation – particle becomes more positive – oxidation # increases

What Are Oxidation and Reduction? • Carbon is oxidized when charcoal burns.

What Are Oxidation and Reduction? • Iron is oxidized when it rusts.

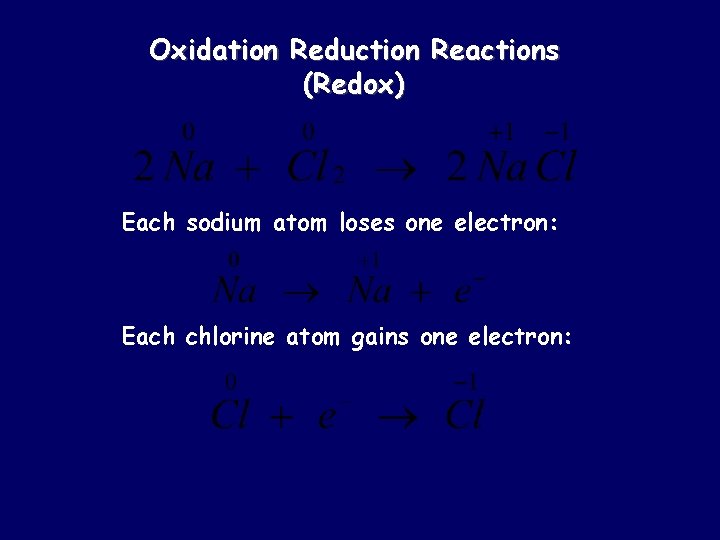
Oxidation Reduction Reactions (Redox) Each sodium atom loses one electron: Each chlorine atom gains one electron:

Reduction • • Compound loses oxygen GER – gain electrons reduction Oxidation # becomes more negative # decreases

LEO says GER : Lose Electrons = Oxidation Sodium is oxidized Gain Electrons = Reduction Chlorine is reduced

Assigning Oxidation Numbers • An oxidation number is a positive or negative number assigned to an atom to indicate its degree of oxidation or reduction.

Assigning Oxidation Numbers • Oxidation numbers are often written above the chemical symbols in a formula.

Rules for Assigning Oxidation Numbers Rules 1 & 2 1. The oxidation number of any uncombined element is zero 2. The oxidation number of a monatomic ion equals its charge

Rules for Assigning Oxidation Numbers Rules 3 & 4 3. The oxidation number of oxygen in compounds is -2 4. The oxidation number of hydrogen in compounds is +1

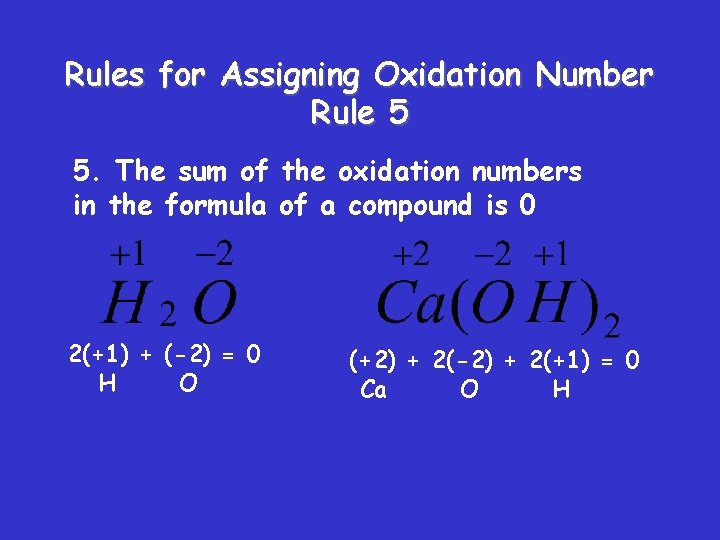
Rules for Assigning Oxidation Number Rule 5 5. The sum of the oxidation numbers in the formula of a compound is 0 2(+1) + (-2) = 0 H O (+2) + 2(-2) + 2(+1) = 0 Ca O H

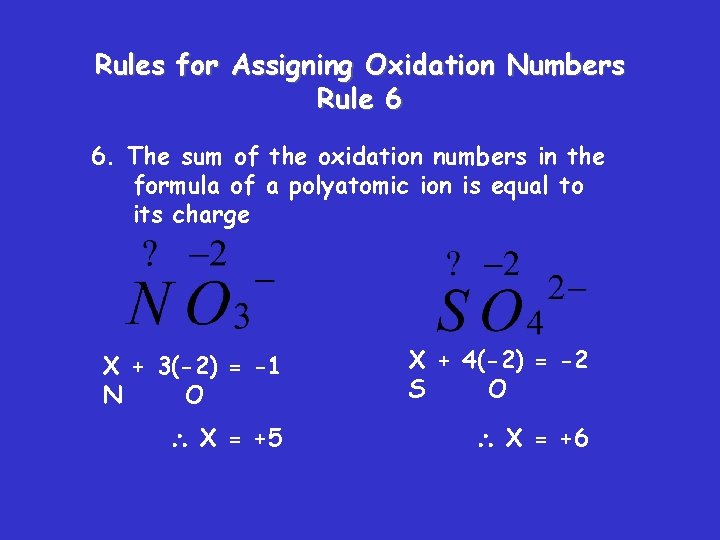
Rules for Assigning Oxidation Numbers Rule 6 6. The sum of the oxidation numbers in the formula of a polyatomic ion is equal to its charge X + 3(-2) = -1 N O X + 4(-2) = -2 S O X = +5 X = +6

Assigning Oxidation Numbers K 2 Cr. O 4 Cr oxidation number = +6 Cr 2 O 3 Cr oxidation number = +3

Trends in Oxidation and Reduction Active metals: Lose electrons easily Are easily oxidized Are strong reducing agents Active nonmetals: Gain electrons easily Are easily reduced Are strong oxidizing agents

Reducing Agents and Oxidizing Agents The substance reduced is the oxidizing agent The substance oxidized is the reducing agent Sodium is oxidized – it is the reducing agent Chlorine is reduced – it is the oxidizing agent

Not All Reactions are Redox Reactions in which there has been no change in oxidation number are not redox rxns. Examples:

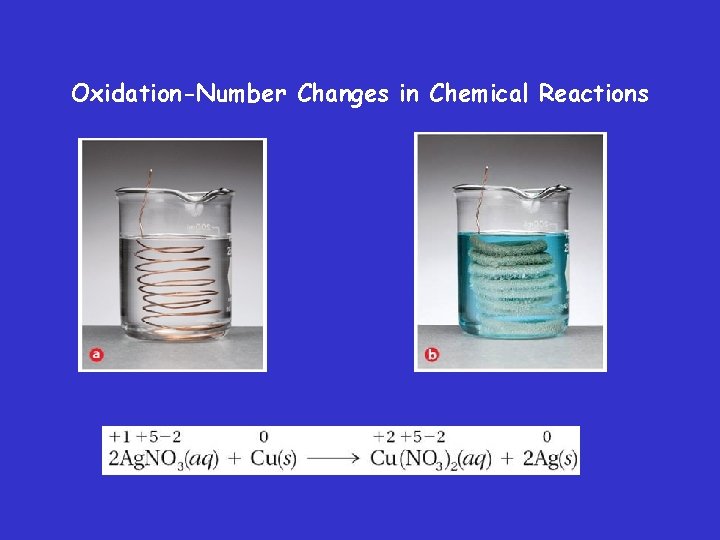
Oxidation-Number Changes in Chemical Reactions


