Chapter 19 Gas Stoichiometry If an excess of


- Slides: 19

Chapter 19 Gas Stoichiometry

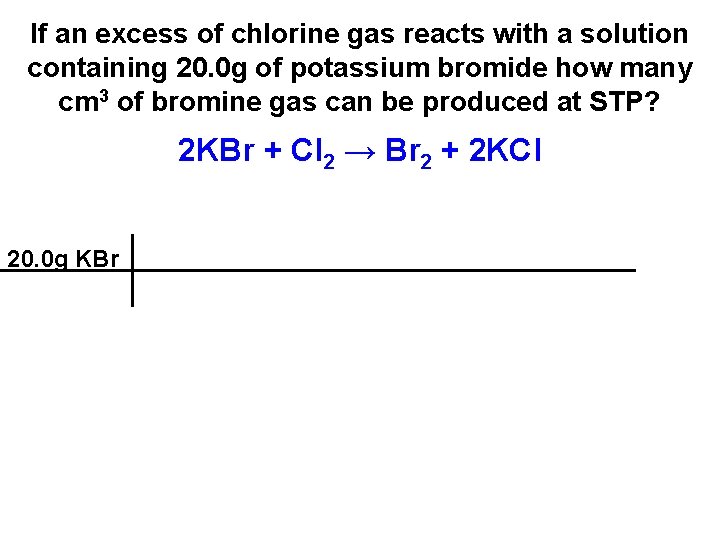
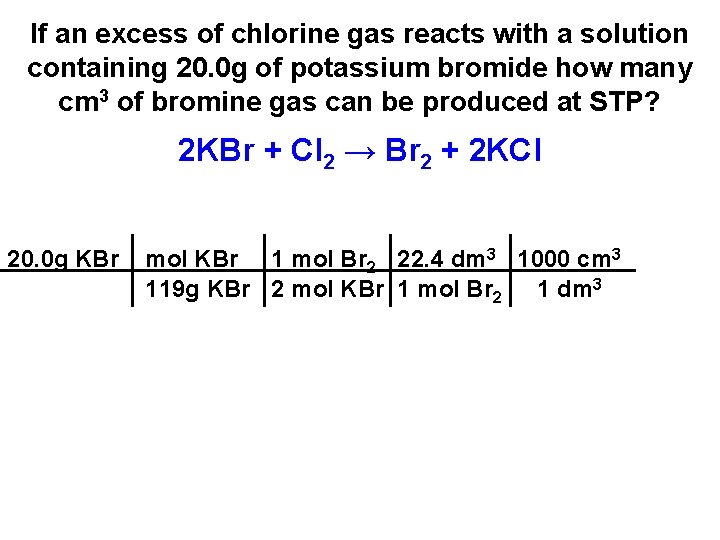
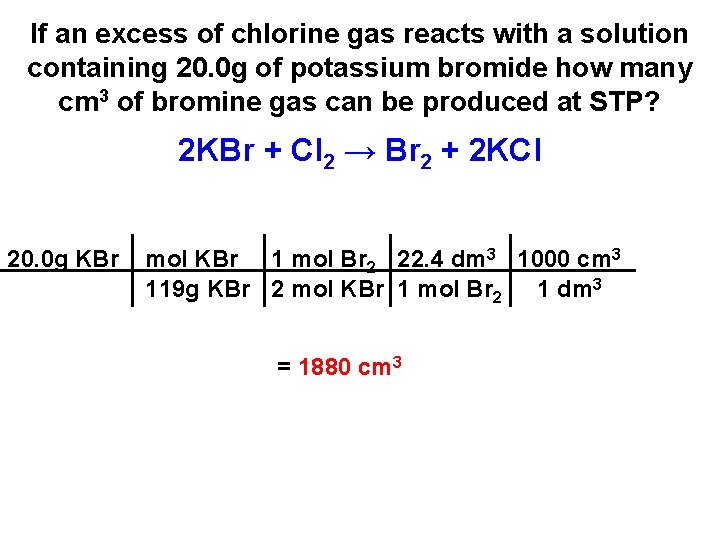
If an excess of chlorine gas reacts with a solution containing 20. 0 g of potassium bromide how many cm 3 of bromine gas can be produced at STP? 2 KBr + Cl 2 → Br 2 + 2 KCl

