Chemical Reactions in Aqueous Solutions 4 1 Major





















- Slides: 77

Chemical Reactions in Aqueous Solutions 4 -1

Major Types of Chemical Reactions Solution Concentration and the Role of Water as a Solvent Ionic Reactions Precipitation Reactions Acid-Base Reactions Oxidation-Reduction (Redox) Reactions Elements in Redox Reactions 4 -2

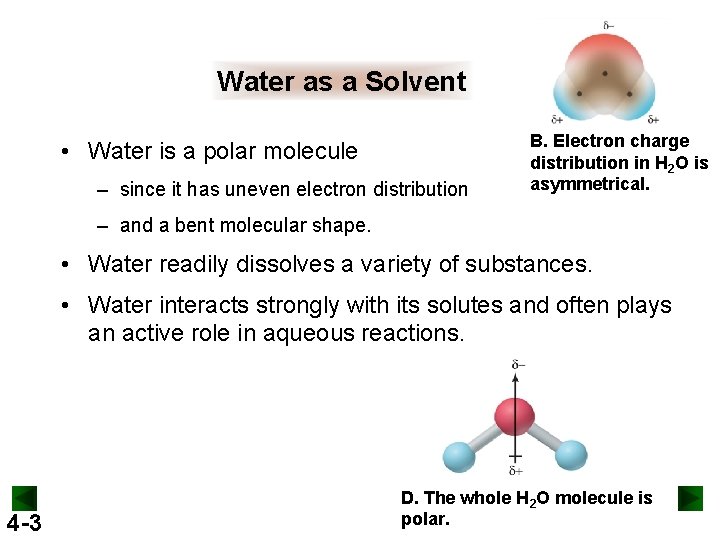
Water as a Solvent • Water is a polar molecule – since it has uneven electron distribution B. Electron charge distribution in H 2 O is asymmetrical. – and a bent molecular shape. • Water readily dissolves a variety of substances. • Water interacts strongly with its solutes and often plays an active role in aqueous reactions. 4 -3 D. The whole H 2 O molecule is polar.

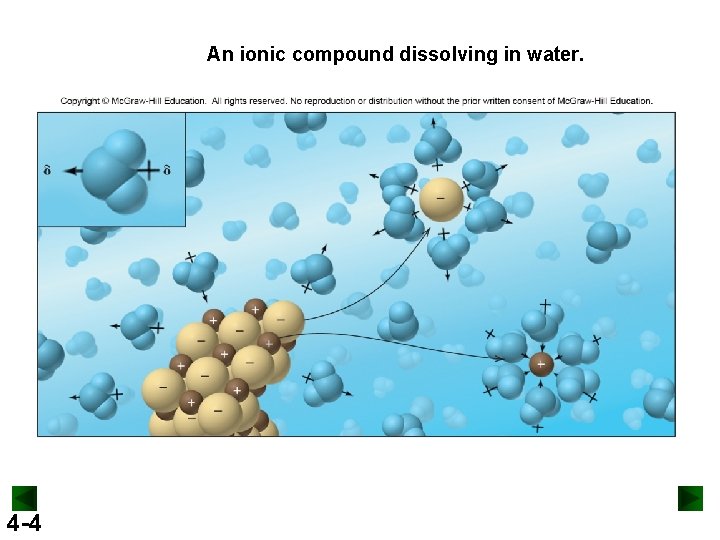
An ionic compound dissolving in water. 4 -4