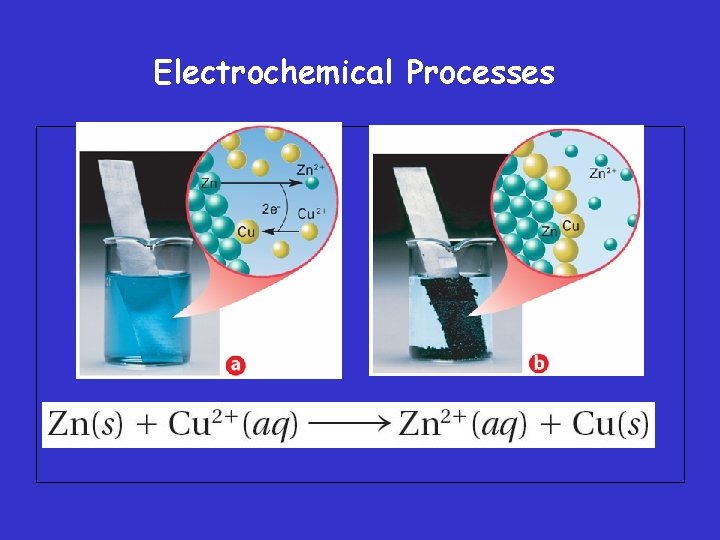
Oxidation-Number Changes in Chemical Reactions • When an iron nail is placed in a copper(II) sulfate solution, the iron reduces Cu 2+ ions in solution and is simultaneously oxidized to Fe 2+ The iron becomes coated with metallic copper.


Electrochemical Processes – For any two metals in an activity series, the more active metal is the more readily oxidized.

Electrochemical Processes

Electrochemical Processes

Electrochemical Processes – Redox Reactions and Electrochemistry • An electrochemical cell involves a chemical reaction and a flow of electrons. There are two types of EC

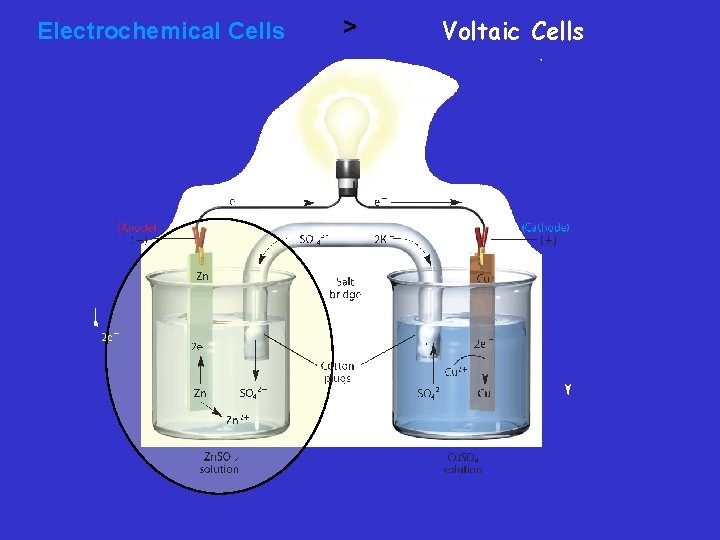
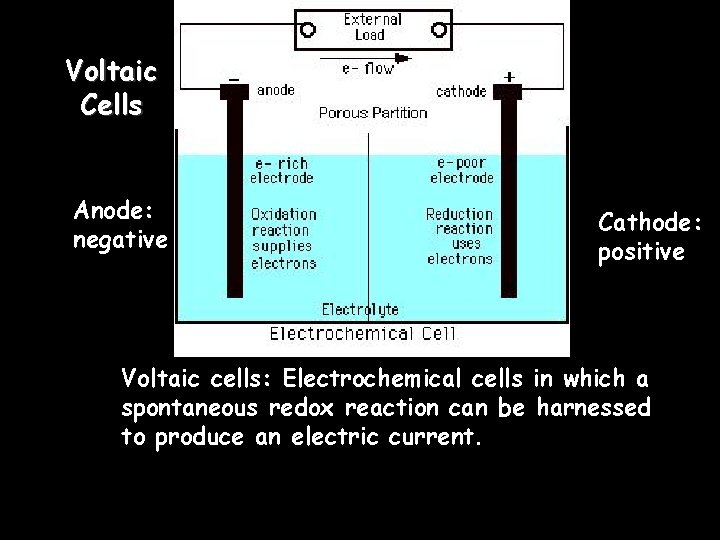
Voltaic Cells • Produces electrical energy by a spontaneous redox reactions within the cell. (flow of electrons) – Voltaic cells (named after their inventor) are electrochemical cells used to convert chemical energy into electrical energy.

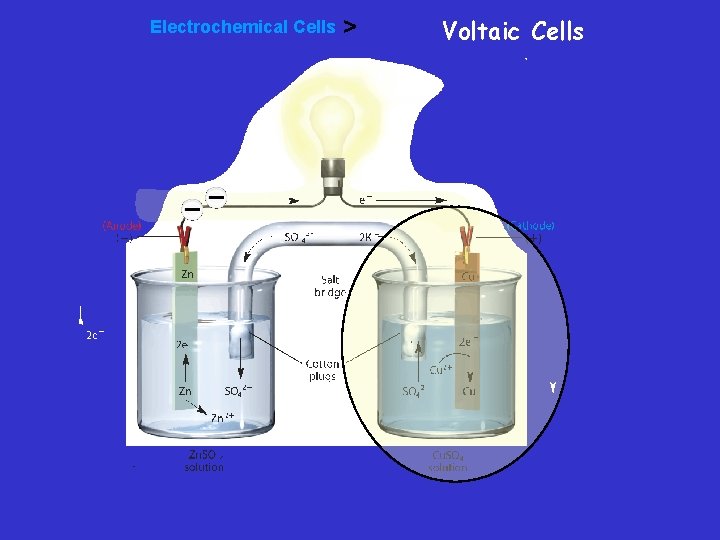
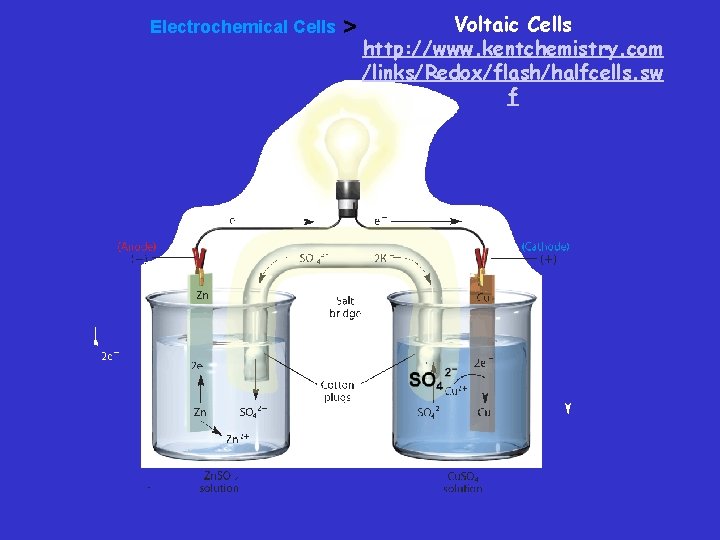
Salt Bridge – Constructing a Voltaic Cell – A half-cell is one part of a voltaic cell in which either oxidation or reduction occurs. – The half-cells are connected by a salt bridge—a tube containing a strong electrolyte, often potassium sulfate (K 2 SO 4). – Salt bridge - Connects two containers and provides a path for the flow of ions between beakers. This completes the circuit and prevents polarization or keeps the solutions neutral

