Introduction s Chlorine is a powerful oxidizing agent


































- Slides: 41


Introduction s Chlorine is a powerful oxidizing agent. This means that it chemically oxidizes or “burns up” those materials it comes into contact with. s In this way it is acting the same way oxygen would in a chemical reaction.

Introduction n It is this property of chlorine that it makes it a powerful disinfectant. In water, regardless of the source chlorine forms two compounds, hypochlorus acid (HOCl) and chlorite ion (OCL-). Hypochlorus acid is the compound that is a powerful disinfectant, chlorite ion is not.

s The balance between chlorite ions and hypochlorus acid is dependent on the p. H of the solution. s Hypochlorus acid is also know as “Available Chlorine”. s The optimum p. H for chlorine applications is between 6. 5 and 7. 0. s Below 6 - gas is released into the air. s At 9 almost no chlorine is available.

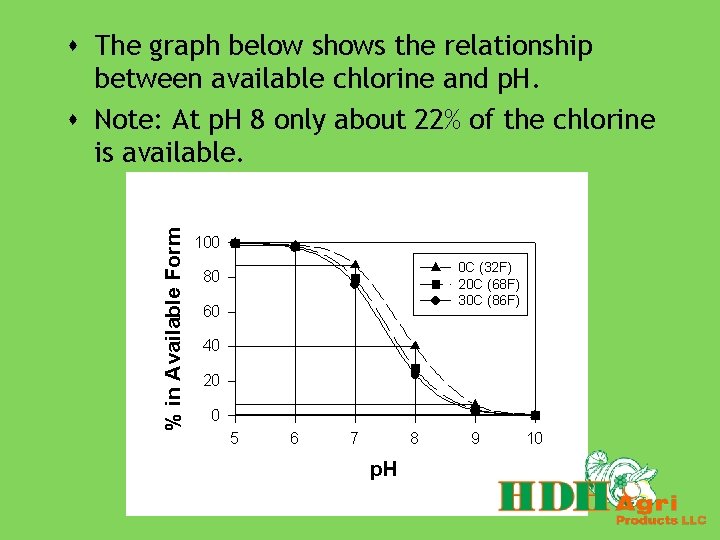
s The graph below shows the relationship between available chlorine and p. H. s Note: At p. H 8 only about 22% of the chlorine is available.

p. H § As supplied the p. H of Sodium hypochlorite is about 10. 0. § When diluted the p. H will be between 8 -9. § It is necessary to adjust the p. H to about 7. 0 § Several acids can be used to accomplish this.

Acids w Citric w Hydrochloric (Muriatic) w Carbon Dioxide w Phosphoric

Hydrochloric Acid • Also known as Muriatic Acid. • Usually supplied as 20% Solution (6. 0 N). • Gives of fumes that are hazardous to humans and corrosive to metals. • Requires 1. 4 gallons to replace 1 gallon of 24% Phosphoric Acid.

Citric Acid • Common Food Acid • Supplied as 30% Solution (5. 3 N). • Best with systems using Calcium Hypochlorite Tablets. • Requires 1. 6 gallons to replace one gallon of 24% Phosphoric Acid. • May interfere with the lethal action of hypochlorus acid at some levels*. * Suslow, Trevor V. 2004. Oxidation-Reduction Potential (ORP) for water disinfection Monitoring, Control, and Documentation. Univ. Cal. Div. Agr. Nat. Resources. Pub. 8149.

Carbon Dioxide § Supplied as a gas in cylinders. § Requires complex gas handling system for use. § Broken cylinders will release a suffocating gas. § Approximately 3 - 150 lb cylinders replace one drum of 24% Phosphoric Acid.

Phosphoric Acid l. A food grade acid. l Does not release hazardous gasses. l Phosphates tend to passivate mild steel and iron. l Available in 24% (8. 5 N) and 85% (30. 1 N).

Chlorine in Processing ü 1) 2) 3) 4) Chlorine use is dependent upon several factors. Produce treated. Temperature of process water. Organisms to be controlled. Legal restraints.

Controlling the Application With concentration, time and temperature determined, the next important consideration is control. There are two methods to control chlorine applications 1. Manual control by the use of test kits, test papers or hand held instruments. 2. Use automatic controllers. ORP and p. H.

