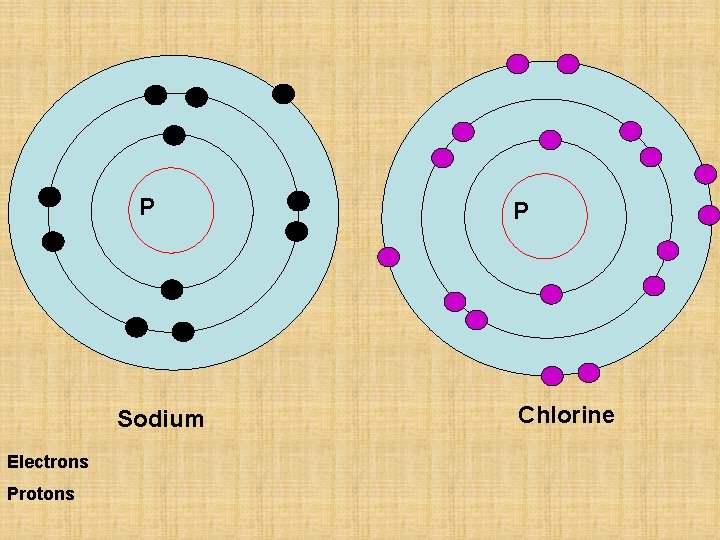
Sodium Chlorine P P N N Sodium Chlorine





- Slides: 20

Sodium Chlorine

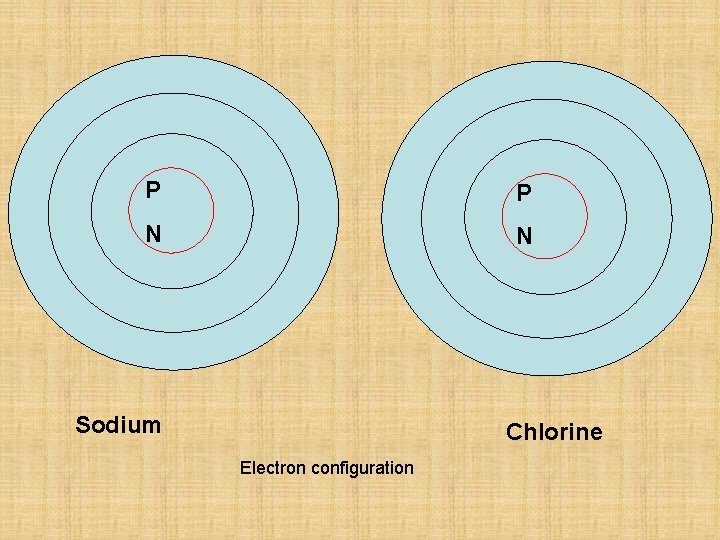
P P N N Sodium Chlorine Electron configuration

