Oxidizing Reducing Agents Oxidizing Agents An oxidizing agent


- Slides: 11

Oxidizing & Reducing Agents

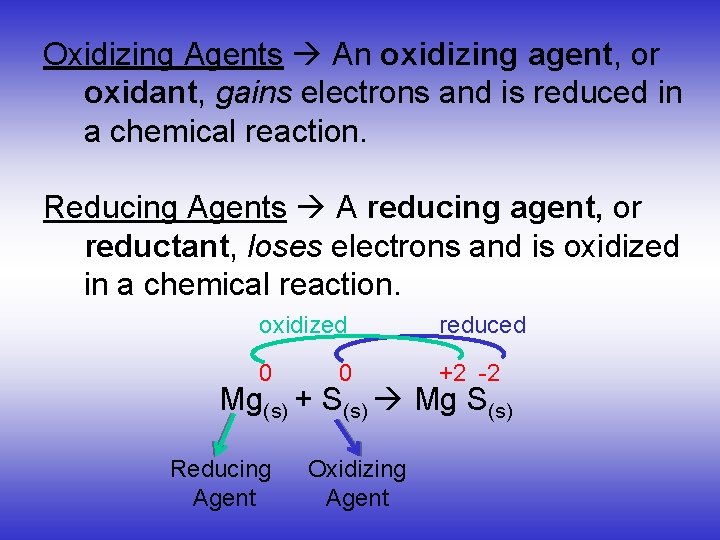
Oxidizing Agents An oxidizing agent, or oxidant, gains electrons and is reduced in a chemical reaction. Reducing Agents A reducing agent, or reductant, loses electrons and is oxidized in a chemical reaction. oxidized reduced 0 +2 -2 Mg(s) + S(s) Mg S(s) Reducing Agent Oxidizing Agent


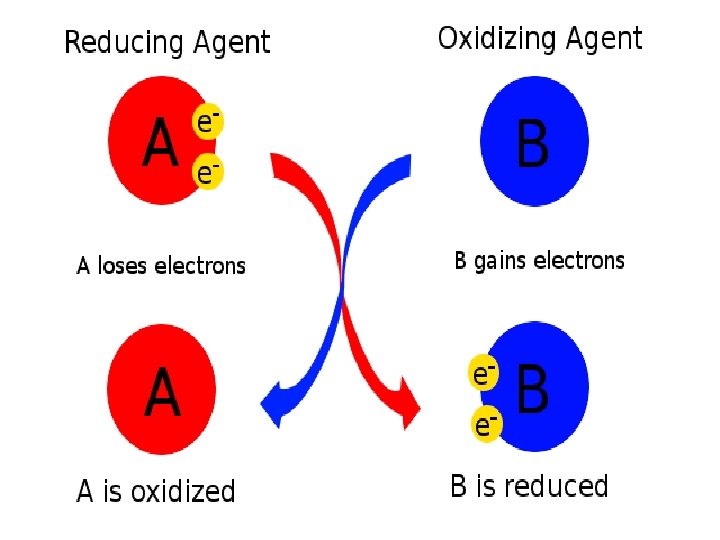
GER Gains Electrons Reduction LEO Loses Electrons Oxidation r. OAR Oxidizing Agent Reduced

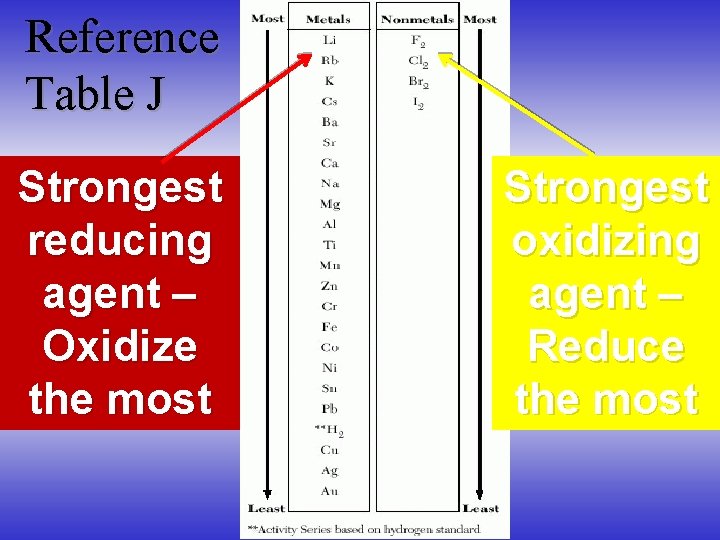
Reference Table J Strongest reducing agent – Oxidize the most Strongest oxidizing agent – Reduce the most