Manual Control Testing • There are three methods to test for chlorine concentration in process waters manually. I. The quick dip test using test papers. II. The vacuette test - A color comparison method. III. By the use of hand held instruments

Test Paper • Based on Iodine starch reaction. • Paper impregnated with Potassium Iodide and Starch. • Available Chlorine frees Iodine which reacts with starch producing a blue-black color. • Color concentration is related to amount of chlorine. • Range 0 - 200 ppm. (Estimated)

Test Paper § § § § Quick and easy. 1 - 2” strip. Dip 5 seconds. Compare to chart. Upper @ 50 ppm Lower 200 ppm + Answer is estimate.

Vacuette® Kit The kit comes with a color standard and a box of Vacuetts® In addition there a”Snapper” cup, a micro tube, and a capillary tube attached to the ampoule.

Vacuette Test Kit • • Based on DDPD reaction with Hypochlorus Acid. VACUette ampoule filled with reagent. Micro sample used. VACUette ampoule automatically draws sample and dilution water into test reagent. • Blue color develops in 1 minute. • Color concentration is related to amount of chlorine. • Range 0 - 250 ppm. (Reasonably Accurate)

VACUette® Procedure n Fill Diluter-Snapper cup with chlorine free water. n Use bottled drinking, distilled or purified water.

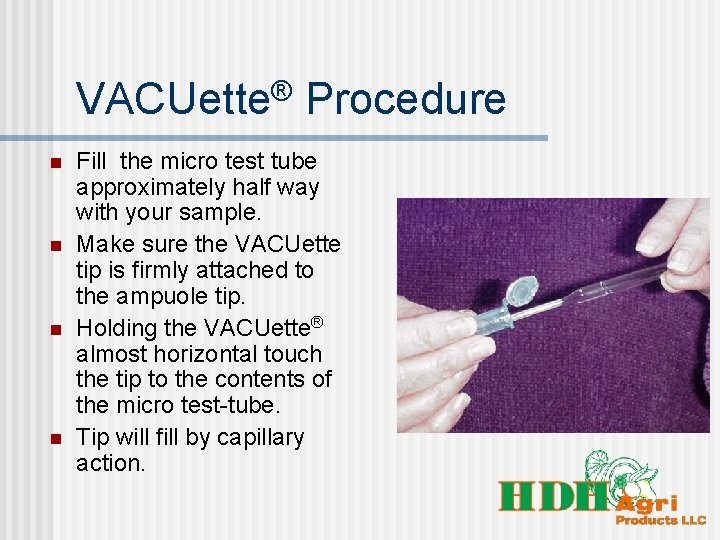
VACUette® Procedure n n Fill the micro test tube approximately half way with your sample. Make sure the VACUette tip is firmly attached to the ampuole tip. Holding the VACUette® almost horizontal touch the tip to the contents of the micro test-tube. Tip will fill by capillary action.

VACUette® Procedure n n n Place the VACUette® in the snapper cup and snap the tip of the ampoule. Mix and wait one minute. NOTE: A blue color begins to develop immediately.

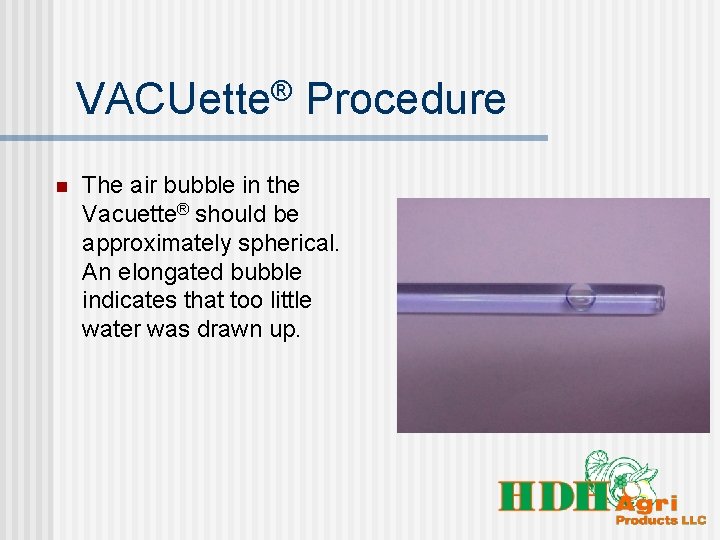
VACUette® Procedure n The air bubble in the Vacuette® should be approximately spherical. An elongated bubble indicates that too little water was drawn up.

