Relative Strengths of Oxidizing and Reducing Agents metals

- Slides: 12

Relative Strengths of Oxidizing and Reducing Agents

metals: lose electrons and are good reducing agents. non-metals: gain electrons and are good oxidizing agents.

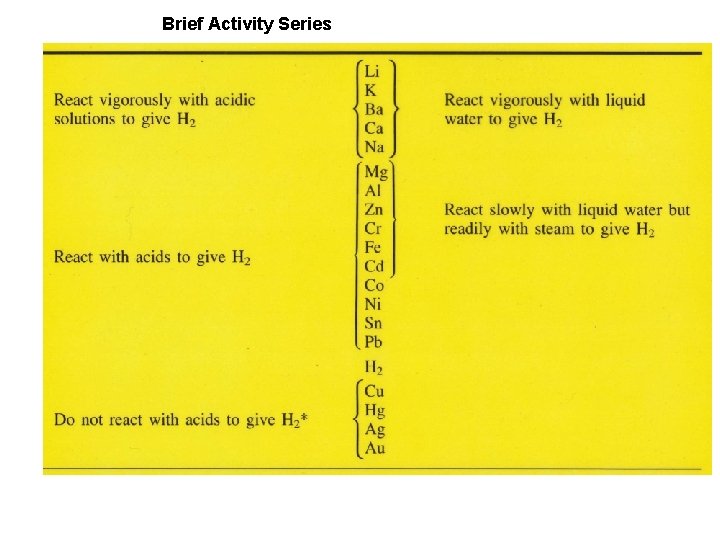
Brief Activity Series

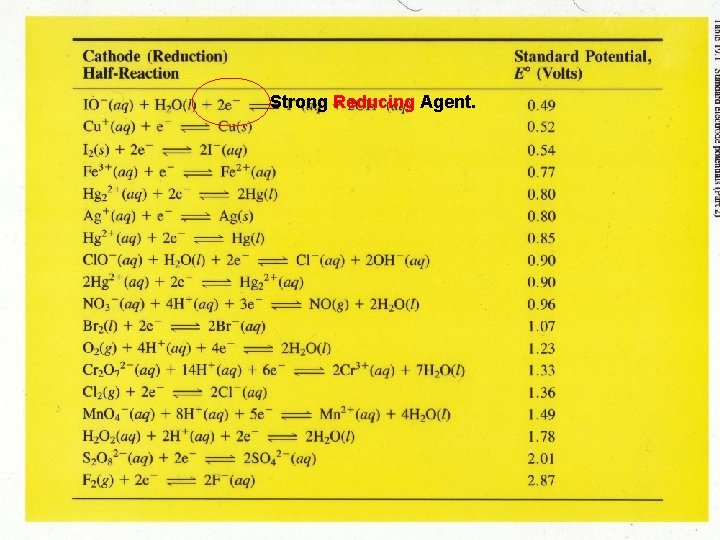
Strong Reducing Agent.