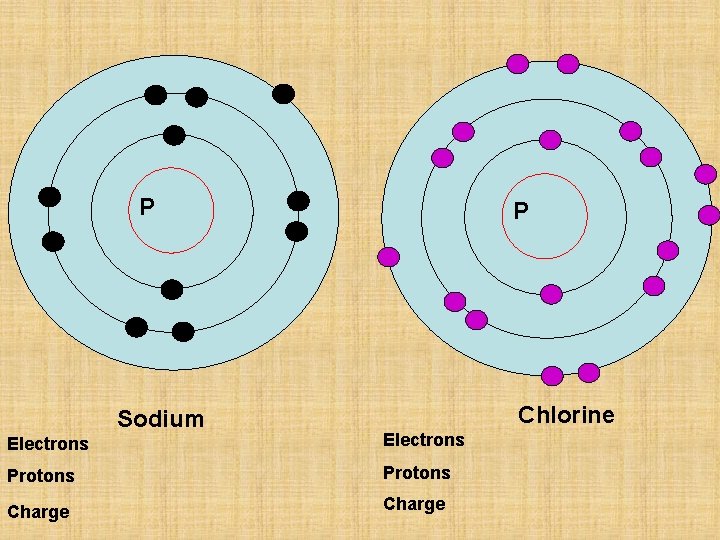
P Sodium Electrons Protons P Chlorine

P Sodium P Chlorine Electrons Protons Charge

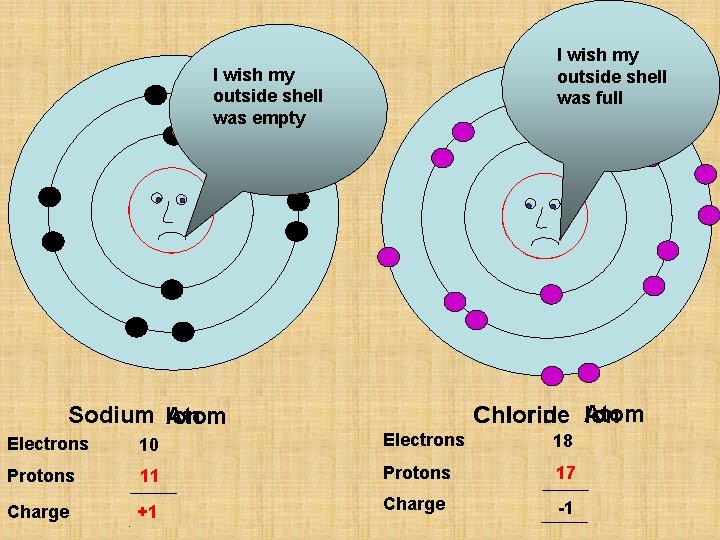
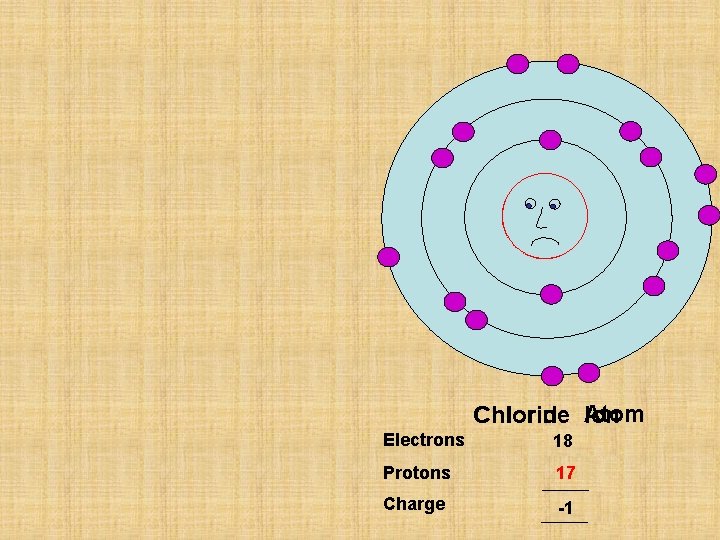
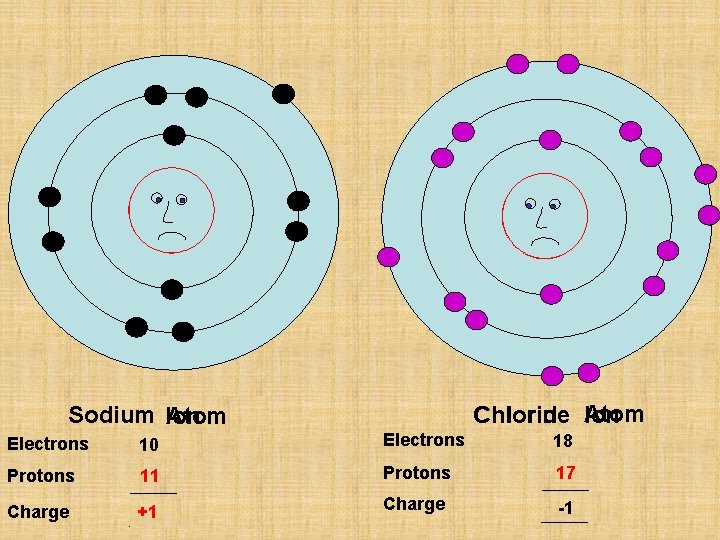
I wish my outside shell was full I wish my outside shell was empty P 11 P 17 Chlorine Chloride Atom Ion Sodium Ion Atom Electrons 11 10 Electrons 17 18 Protons 11 Protons 17 Charge 0 +1 Charge 0 -1