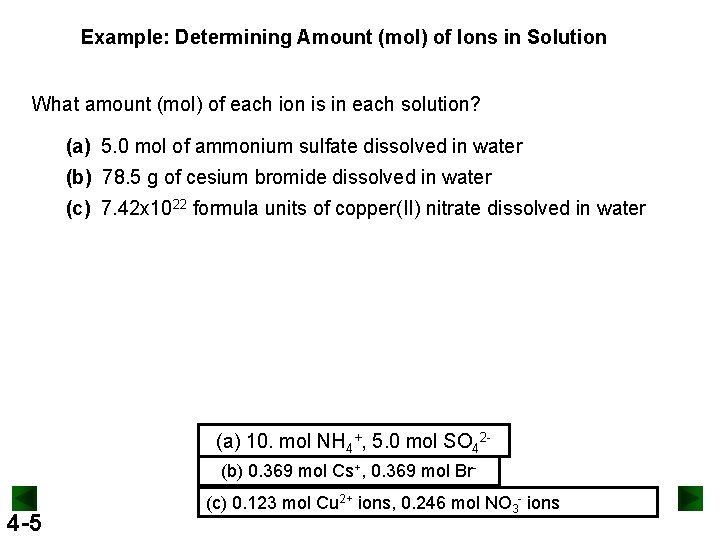
Example: Determining Amount (mol) of Ions in Solution What amount (mol) of each ion is in each solution? (a) 5. 0 mol of ammonium sulfate dissolved in water (b) 78. 5 g of cesium bromide dissolved in water (c) 7. 42 x 1022 formula units of copper(II) nitrate dissolved in water (a) 10. mol NH 4+, 5. 0 mol SO 42(b) 0. 369 mol Cs+, 0. 369 mol Br- 4 -5 (c) 0. 123 mol Cu 2+ ions, 0. 246 mol NO 3 - ions

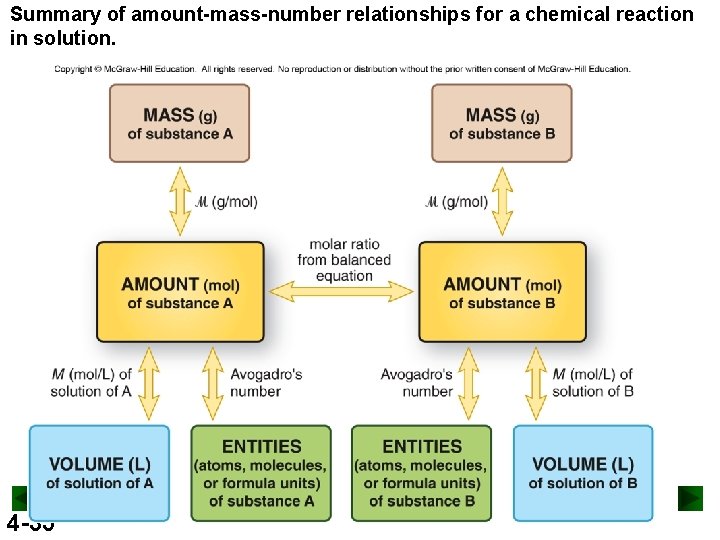
Summary of mass-mole-number-volume relationships in solution. 4 -6

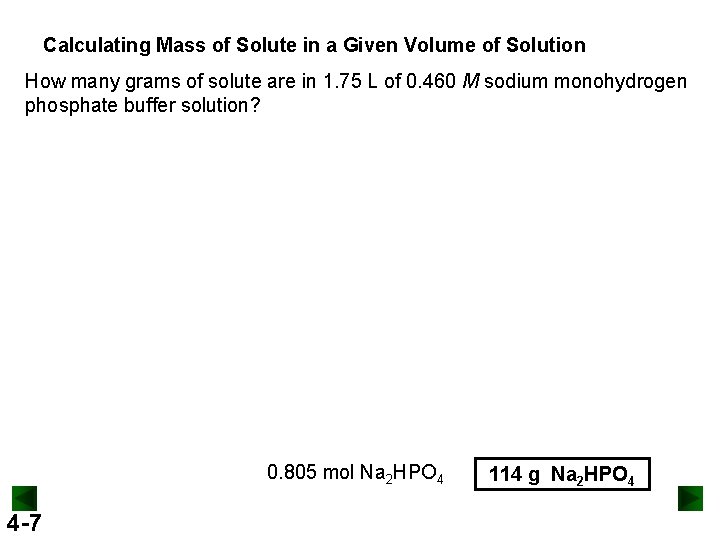
Calculating Mass of Solute in a Given Volume of Solution How many grams of solute are in 1. 75 L of 0. 460 M sodium monohydrogen phosphate buffer solution? 0. 805 mol Na 2 HPO 4 4 -7 114 g Na 2 HPO 4