Voltaic Cells • An electrode is the site of oxidation or reduction – The electrode at which oxidation occurs is called the anode. » AN OX – The electrode at which reduction occurs is called the cathode. » RED CAT

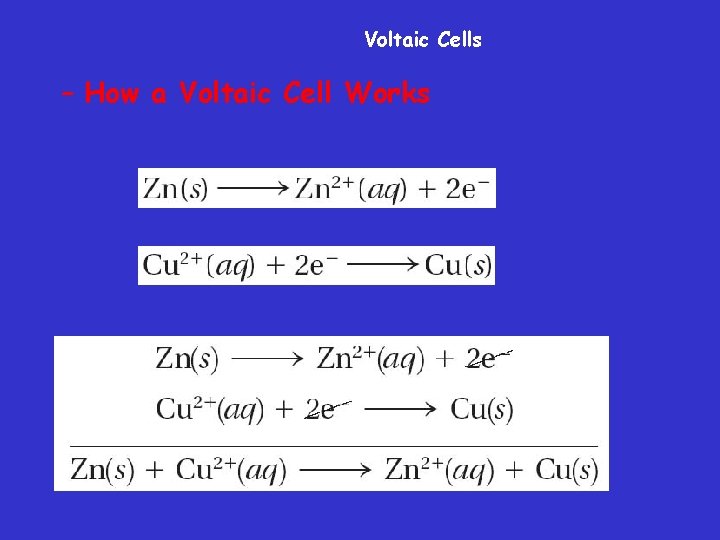
Voltaic Cells – How a Voltaic Cell Works

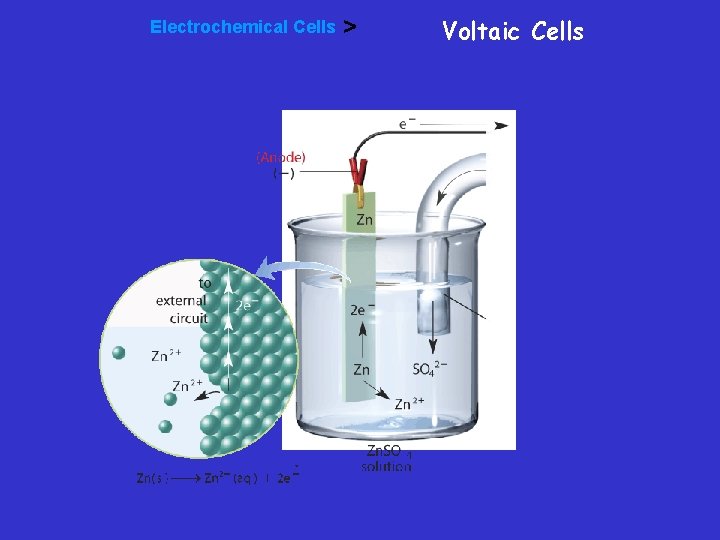
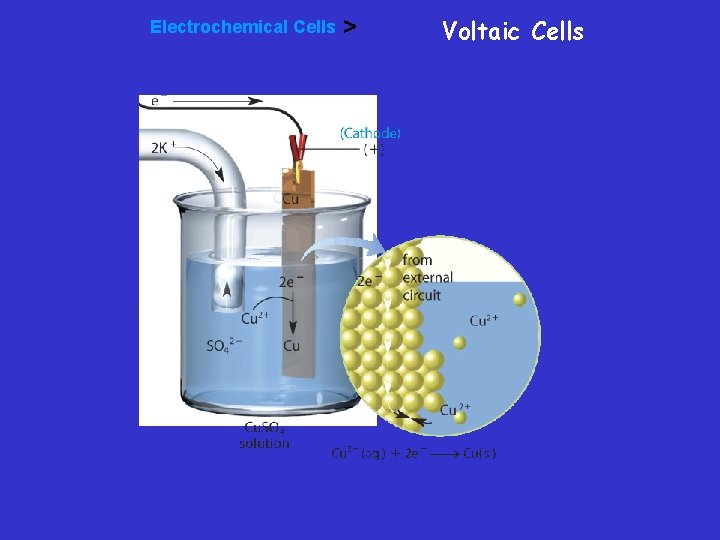
Voltaic cell • In this voltaic cell, the electrons generated from the oxidation of Zn to Zn 2+ flow through the external circuit (the wire) into the copper strip. These electrons reduce the surrounding Cu 2+ to Cu. To maintain neutrality in the electrolytes, anions flow through the salt bridge

Electrochemical Cells > Voltaic Cells

Electrochemical Cells > Voltaic Cells

Electrochemical Cells > Voltaic Cells

Electrochemical Cells > Voltaic Cells

Electrochemical Cells > Voltaic Cells http: //www. kentchemistry. com /links/Redox/flash/halfcells. sw f

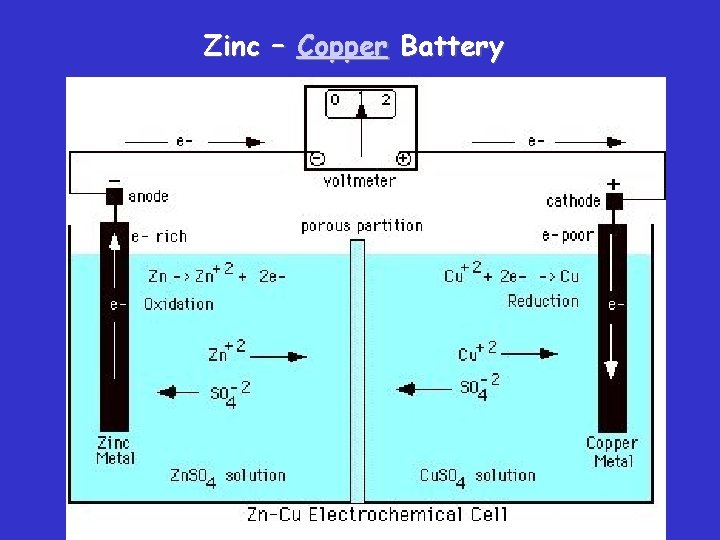
Zinc – Copper Battery

Using Voltaic Cells as Energy Sources – Current technologies that use electrochemical processes to produce electrical energy include dry cells, lead storage batteries, and fuel cells.