If an excess of chlorine gas reacts with a solution containing 20. 0 g of potassium bromide how many cm 3 of bromine gas can be produced at STP? 2 KBr + Cl 2 → Br 2 + 2 KCl 20. 0 g KBr

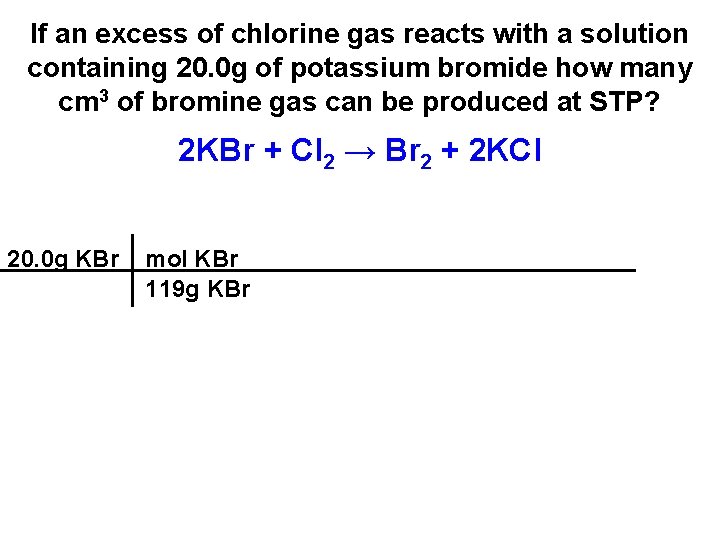
If an excess of chlorine gas reacts with a solution containing 20. 0 g of potassium bromide how many cm 3 of bromine gas can be produced at STP? 2 KBr + Cl 2 → Br 2 + 2 KCl 20. 0 g KBr mol KBr 119 g KBr

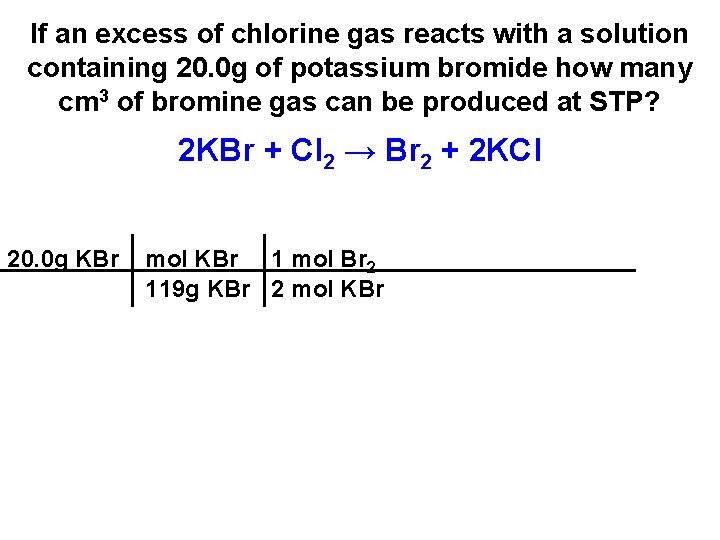
If an excess of chlorine gas reacts with a solution containing 20. 0 g of potassium bromide how many cm 3 of bromine gas can be produced at STP? 2 KBr + Cl 2 → Br 2 + 2 KCl 20. 0 g KBr mol KBr 1 mol Br 2 119 g KBr 2 mol KBr