Determining Molarity of in a Solution Each can of soda (355 m. L) contains 40. gram sugar (C 12 H 22 O 11). Calculate the molarity of sugar in soda. Assume the density of solution same as pure water. Assume exactly 100 g solution. Molar mass = 342. g/mol 4 -8 0. 355 L solution 0. 117 mol 0. 33 M

Determining Amount (mol) of Ions in a Solution What is the amount (mol) of each ion in 35 m. L 0. 84 M zinc chloride? 4 -9 2. 9 x 10– 2 mol Zn. Cl 2 2. 9 x 10– 2 mol Zn 2+ 5. 8 x 10– 2 mol Cl–

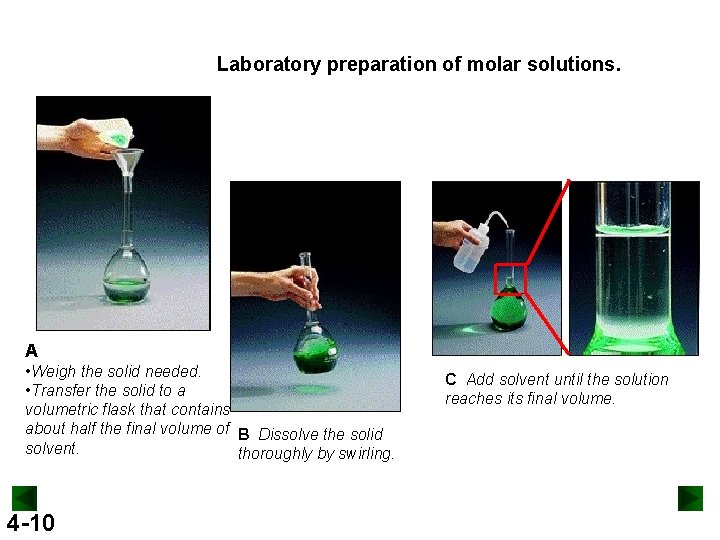
Laboratory preparation of molar solutions. A • Weigh the solid needed. • Transfer the solid to a volumetric flask that contains about half the final volume of B Dissolve the solid solvent. thoroughly by swirling. 4 -10 C Add solvent until the solution reaches its final volume.

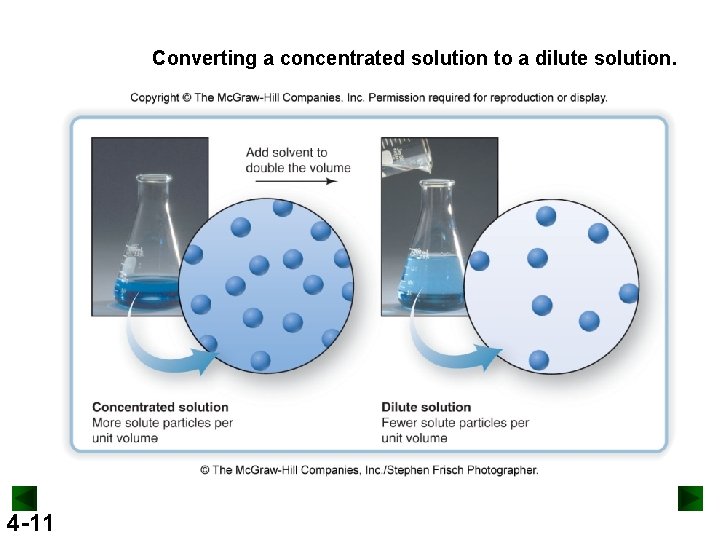
Converting a concentrated solution to a dilute solution. 4 -11