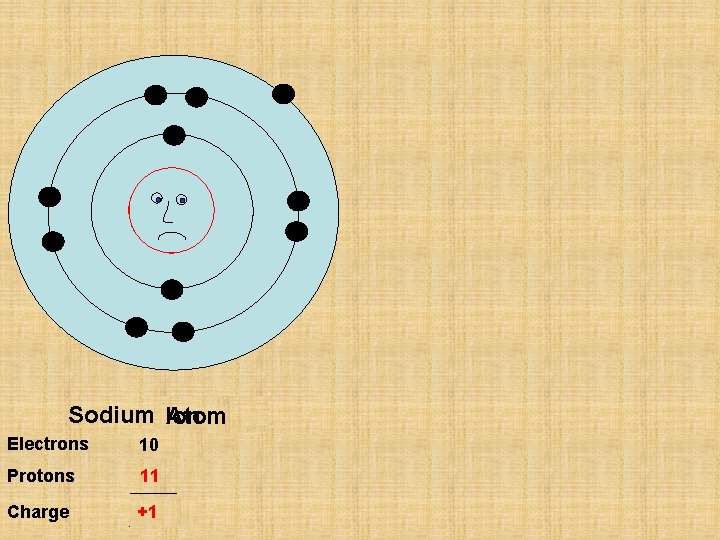
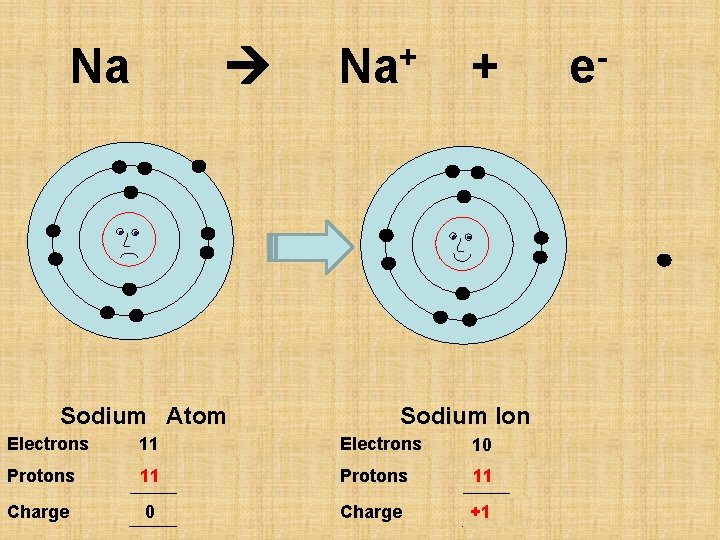
P 11 Sodium Ion Atom Electrons 11 10 Protons 11 Charge 0 +1