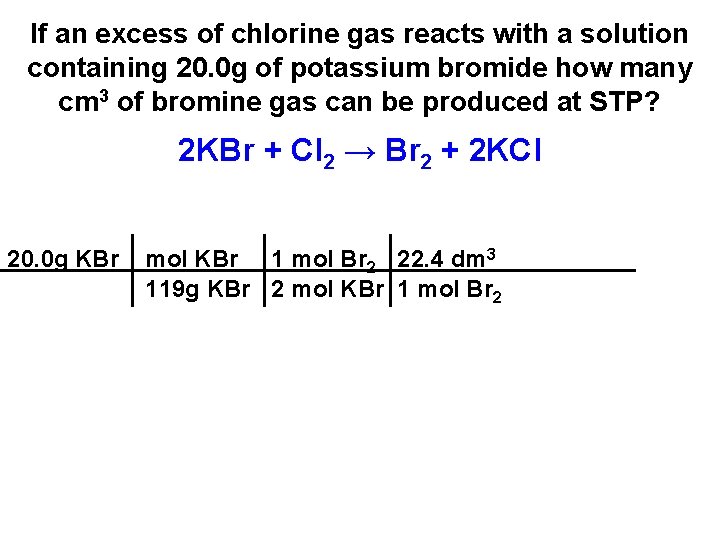
If an excess of chlorine gas reacts with a solution containing 20. 0 g of potassium bromide how many cm 3 of bromine gas can be produced at STP? 2 KBr + Cl 2 → Br 2 + 2 KCl 20. 0 g KBr mol KBr 1 mol Br 2 22. 4 dm 3 119 g KBr 2 mol KBr 1 mol Br 2

If an excess of chlorine gas reacts with a solution containing 20. 0 g of potassium bromide how many cm 3 of bromine gas can be produced at STP? 2 KBr + Cl 2 → Br 2 + 2 KCl 20. 0 g KBr mol KBr 1 mol Br 2 22. 4 dm 3 1000 cm 3 119 g KBr 2 mol KBr 1 mol Br 2 1 dm 3

If an excess of chlorine gas reacts with a solution containing 20. 0 g of potassium bromide how many cm 3 of bromine gas can be produced at STP? 2 KBr + Cl 2 → Br 2 + 2 KCl 20. 0 g KBr mol KBr 1 mol Br 2 22. 4 dm 3 1000 cm 3 119 g KBr 2 mol KBr 1 mol Br 2 1 dm 3 = 1880 cm 3