Preparing a Dilute Solution from a Concentrated Solution: M 1 x V 1 = M 2 x V 2 (= mol solute) “Isotonic saline” is a 0. 15 M aqueous solution of Na. Cl. How would you prepare 0. 80 L of isotonic saline from a 6. 0 M stock solution? Mdil x Vdil = # mol solute = Mconc x Vconc A 0. 020 L portion of the concentrated solution must be diluted to a final volume of 0. 80 L. 4 -12

Writing Equations for Aqueous Ionic Reactions 4 -13

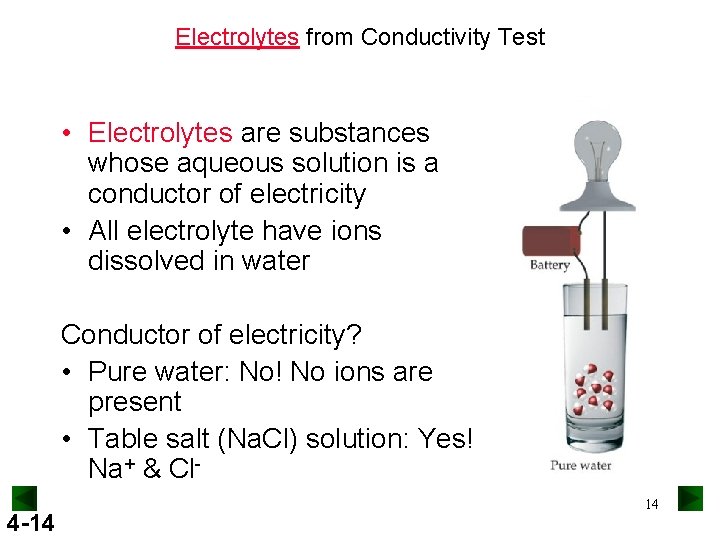
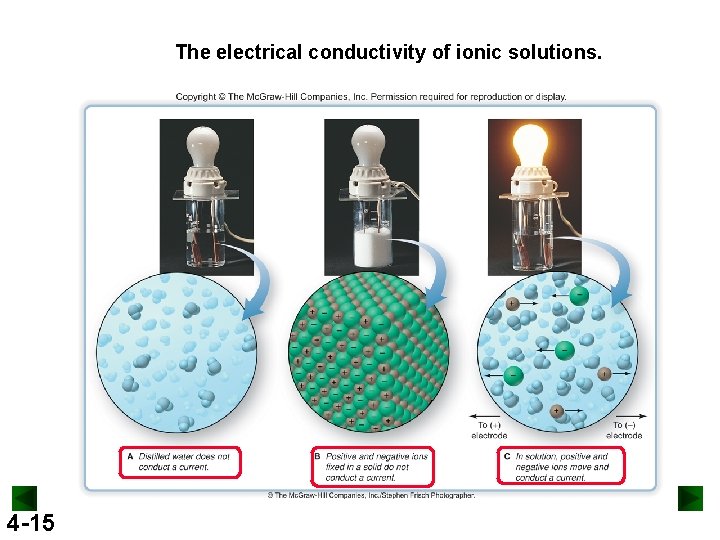
Electrolytes from Conductivity Test • Electrolytes are substances whose aqueous solution is a conductor of electricity • All electrolyte have ions dissolved in water Conductor of electricity? • Pure water: No! No ions are present • Table salt (Na. Cl) solution: Yes! Na+ & Cl 4 -14 14