Na Sodium Atom Na+ + Sodium Ion Electrons 11 10 Protons 11 Charge 0 +1 e-

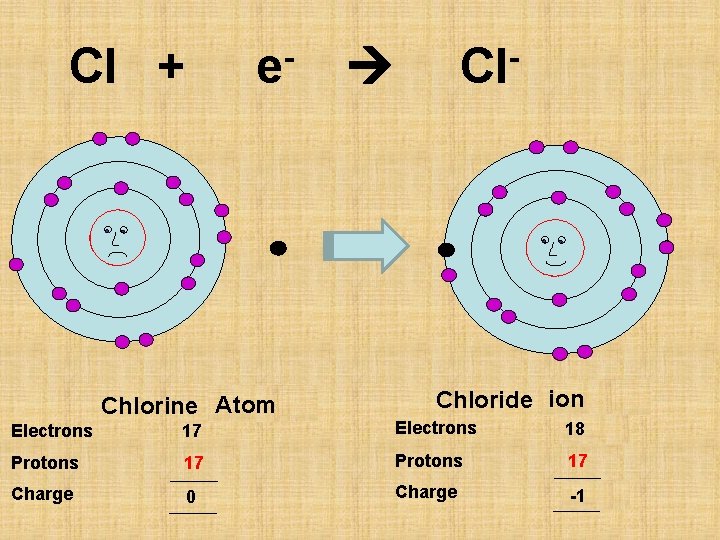
P 17 Chlorine Chloride Atom Ion Electrons 17 18 Protons 17 Charge 0 -1

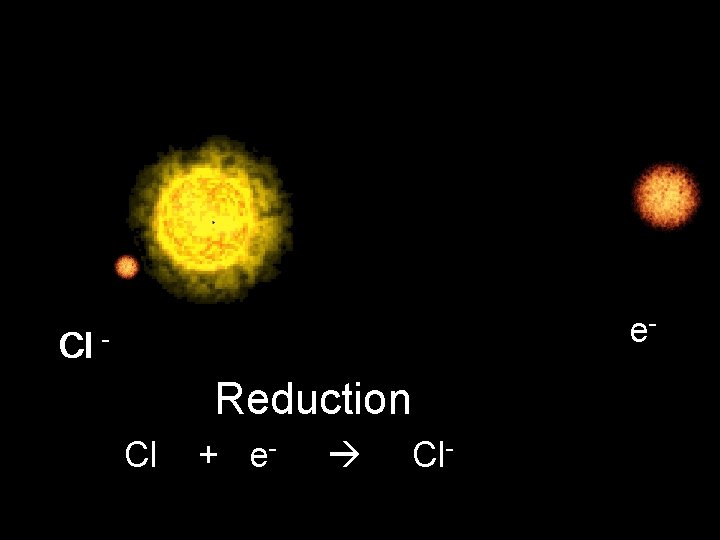
Cl + e- Chlorine Atom Cl- Chloride ion Electrons 17 18 Protons 17 Charge 0 -1

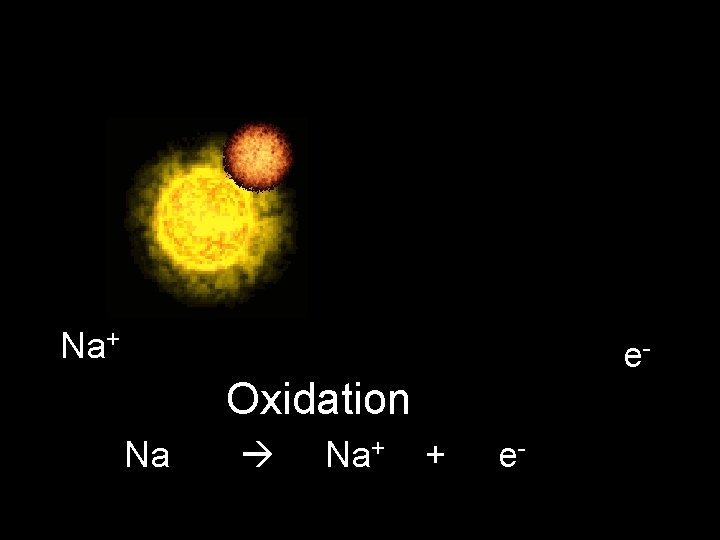
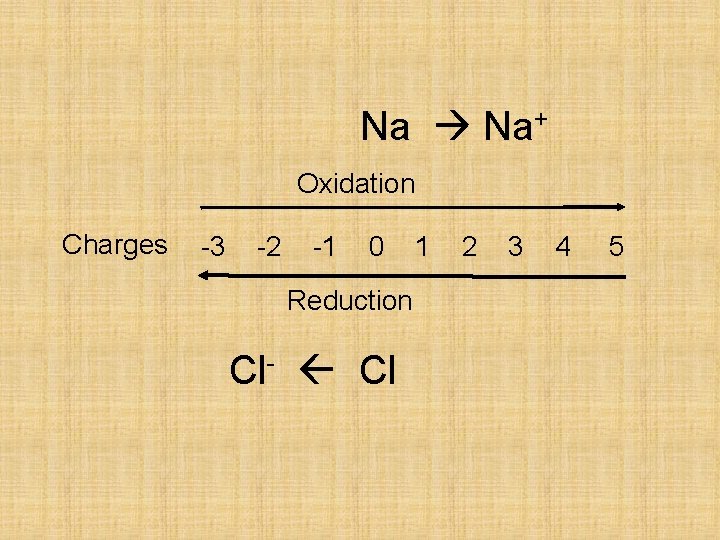
Na+ e- Oxidation Na Na+ + e-