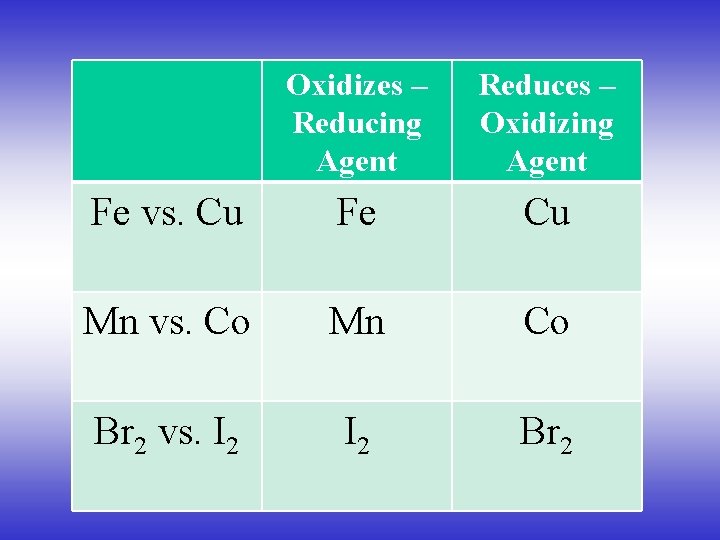
Oxidizes – Reducing Agent Reduces – Oxidizing Agent Fe vs. Cu Fe Cu Mn vs. Co Mn Co Br 2 vs. I 2 Br 2

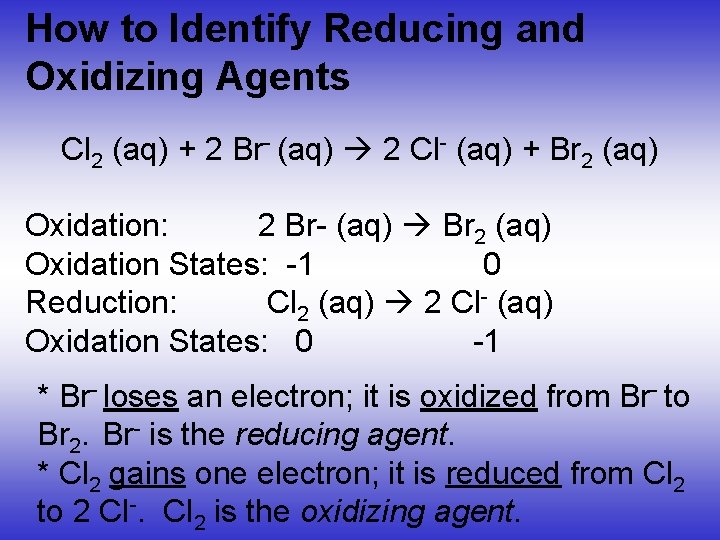
How to Identify Reducing and Oxidizing Agents Cl 2 (aq) + 2 Br- (aq) 2 Cl- (aq) + Br 2 (aq) Oxidation: 2 Br- (aq) Br 2 (aq) Oxidation States: -1 0 Reduction: Cl 2 (aq) 2 Cl- (aq) Oxidation States: 0 -1 * Br- loses an electron; it is oxidized from Br- to Br 2. Br- is the reducing agent. * Cl 2 gains one electron; it is reduced from Cl 2 to 2 Cl-. Cl 2 is the oxidizing agent.

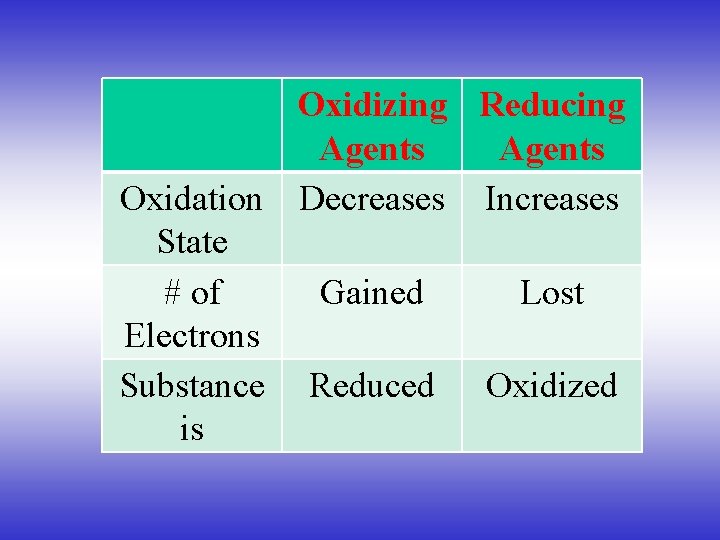
Oxidizing Reducing Agents Oxidation Decreases Increases State # of Gained Lost Electrons Substance Reduced Oxidized is