The electrical conductivity of ionic solutions. 4 -15

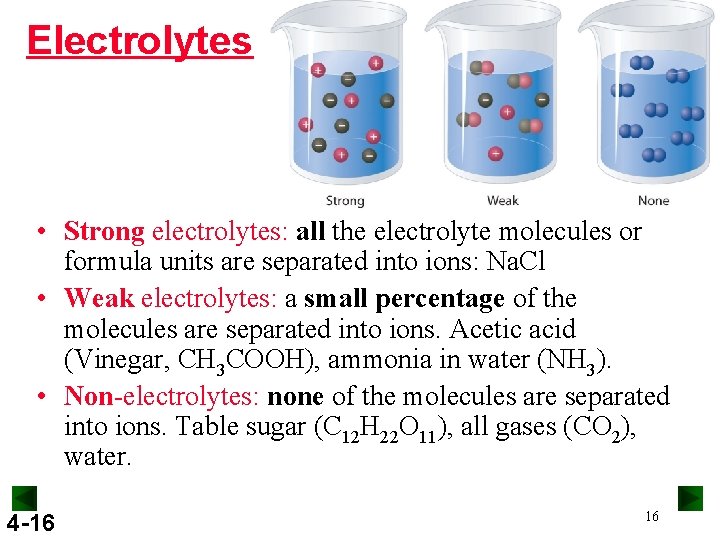
Electrolytes • Strong electrolytes: all the electrolyte molecules or formula units are separated into ions: Na. Cl • Weak electrolytes: a small percentage of the molecules are separated into ions. Acetic acid (Vinegar, CH 3 COOH), ammonia in water (NH 3). • Non-electrolytes: none of the molecules are separated into ions. Table sugar (C 12 H 22 O 11), all gases (CO 2), water. 4 -16 16

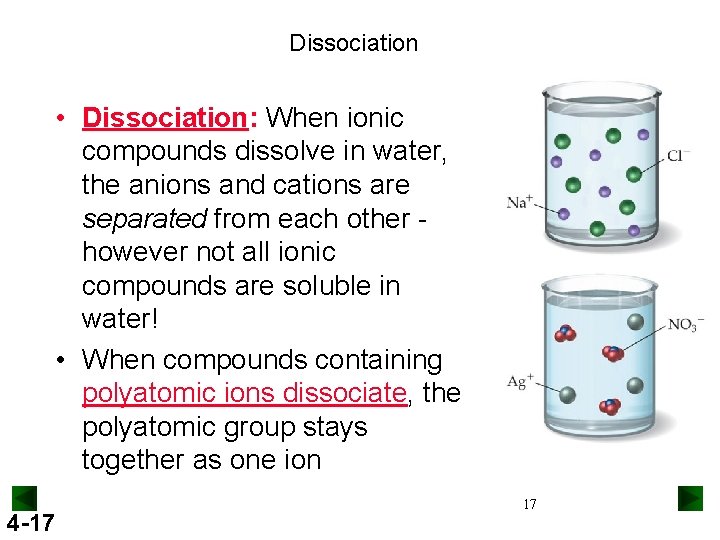
Dissociation • Dissociation: When ionic compounds dissolve in water, the anions and cations are separated from each other however not all ionic compounds are soluble in water! • When compounds containing polyatomic ions dissociate, the polyatomic group stays together as one ion 4 -17 17

Dissociation • Potassium iodide dissociates in water into potassium cations and iodide anions KI(aq) K+(aq) + I-(aq) K I K+ I- • Copper(II) sulfate dissociates in water into copper(II) cations and sulfate anions Cu. SO 4(aq) Cu 2+(aq) + SO 42 -(aq) Cu SO 4 Cu 2+ SO 4218 4 -18