e- Cl - Reduction Cl + e- Cl-

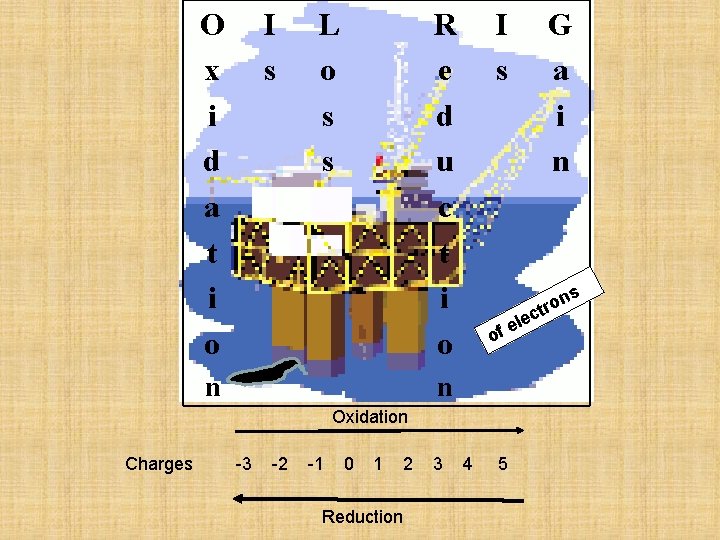
O x i d a t i o I s L o s s R e d u c t i o n I s s n -3 -2 -1 0 1 Reduction 2 3 on r t c le e of Oxidation Charges G a i n 4 5

. . “LEO says GER!” Lose Electrons Oxidation Gain Electrons Reduction

P 11 P 17 Chlorine Chloride Atom Ion Sodium Ion Atom Electrons 11 10 Electrons 17 18 Protons 11 Protons 17 Charge 0 +1 Charge 0 -1