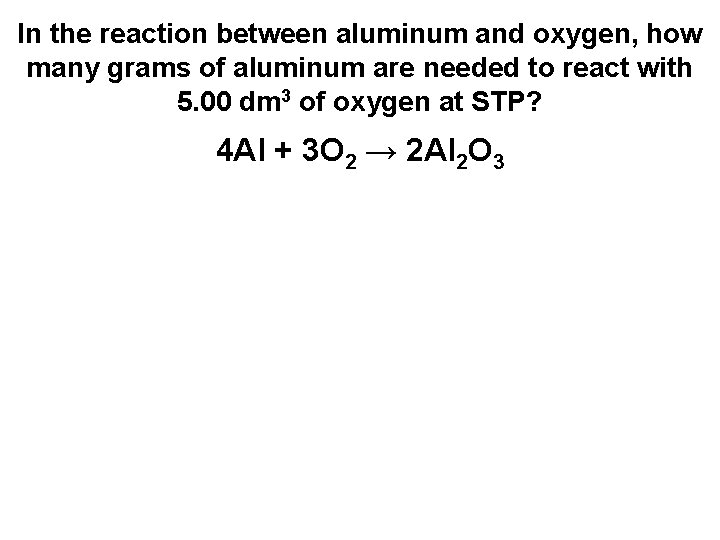
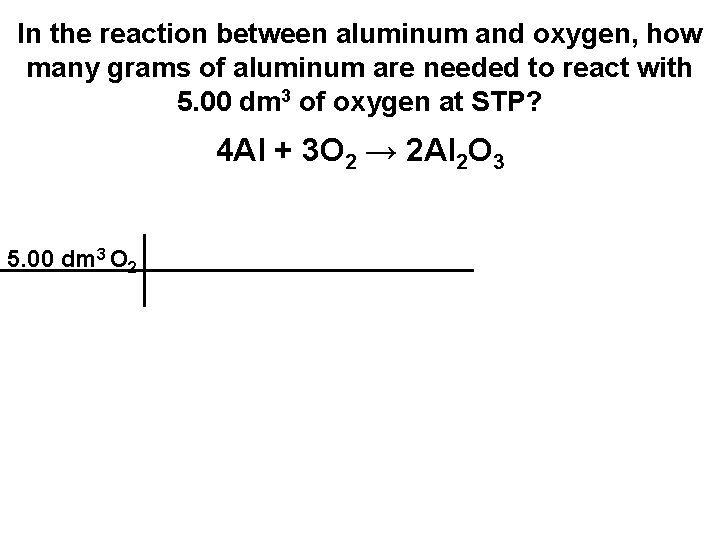
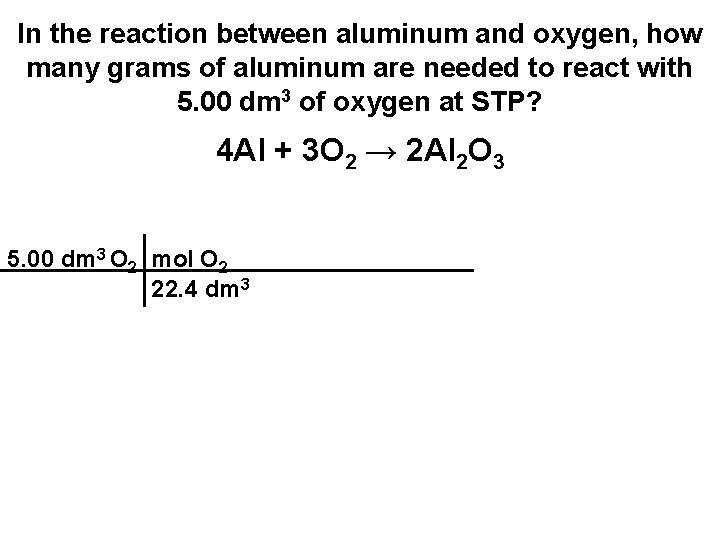
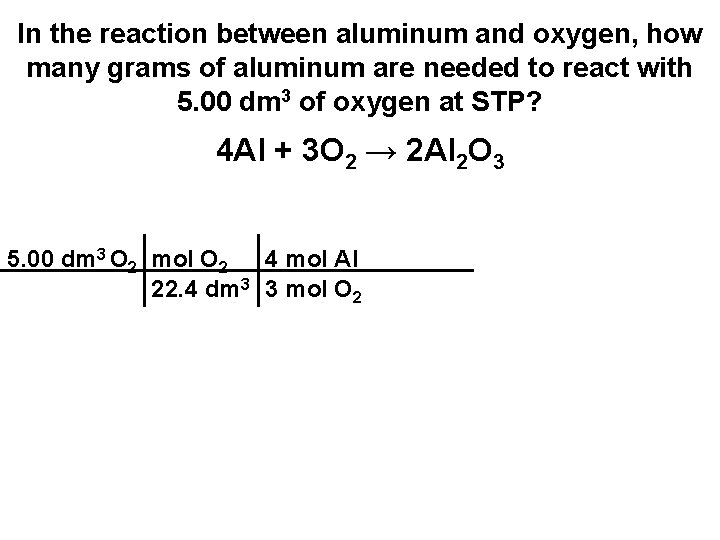
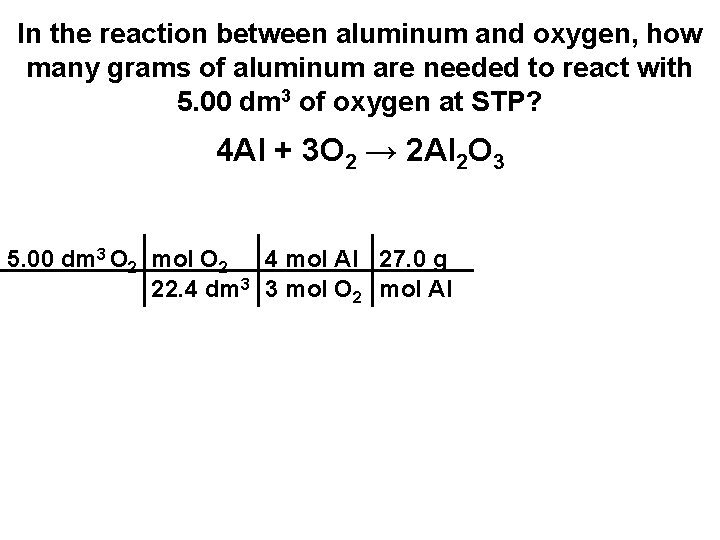
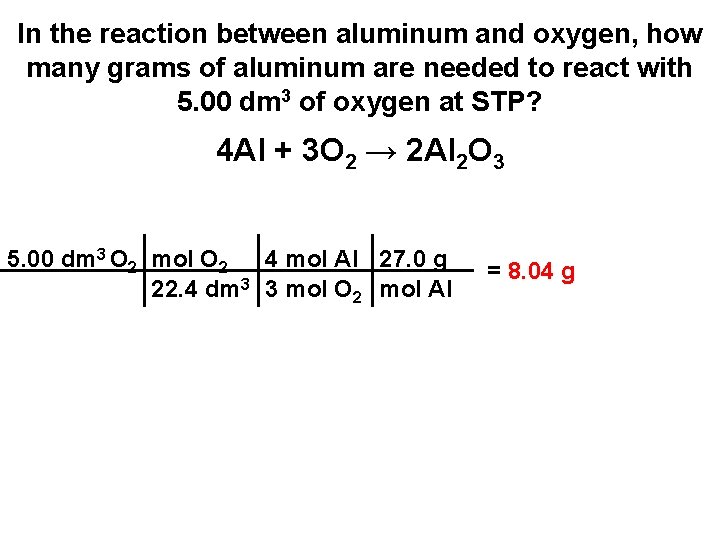
In the reaction between aluminum and oxygen, how many grams of aluminum are needed to react with 5. 00 dm 3 of oxygen at STP? 4 Al + 3 O 2 → 2 Al 2 O 3