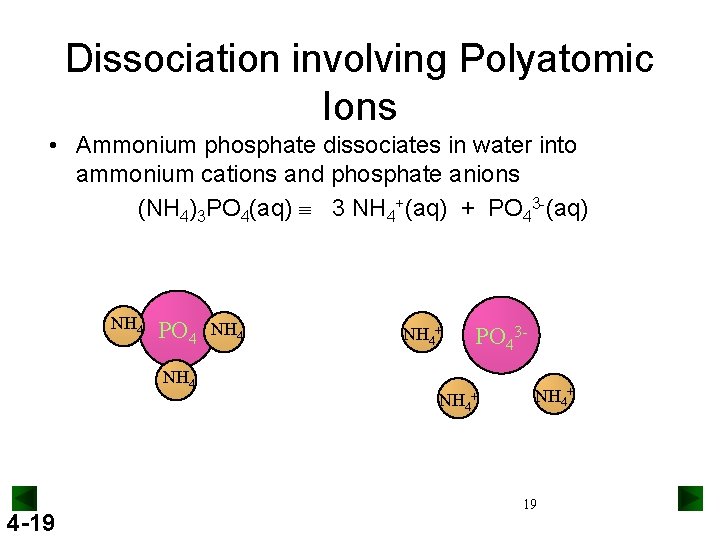
Dissociation involving Polyatomic Ions • Ammonium phosphate dissociates in water into ammonium cations and phosphate anions (NH 4)3 PO 4(aq) 3 NH 4+(aq) + PO 43 -(aq) NH 4 PO 4 NH 4+ PO 43 - NH 4+ 4 -19 NH 4+ 19

Strong Electrolytes (remember!!!) • Salts = water soluble ionic compounds – Na. Cl, NH 4 C 2 H 3 O 2, Ba(NO 3)2, Fe. Br 3, Cu. SO 4 • Strong Acids = completely dissociate to form H+ ions in water solution - HCl, HBr, HI, HNO 3, H 2 SO 4, HCl. O 4 • Strong Bases = water soluble metal hydroxides (OH-) – Metal hydroxide of ALL Group IA metals such as Na. OH, KOH, etc. – Ca(OH)2), Sr(OH)2, Ba(OH)2 4 -20 20

Weak Electrolytes and Nonelectrolytes Dissociate Poorly • Weak acids (acetic acid, nitrous acid, hydrofluoric acid, etc), Weak base (ammonium hydroxide, etc), HC 2 H 3 O 2(aq) H+(aq) + C 2 H 3 O 2 -(aq) NH 4 OH(aq) NH 4+(aq) + OH-(aq) • Insoluble salts largely remain undissociated in water Pb. Cl 2(s) Ca. CO 3(s) Ba. SO 4(s) • Pure gas, liquid, or solid do not dissociate in solution: CO 2(g), H 2 S(g), H 2 O(l), Al(s), Hg(s) 4 -21 21

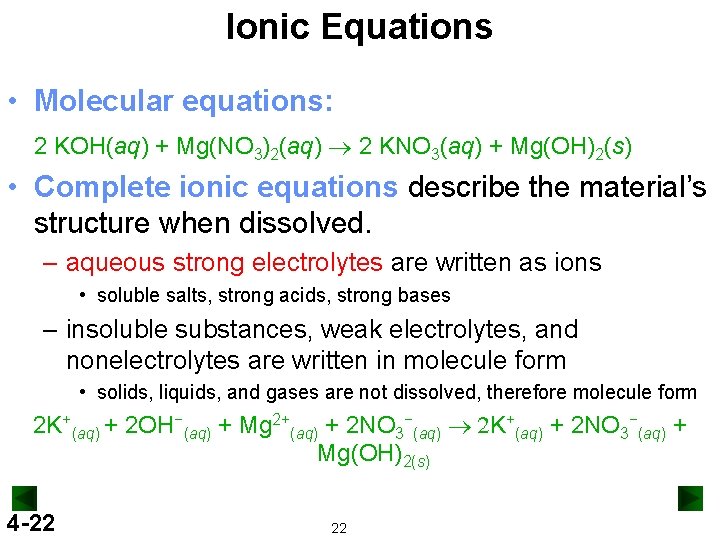
Ionic Equations • Molecular equations: 2 KOH(aq) + Mg(NO 3)2(aq) ® 2 KNO 3(aq) + Mg(OH)2(s) • Complete ionic equations describe the material’s structure when dissolved. – aqueous strong electrolytes are written as ions • soluble salts, strong acids, strong bases – insoluble substances, weak electrolytes, and nonelectrolytes are written in molecule form • solids, liquids, and gases are not dissolved, therefore molecule form 2 K+(aq) + 2 OH−(aq) + Mg 2+(aq) + 2 NO 3−(aq) ® 2 K+(aq) + 2 NO 3−(aq) + Mg(OH)2(s) 4 -22 22