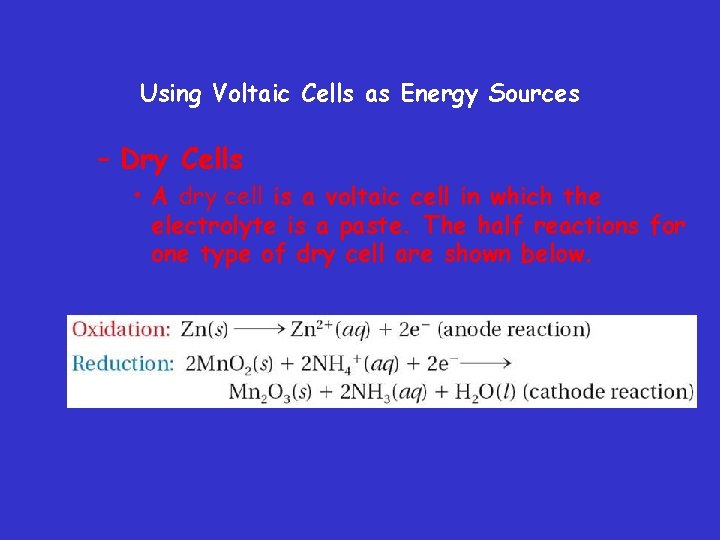
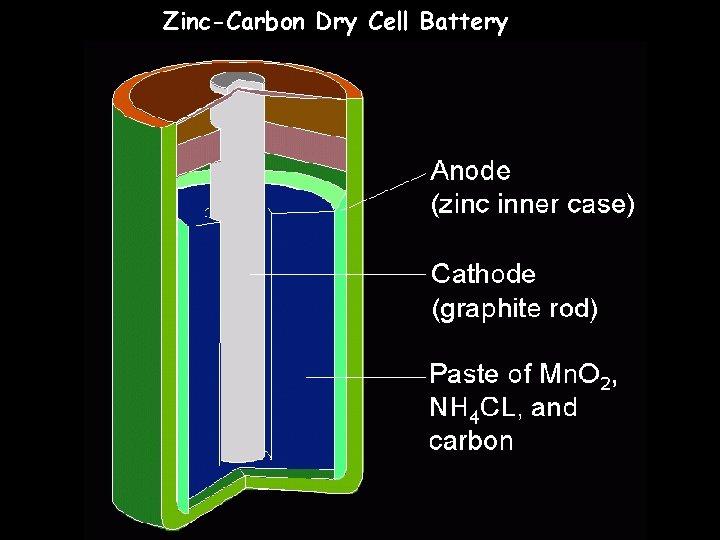
Using Voltaic Cells as Energy Sources – Dry Cells • A dry cell is a voltaic cell in which the electrolyte is a paste. The half reactions for one type of dry cell are shown below.

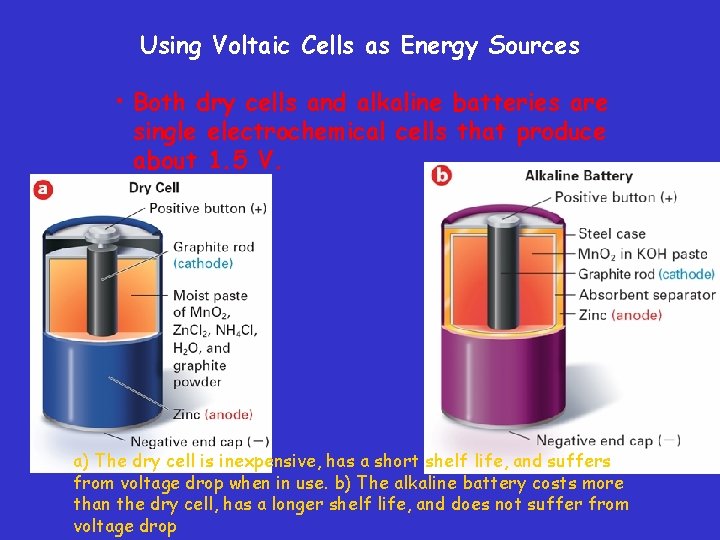
Using Voltaic Cells as Energy Sources • Both dry cells and alkaline batteries are single electrochemical cells that produce about 1. 5 V. a) The dry cell is inexpensive, has a short shelf life, and suffers from voltage drop when in use. b) The alkaline battery costs more than the dry cell, has a longer shelf life, and does not suffer from voltage drop

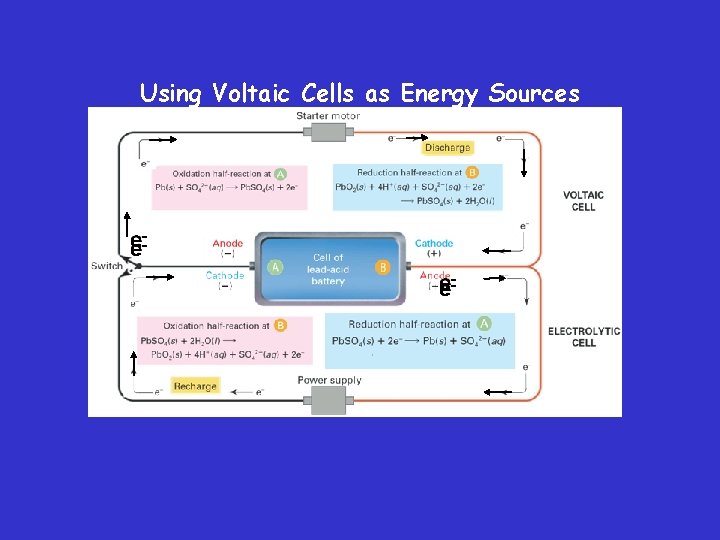
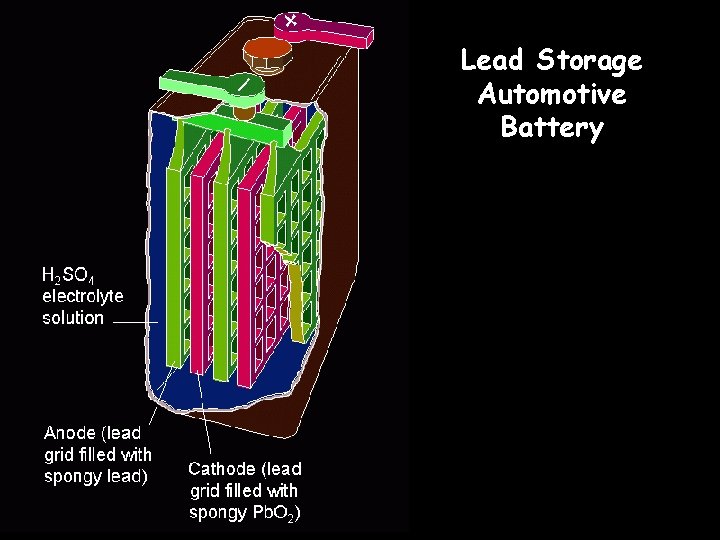
Using Voltaic Cells as Energy Sources – Lead Storage Batteries • A battery is a group of cells connected together. The half-reactions for a lead storage battery are as follows.

Using Voltaic Cells as Energy Sources • A 12 -V car battery consists of six voltaic cells connected together. One cell of a 12 -V lead storage battery is illustrated here.

Using Voltaic Cells as Energy Sources

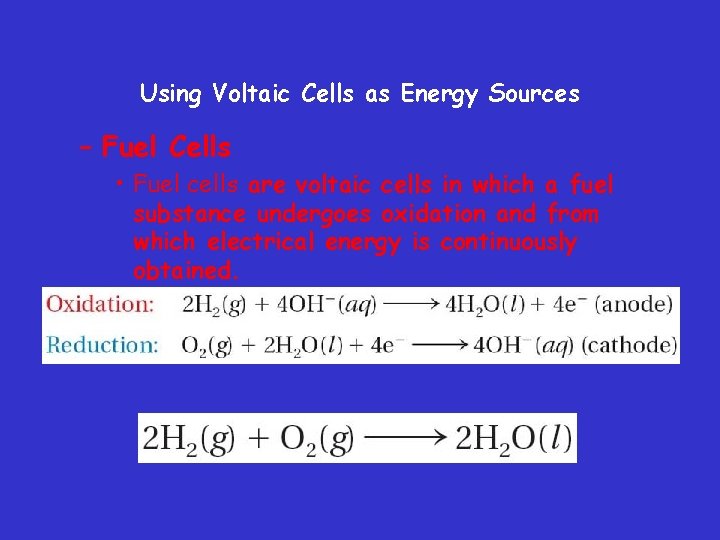
Using Voltaic Cells as Energy Sources – Fuel Cells • Fuel cells are voltaic cells in which a fuel substance undergoes oxidation and from which electrical energy is continuously obtained.