In the reaction between aluminum and oxygen, how many grams of aluminum are needed to react with 5. 00 dm 3 of oxygen at STP? 4 Al + 3 O 2 → 2 Al 2 O 3 5. 00 dm 3 O 2

In the reaction between aluminum and oxygen, how many grams of aluminum are needed to react with 5. 00 dm 3 of oxygen at STP? 4 Al + 3 O 2 → 2 Al 2 O 3 5. 00 dm 3 O 2 mol O 2 22. 4 dm 3

In the reaction between aluminum and oxygen, how many grams of aluminum are needed to react with 5. 00 dm 3 of oxygen at STP? 4 Al + 3 O 2 → 2 Al 2 O 3 5. 00 dm 3 O 2 mol O 2 4 mol Al 22. 4 dm 3 3 mol O 2

In the reaction between aluminum and oxygen, how many grams of aluminum are needed to react with 5. 00 dm 3 of oxygen at STP? 4 Al + 3 O 2 → 2 Al 2 O 3 5. 00 dm 3 O 2 mol O 2 4 mol Al 27. 0 g 22. 4 dm 3 3 mol O 2 mol Al

In the reaction between aluminum and oxygen, how many grams of aluminum are needed to react with 5. 00 dm 3 of oxygen at STP? 4 Al + 3 O 2 → 2 Al 2 O 3 5. 00 dm 3 O 2 mol O 2 4 mol Al 27. 0 g 22. 4 dm 3 3 mol O 2 mol Al = 8. 04 g