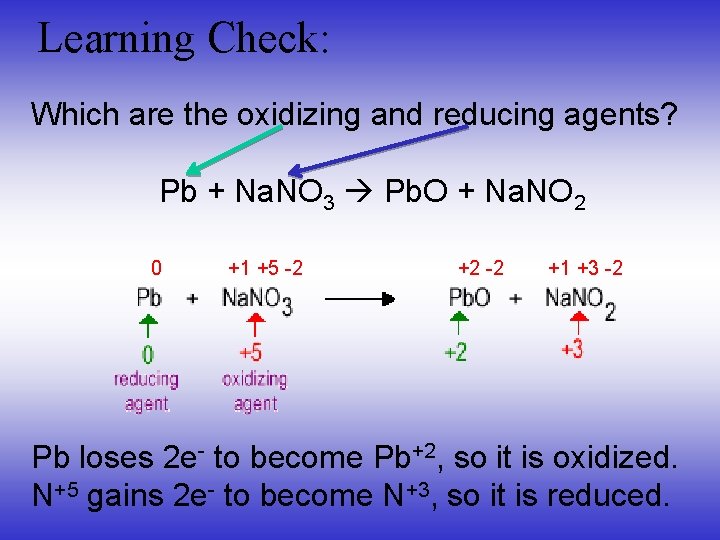
Learning Check: Which are the oxidizing and reducing agents? Pb + Na. NO 3 Pb. O + Na. NO 2 0 +1 +5 -2 +2 -2 +1 +3 -2 Pb loses 2 e- to become Pb+2, so it is oxidized. N+5 gains 2 e- to become N+3, so it is reduced.

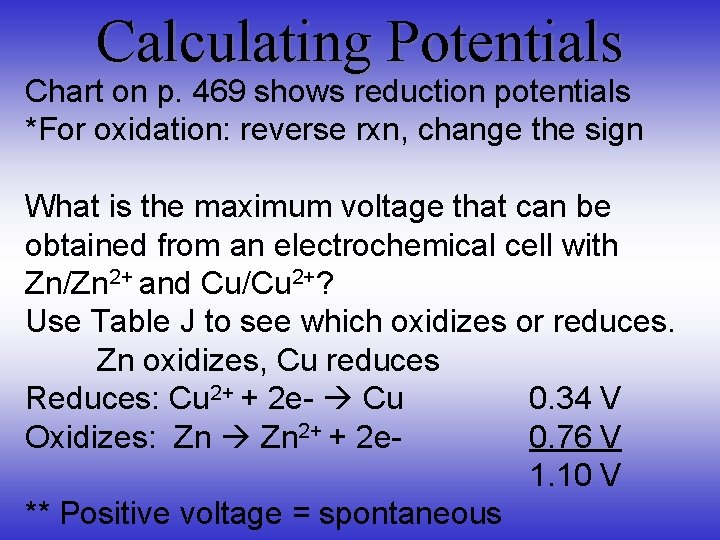
Calculating Potentials Chart on p. 469 shows reduction potentials *For oxidation: reverse rxn, change the sign What is the maximum voltage that can be obtained from an electrochemical cell with Zn/Zn 2+ and Cu/Cu 2+? Use Table J to see which oxidizes or reduces. Zn oxidizes, Cu reduces Reduces: Cu 2+ + 2 e- Cu 0. 34 V Oxidizes: Zn 2+ + 2 e- 0. 76 V 1. 10 V ** Positive voltage = spontaneous

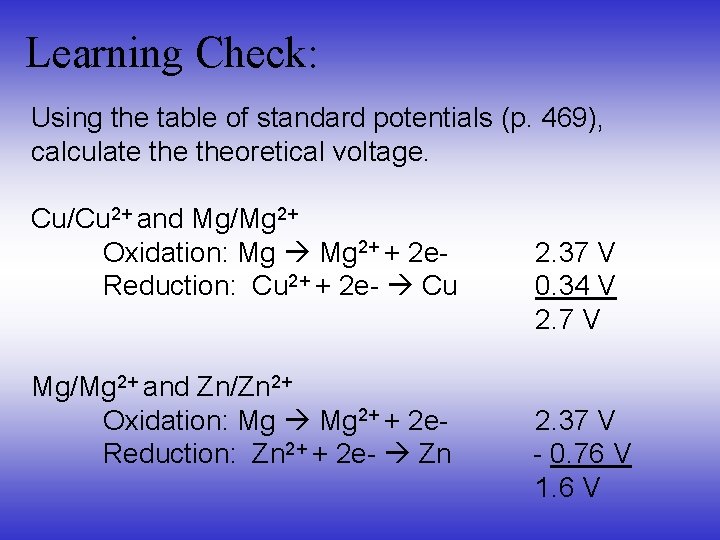
Learning Check: Using the table of standard potentials (p. 469), calculate theoretical voltage. Cu/Cu 2+ and Mg/Mg 2+ Oxidation: Mg 2+ + 2 e. Reduction: Cu 2+ + 2 e- Cu 2. 37 V 0. 34 V 2. 7 V Mg/Mg 2+ and Zn/Zn 2+ Oxidation: Mg 2+ + 2 e 2. 37 V Reduction: Zn 2+ + 2 e- Zn - 0. 76 V 1. 6 V