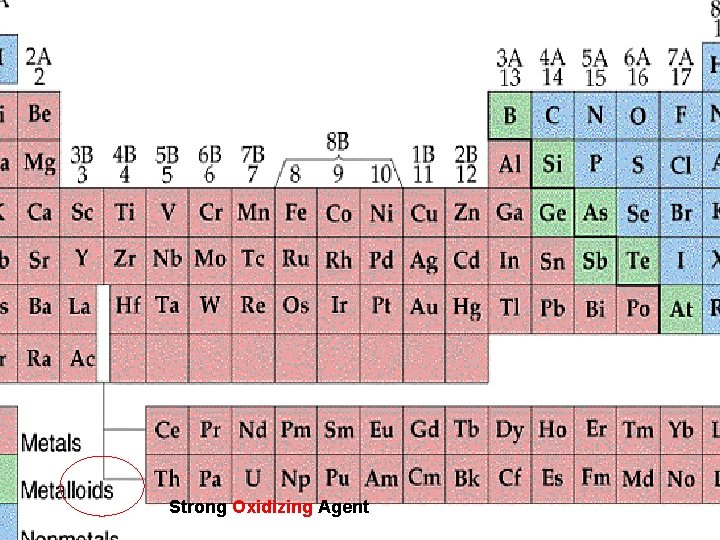
Strong Oxidizing Agent

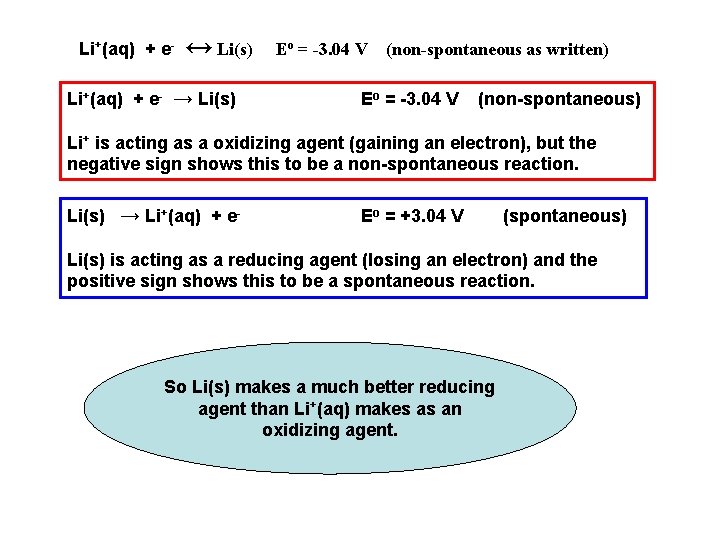
Li+(aq) + e- ↔ Li(s) Li+(aq) + e- → Li(s) Eo = -3. 04 V (non-spontaneous as written) Eo = -3. 04 V (non-spontaneous) Li+ is acting as a oxidizing agent (gaining an electron), but the negative sign shows this to be a non-spontaneous reaction. Li(s) → Li+(aq) + e- Eo = +3. 04 V (spontaneous) Li(s) is acting as a reducing agent (losing an electron) and the positive sign shows this to be a spontaneous reaction. So Li(s) makes a much better reducing agent than Li+(aq) makes as an oxidizing agent.

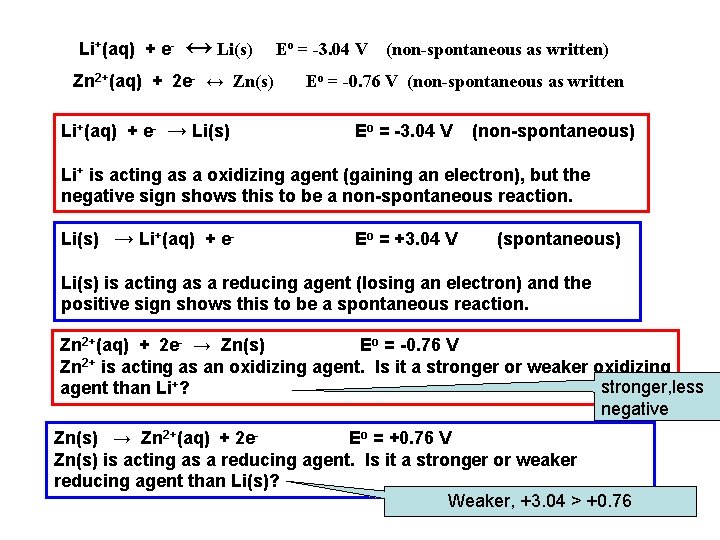
Li+(aq) + e- ↔ Li(s) Zn 2+(aq) + 2 e- ↔ Zn(s) Li+(aq) + e- → Li(s) Eo = -3. 04 V (non-spontaneous as written) Eo = -0. 76 V (non-spontaneous as written Eo = -3. 04 V (non-spontaneous) Li+ is acting as a oxidizing agent (gaining an electron), but the negative sign shows this to be a non-spontaneous reaction. Li(s) → Li+(aq) + e- Eo = +3. 04 V (spontaneous) Li(s) is acting as a reducing agent (losing an electron) and the positive sign shows this to be a spontaneous reaction. Zn 2+(aq) + 2 e- → Zn(s) Eo = -0. 76 V Zn 2+ is acting as an oxidizing agent. Is it a stronger or weaker oxidizing stronger, less agent than Li+? negative Zn(s) → Zn 2+(aq) + 2 e. Eo = +0. 76 V Zn(s) is acting as a reducing agent. Is it a stronger or weaker reducing agent than Li(s)? Weaker, +3. 04 > +0. 76