Na Na+ Oxidation Charges -3 -2 -1 0 Reduction Cl- Cl 1 2 3 4 5

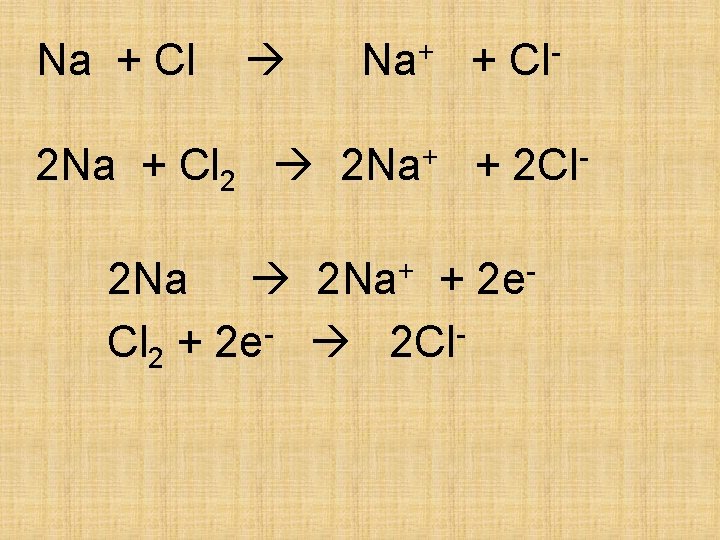
Na + Cl Na+ + Cl- 2 Na + Cl 2 2 Na+ + 2 Cl 2 Na+ + 2 e. Cl 2 + 2 e- 2 Cl-

Decide in each case if the element undergoes oxidation or reduction + Na Mg Mg+2 S S-2 F 2 2 F-1 -2 O 2 2 O

Insert the missing electrons into the following ½ equation Ca Ca+2 Step 1 Assign total ionic charge to both sides Ca 0 Ca+2 +2 Step 2 Add e- to most positive side Ca 0 Ca+2 + 2 e+2 + -2 Step 3 check both sides balance

Insert the missing electrons into the following ½ equation Cl 2 2 Cl. Step 1 Cl 2 2 Cl 0 -2 Step 2 Cl 2 + 2 e- 2 Cl-2 -2 Step 3 Check.

Insert the missing electrons into each of the half equations below Na Na+ +2 Mg -2 S S F 2 2 F-1 O 2 2 O-2
 Bromine sodium iodide
Bromine sodium iodide Oxygen bleach
Oxygen bleach Sodium carbonate e500
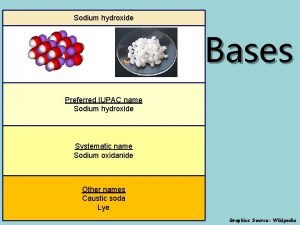
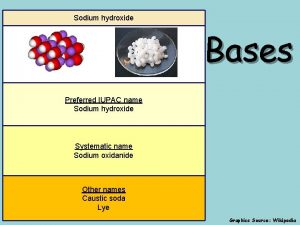
Sodium carbonate e500 What is the iupac name of the base naoh?
What is the iupac name of the base naoh? Iodine and sodium thiosulfate
Iodine and sodium thiosulfate Hydroxide catalysis bonding
Hydroxide catalysis bonding Word equation for calcium chloride
Word equation for calcium chloride Cl- molar mass
Cl- molar mass Chlorine uses
Chlorine uses Bohr's model neon
Bohr's model neon Chlorine charge ion
Chlorine charge ion Orbital notation worksheet
Orbital notation worksheet Helium + chlorine
Helium + chlorine Cl bohr model
Cl bohr model Atomic mass of chlorine
Atomic mass of chlorine Chlorine gas and potassium bromide
Chlorine gas and potassium bromide Covalent network vs covalent molecular
Covalent network vs covalent molecular Potassium chloride and bromine equation
Potassium chloride and bromine equation Chlorine + water balanced equation
Chlorine + water balanced equation 40g of calcium reacts with 71g of chlorine to produce
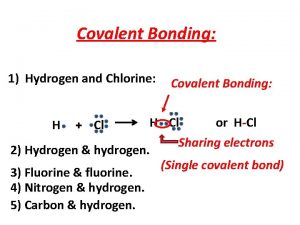
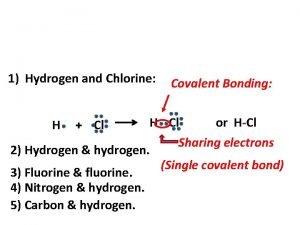
40g of calcium reacts with 71g of chlorine to produce Covalent bond of hydrogen and chlorine
Covalent bond of hydrogen and chlorine