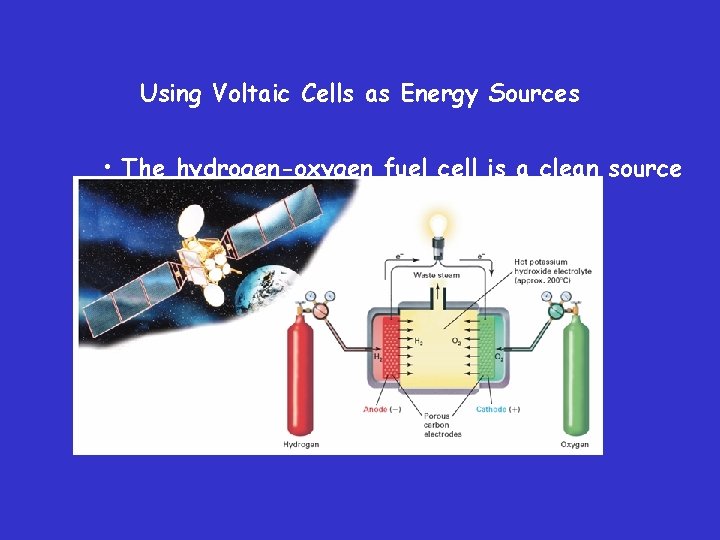
Using Voltaic Cells as Energy Sources • The hydrogen-oxygen fuel cell is a clean source of power. Such cells are often used in spacecraft.

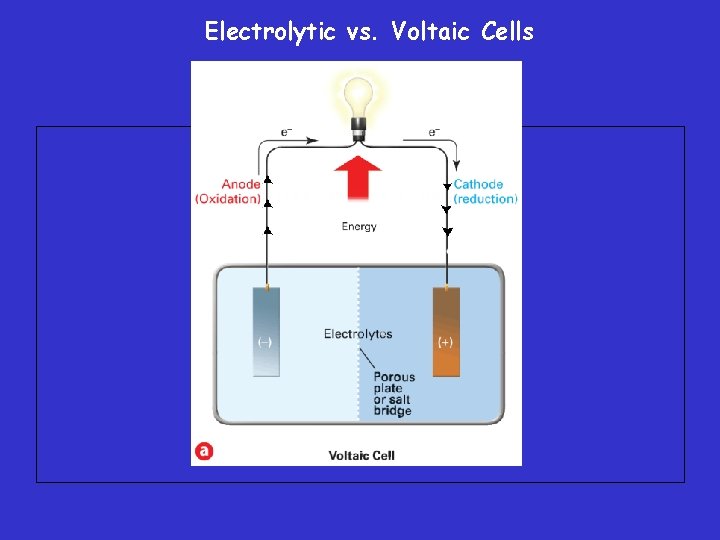
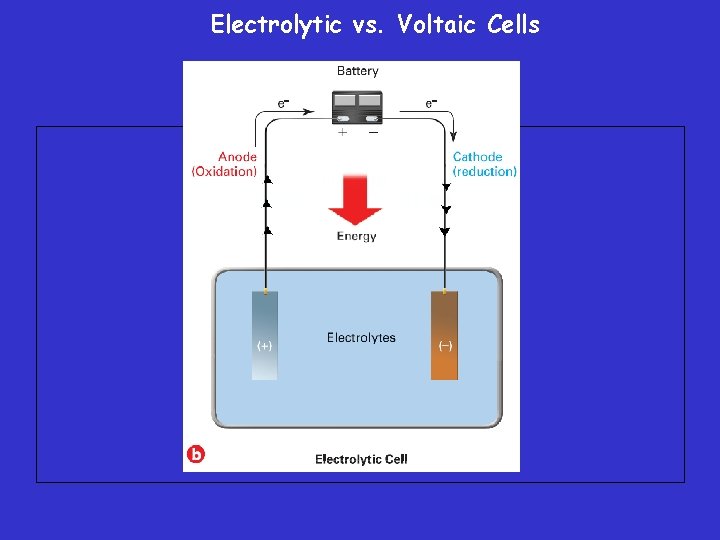
Electrolytic vs. Voltaic Cells • An electrolytic cell is an electrochemical cell used to cause a chemical change through the application of electrical energy. • The process in which electrical energy is used to bring about such a chemical change is called electrolysis.

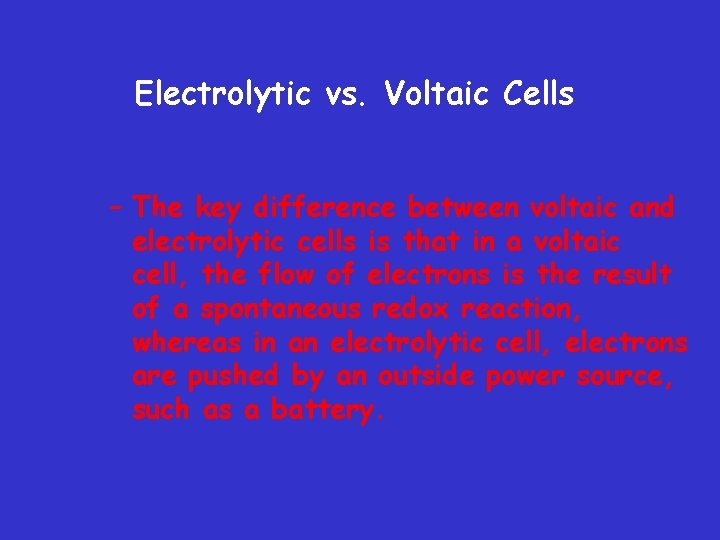
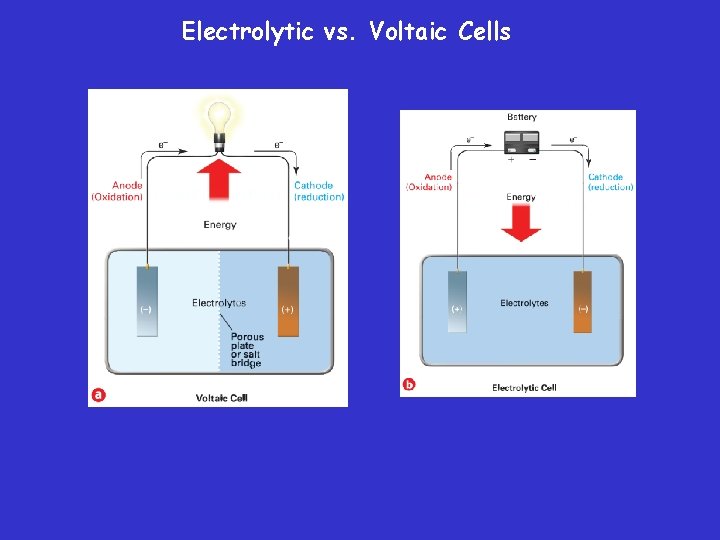
Electrolytic vs. Voltaic Cells – The key difference between voltaic and electrolytic cells is that in a voltaic cell, the flow of electrons is the result of a spontaneous redox reaction, whereas in an electrolytic cell, electrons are pushed by an outside power source, such as a battery.