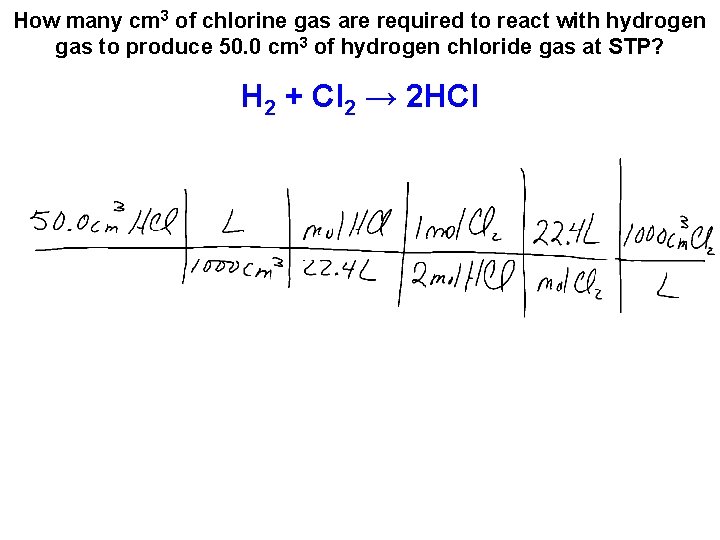
How many cm 3 of chlorine gas are required to react with hydrogen gas to produce 50. 0 cm 3 of hydrogen chloride gas at STP? H 2 + Cl 2 → 2 HCl

How many cm 3 of chlorine gas are required to react with hydrogen gas to produce 50. 0 cm 3 of hydrogen chloride gas at STP? H 2 + Cl 2 → 2 HCl

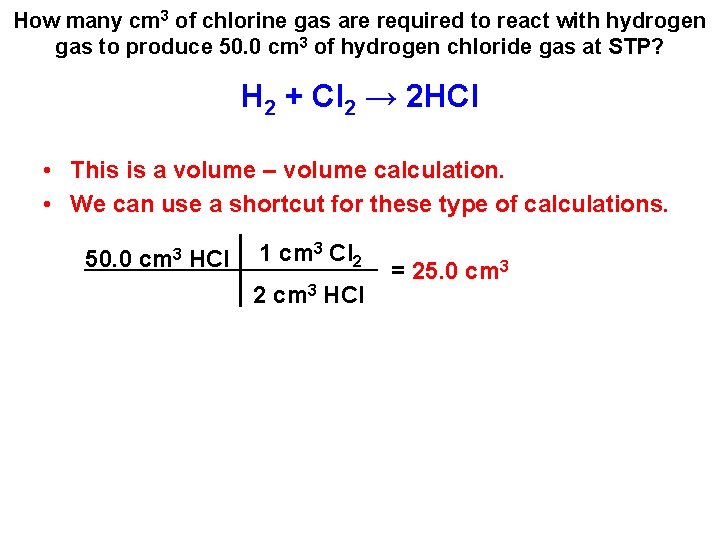
How many cm 3 of chlorine gas are required to react with hydrogen gas to produce 50. 0 cm 3 of hydrogen chloride gas at STP? H 2 + Cl 2 → 2 HCl • This is a volume – volume calculation. • We can use a shortcut for these type of calculations. 50. 0 cm 3 HCl

How many cm 3 of chlorine gas are required to react with hydrogen gas to produce 50. 0 cm 3 of hydrogen chloride gas at STP? H 2 + Cl 2 → 2 HCl • This is a volume – volume calculation. • We can use a shortcut for these type of calculations. 50. 0 cm 3 HCl 1 cm 3 Cl 2 2 cm 3 HCl = 25. 0 cm 3

Homework • Worksheet: Gas Stoichiometry • Lab Summary: “Molecular Mass of a Gas”