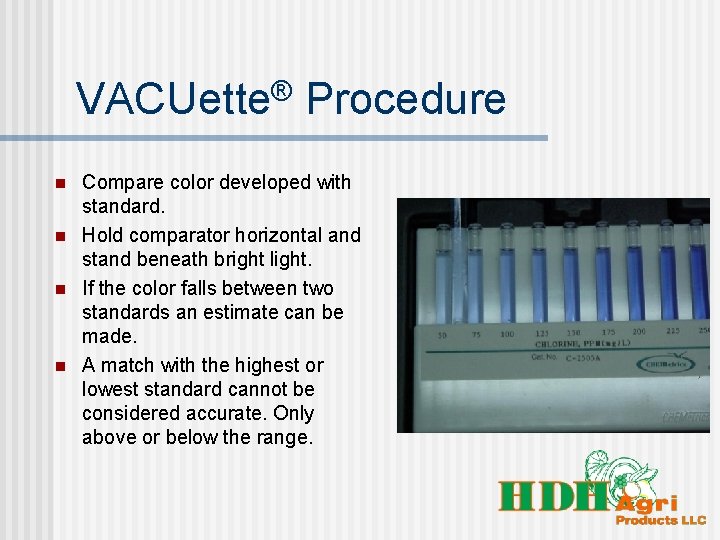
VACUette® Procedure n n Compare color developed with standard. Hold comparator horizontal and stand beneath bright light. If the color falls between two standards an estimate can be made. A match with the highest or lowest standard cannot be considered accurate. Only above or below the range.

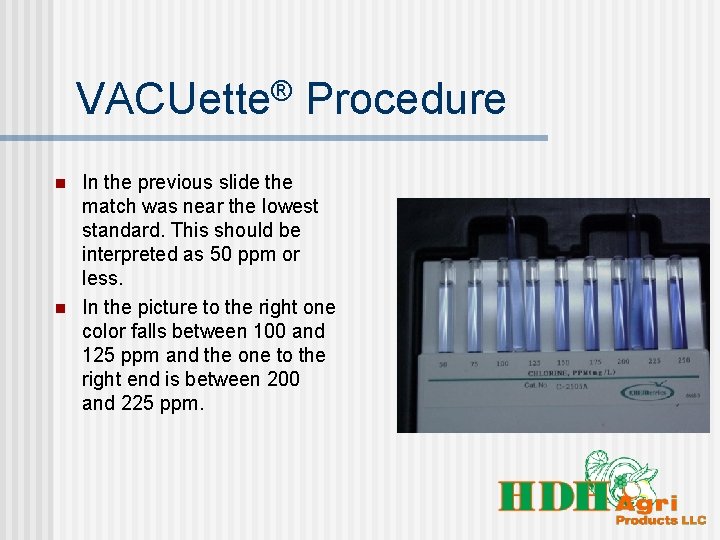
VACUette® Procedure n n In the previous slide the match was near the lowest standard. This should be interpreted as 50 ppm or less. In the picture to the right one color falls between 100 and 125 ppm and the one to the right end is between 200 and 225 ppm.

Portable Meters Hand held testers for both p. H and ORP are available. l In the photo, the unit on the left will measure p. H. l The unit on the right will measure ORP. l As with automatic controllers, calibration should be checked weekly. l

Oxidation - Reduction Whenever a chemical reaction takes place there is a movement of electrons from one atom to another. This movement results in a measurable microvoltage. If the voltage is positive, it is an oxidation reaction. An ORP (Oxidation-Reduction Potential) meter is used to measure this. The higher the ORP reading the greater the chlorine activity.