Electrolytic vs. Voltaic Cells

Electrolytic vs. Voltaic Cells

Electrolytic vs. Voltaic Cells

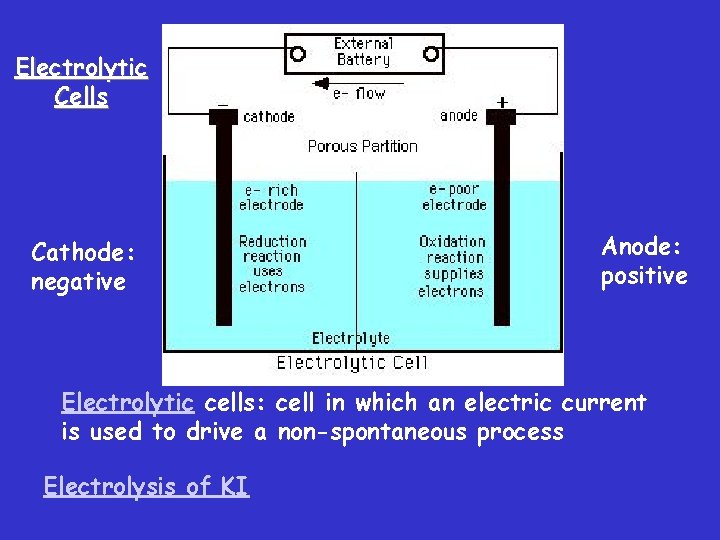
Electrolytic Cells Cathode: negative Anode: positive Electrolytic cells: cell in which an electric current is used to drive a non-spontaneous process Electrolysis of KI

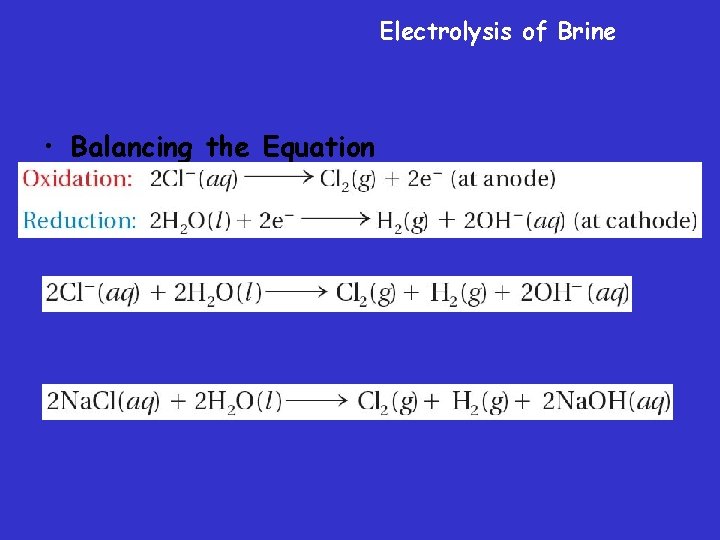
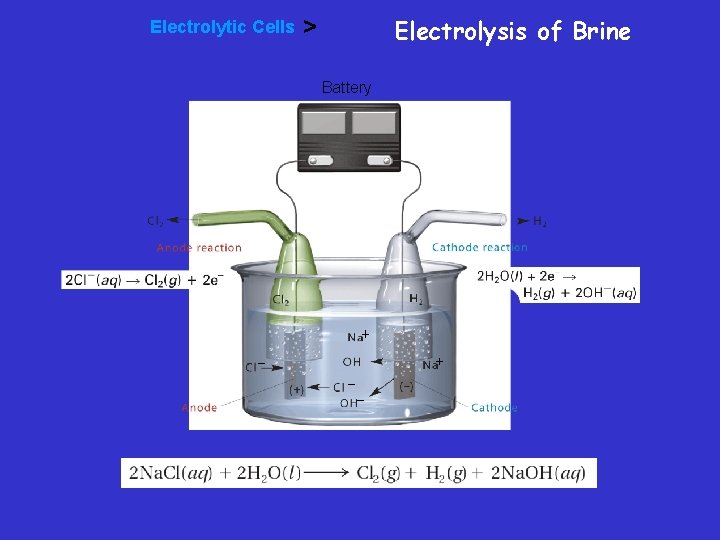
Electrolysis of Brine – During electrolysis of brine, chloride ions are oxidized to produce chlorine gas at the anode. Water is reduced to produce hydrogen gas at the cathode.

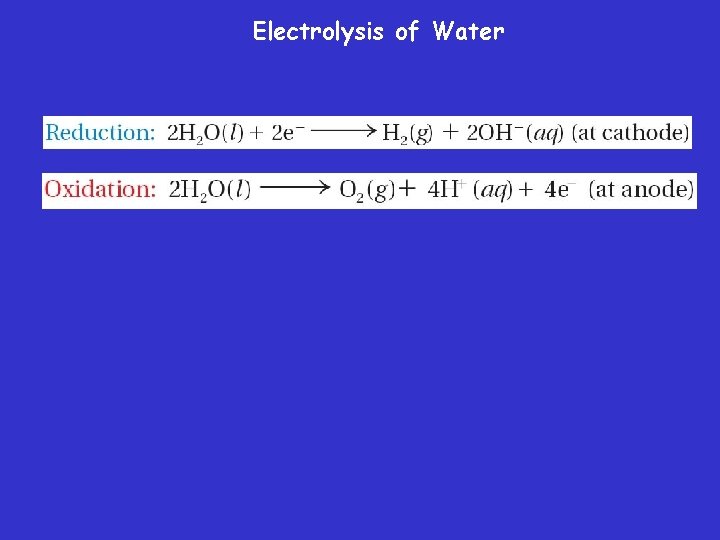
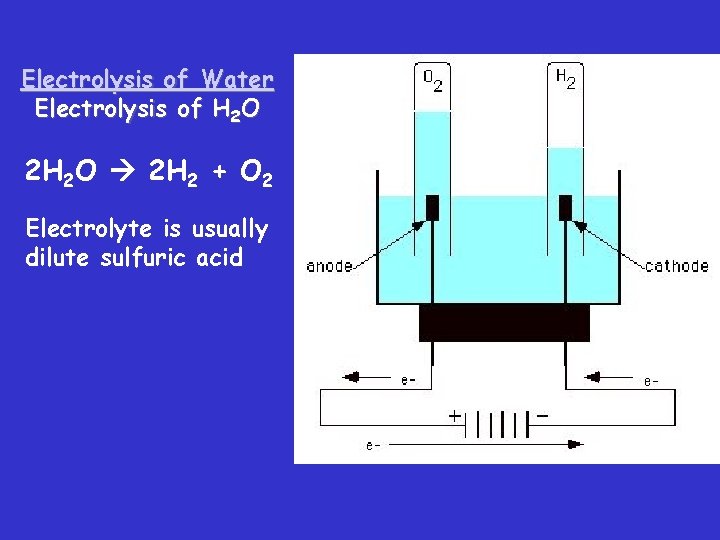
Electrolysis of Water

Electrolysis of Water Electrolysis of H 2 O 2 H 2 O 2 H 2 + O 2 Electrolyte is usually dilute sulfuric acid

Electrolysis of Brine • Balancing the Equation

Electrolytic Cells Electrolysis of Brine > Battery

Electrolysis of Brine • To produce chlorine and sodium hydroxide in electrolytic cells, electricity is passed through brine, a sodium chloride solution.