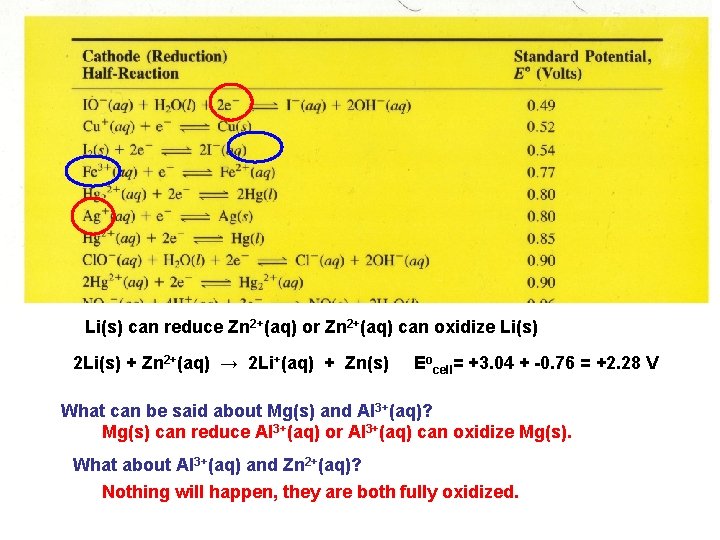
Li(s) can reduce Zn 2+(aq) or Zn 2+(aq) can oxidize Li(s) 2 Li(s) + Zn 2+(aq) → 2 Li+(aq) + Zn(s) Eocell= +3. 04 + -0. 76 = +2. 28 V What can be said about Mg(s) and Al 3+(aq)? Mg(s) can reduce Al 3+(aq) or Al 3+(aq) can oxidize Mg(s). What about Al 3+(aq) and Zn 2+(aq)? Nothing will happen, they are both fully oxidized.


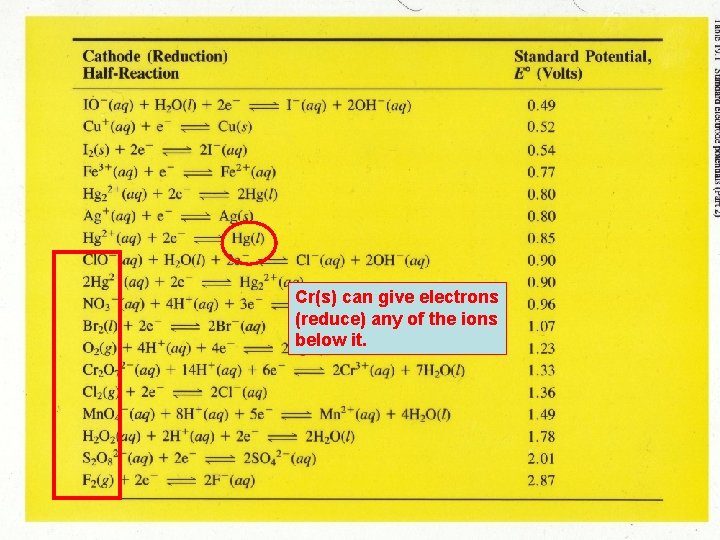
Cr(s) can give electrons (reduce) any of the ions below it.

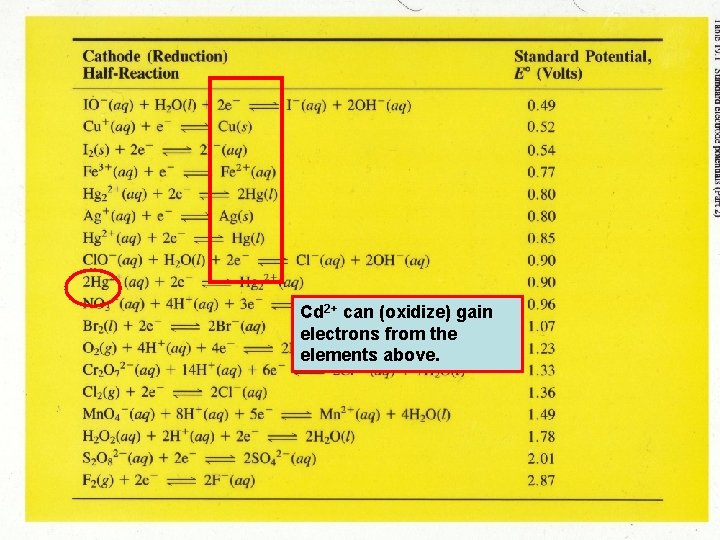
Cd 2+ can (oxidize) gain electrons from the elements above.