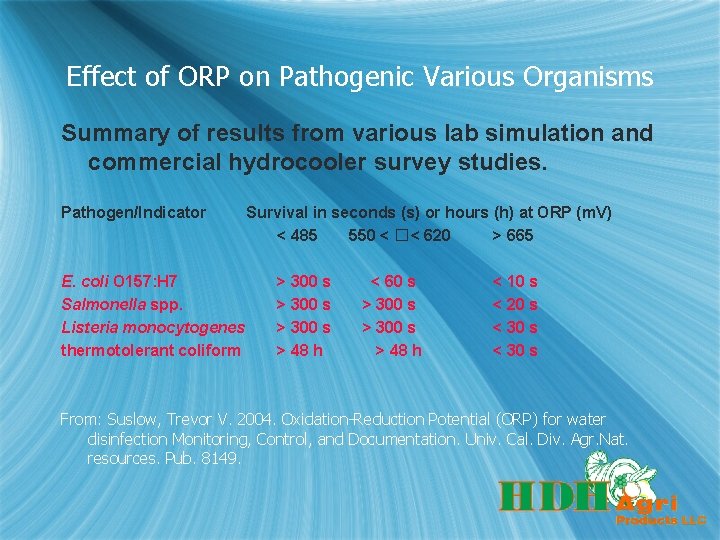
Effect of ORP on Pathogenic Various Organisms Summary of results from various lab simulation and commercial hydrocooler survey studies. Pathogen/Indicator E. coli O 157: H 7 Salmonella spp. Listeria monocytogenes thermotolerant coliform Survival in seconds (s) or hours (h) at ORP (m. V) < 485 550 < �< 620 > 665 > 300 s > 48 h < 60 s > 300 s > 48 h < 10 s < 20 s < 30 s From: Suslow, Trevor V. 2004. Oxidation-Reduction Potential (ORP) for water disinfection Monitoring, Control, and Documentation. Univ. Cal. Div. Agr. Nat. resources. Pub. 8149.

ORP and Chlorine An ORP meter can be used to measure available chlorine activity. Above 20 ppm there is little correlation between ORP and ppm available chlorine. When the p. H is about 7. 0 and the Chlorine is about 200 ppm the ORP will be between 650 and 750. This is more an indication of activity than concentration.

Disadvantages of ORP Systems n n n n n Sensors become fouled and need regular cleaning Sensors must be replaced every 6 to 12 months. Systems need regular calibration Sensor probes can become temporarily desensitized by over-injection. Sensor probes are very fragile and can be damaged in cleaning. Response time may become slowed. Back-up testing is needed. Need Backup - Hand Held p. H and ORP Meters Need Backup - Test Kits.

IMPORTANT Controlling ORP alone is not sufficient. The chlorine concentration vs ORP is dependent upon the p. H of the solution. A solution at p. H 8 having the same ORP as one at p. H 7, will require about 3 1/2 times as much chlorine.

Advantages of ORP Systems n n n Does not require constant attention by packinghouse personnel. When coupled with p. H control will provide consistent disinfection levels. Recorders are available that will provide the permanent record required by HACCP Remote transmission is available so management may “keep an eye on things” from a more convenient location. Well maintained systems provide maximum economical use of chemicals.

Typical ORP Meter A typical ORP metercontroller. When the ORP falls below the set point the controller will turn on a pump to add more chlorine. Usually a second circuit, with probe, monitors p. H and adds buffer when needed.

Typical ORP Probes Several typical ORP probes. The on the left is designed for high chlorine applications. NOTE: Each has a thin glass bulb or membrane at the tip. These are VERY fragile.

High Chlorine Meters exist that can interpret ORP as ppm available chlorine. Using special probes, they measure both ORP and p. H, then calculate Chlorine. The new meters can report up to 200 ppm. They have the same restrictions as conventional units.

ORP Controllers HDH Agri Products is proud to provide the full line of Pulse Instruments ORP monitors, controllers, and recorders. We provide installation and service in addition to sales support.

Chlorine Choices The user of chlorine has three sources to consider üChlorine Gas üCalcium Hypochlorite Tablets üSodium Hypochlorite Solution. §Why Choose Sodium Hypochlorite?

Legal Restraints There are four major considerations for the packer. a) Is the chlorine registered with the EPA for the concentration to be used. (Very few are. ) b) Does the waste water meet local regulations. c) Are any additives approved for food contact use. d) Employee safety.

Safety § Gas chlorine has caused major evacuations because of leaking or broken cylinders. Gas is poisonous § Chlorine tablets are exothermic when wet. Have been implicated in several fires. § Neither consideration applies to Sodium Hypochlorite.

Food Safety • Recent concerns regarding the potential of fresh produce being a source of food born illnesses raises the possibility that a packer, using chlorine that is not properly registered for his use, could face liability issues. • Read the label. Look for the use you anticipate, and the concentration of chlorine you will be using.

HDH Clor s A safe, convenient, reliable source of chlorine for postharvest applications. s EPA Registered for up to 2000 ppm on fresh fruits and vegetables.

HDH Clor s Available in 55 gallon drums, 300 gallon totes or for bulk delivery. s Available for self maintained applications or as part of a full service package. s Call 352 -343 -3484 for assistance.