Using Electrolysis in Metal Processing – Electrolytic cells are commonly used in the plating, purifying, and refining of metals.

Using Electrolysis in Metal Processing – Electroplating is the deposition of a thin layer of a metal on an object in an electrolytic cell. – Protects the surface of the base metal from corrosion or to make it more attractive – Metals used for plating » Au, Ni, Cr, Cu, Ag

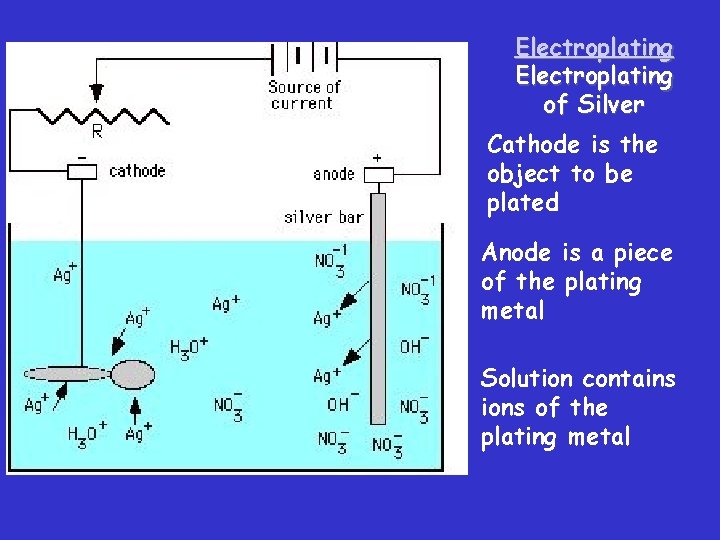
Electroplating of Silver Cathode is the object to be plated Anode is a piece of the plating metal Solution contains ions of the plating metal

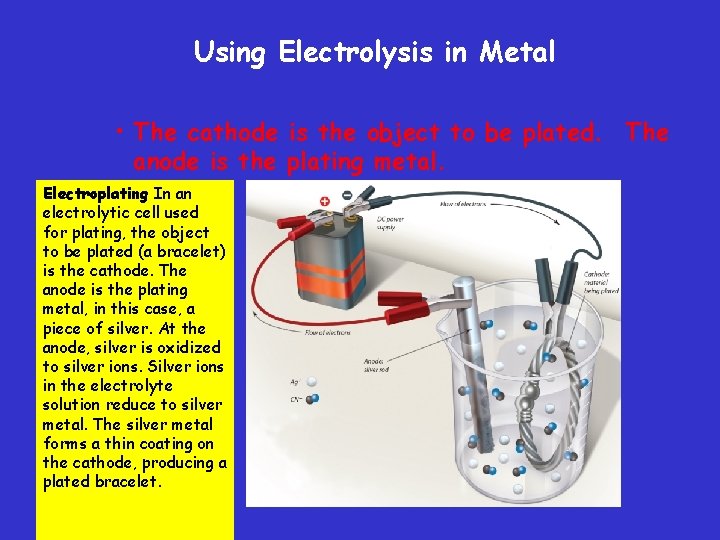
Using Electrolysis in Metal • The cathode is the object to be plated. The anode is the plating metal. Electroplating In an electrolytic cell used for plating, the object to be plated (a bracelet) is the cathode. The anode is the plating metal, in this case, a piece of silver. At the anode, silver is oxidized to silver ions. Silver ions in the electrolyte solution reduce to silver metal. The silver metal forms a thin coating on the cathode, producing a plated bracelet.

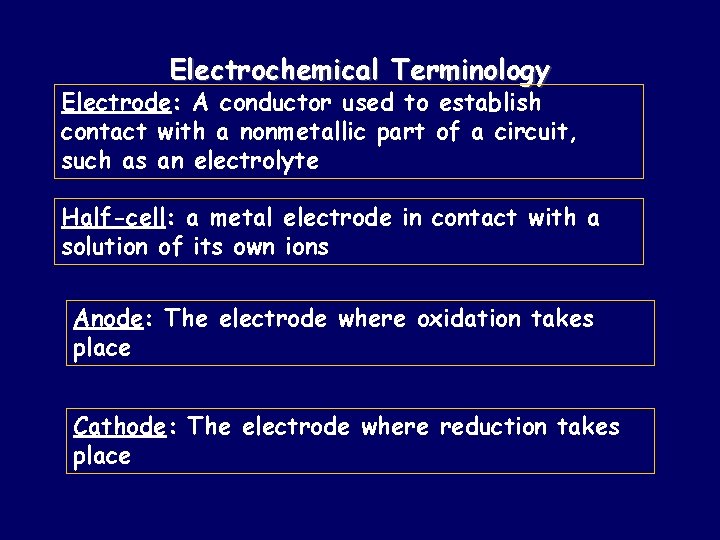
Electrochemical Terminology Electrode: A conductor used to establish contact with a nonmetallic part of a circuit, such as an electrolyte Half-cell: a metal electrode in contact with a solution of its own ions Anode: The electrode where oxidation takes place Cathode: The electrode where reduction takes place

Voltaic Cells Anode: negative Cathode: positive Voltaic cells: Electrochemical cells in which a spontaneous redox reaction can be harnessed to produce an electric current.

Zinc-Carbon Dry Cell Battery

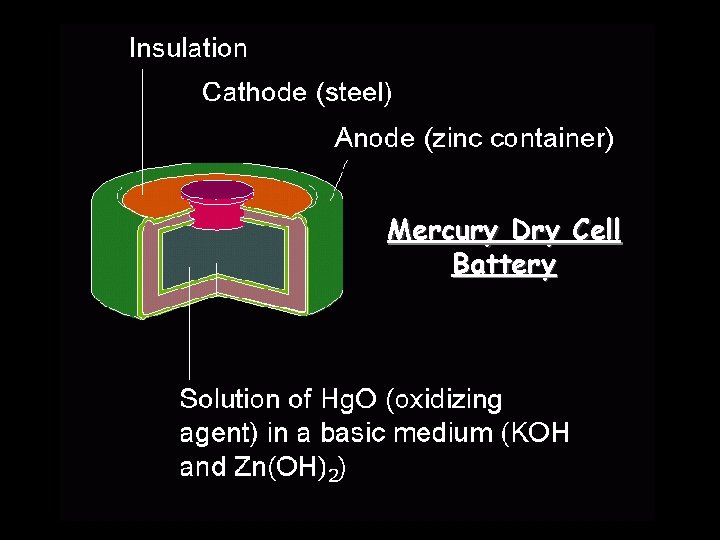
Mercury Dry Cell Battery

Lead Storage Automotive Battery

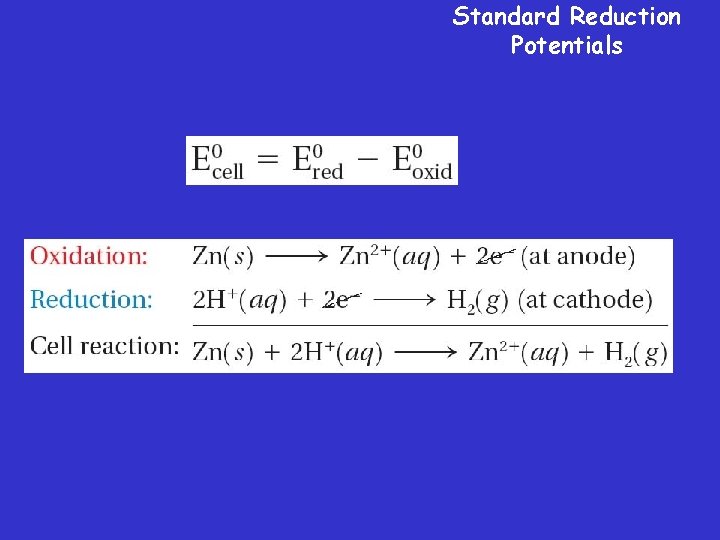
Standard Reduction Potentials

Virtual Labs • Voltaic Cell Virtual Lab • http: //www. kentchemistry. com/movies files/Units/Redox/electrolysis 10. swf

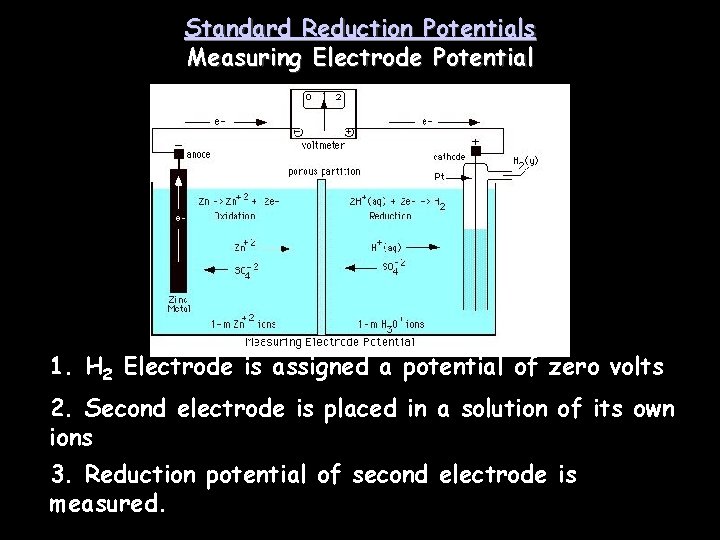
Standard Reduction Potentials Measuring Electrode Potential 1. H 2 Electrode is assigned a potential of zero volts 2. Second electrode is placed in a solution of its own ions 3. Reduction potential of second electrode is measured.

Electrical Potential • The electrical potential of a cell results from a competition for electrons between two halfcells. » The electrical potential of a voltaic cell is a measure of the cell’s ability to produce an electric current. » The tendency of a given half-reaction to occur as a reduction is called the reduction potential.

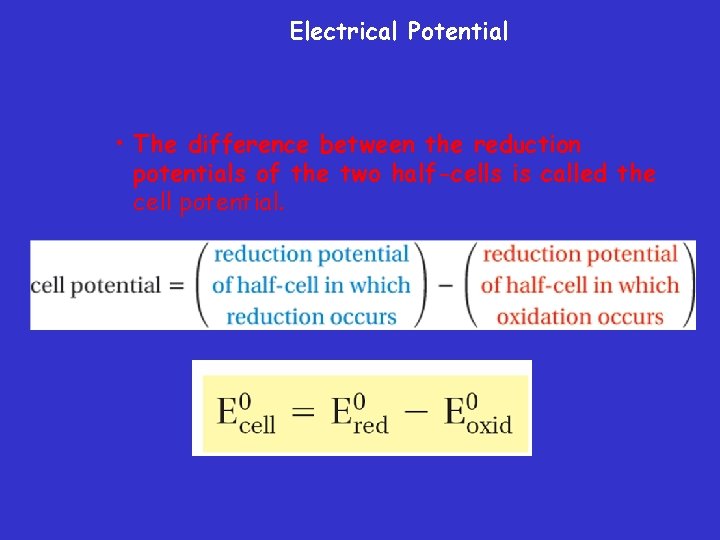
Electrical Potential • The difference between the reduction potentials of the two half-cells is called the cell potential.

Electrical Potential • A working voltaic cell can be constructed using a lemon and strips of copper and zinc.

Standard Cell Potential • The standard cell potential (Eo ) is the measured cell potential when the ion concentrations in the half-cells are 1 M, any gases are at a pressure of 101 k. Pa, and the temperature is 25°C.

Standard Cell Potential • The standard hydrogen electrode is used with other electrodes so the reduction potentials of the other cells can be measured.

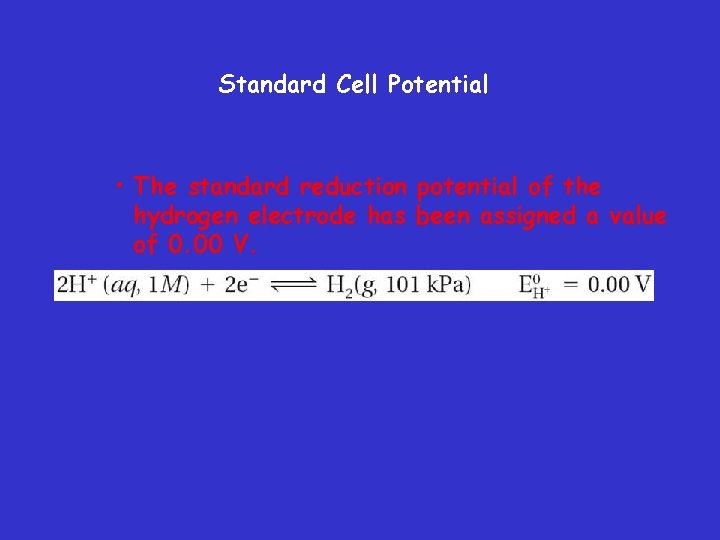
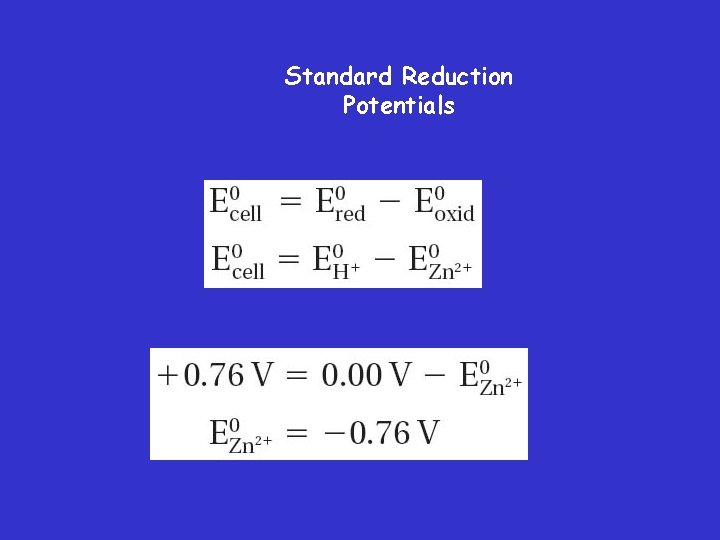
Standard Cell Potential • The standard reduction potential of the hydrogen electrode has been assigned a value of 0. 00 V.

Standard Reduction Potentials • You can determine the standard reduction potential of a half-cell by using a standard hydrogen electrode and the equation for standard cell potential.

Standard Reduction Potentials • Voltaic Cell

Standard Reduction Potentials

Standard Reduction Potentials

Calculating Standard Cell Potentials – If the cell potential for a given redox reaction is positive, then the reaction is spontaneous as written. If the cell potential is negative, then the reaction is nonspontaneous.

Calculating Standard Cell Potentials

Calculating Standard Cell Potentials