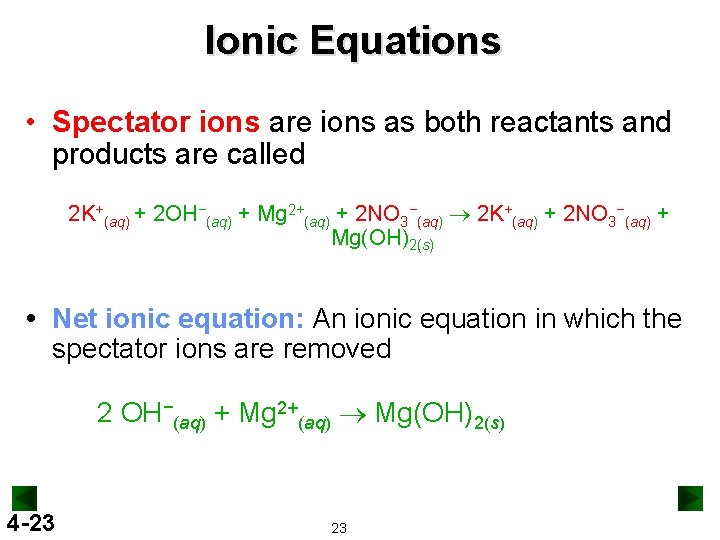
Ionic Equations • Spectator ions are ions as both reactants and products are called 2 K+(aq) + 2 OH−(aq) + Mg 2+(aq) + 2 NO 3−(aq) ® 2 K+(aq) + 2 NO 3−(aq) + Mg(OH)2(s) Net ionic equation: An ionic equation in which the spectator ions are removed 2 OH−(aq) + Mg 2+(aq) ® Mg(OH)2(s) 4 -23 23

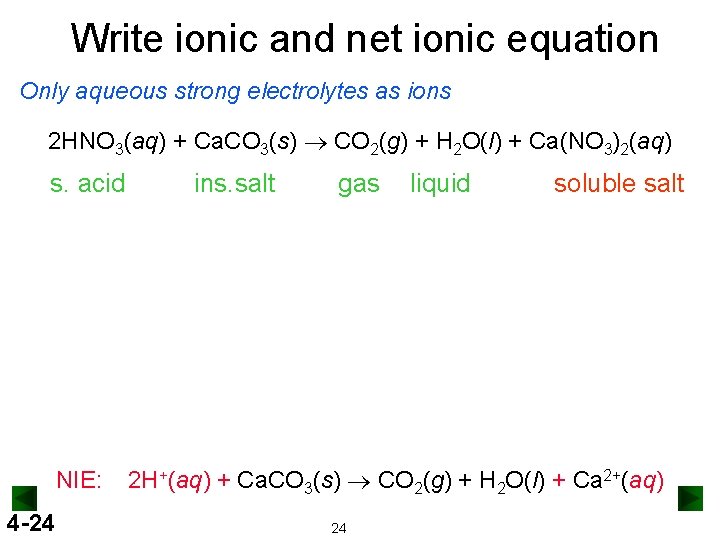
Write ionic and net ionic equation Only aqueous strong electrolytes as ions 2 HNO 3(aq) + Ca. CO 3(s) ® CO 2(g) + H 2 O(l) + Ca(NO 3)2(aq) s. acid NIE: 4 -24 ins. salt gas liquid soluble salt 2 H+(aq) + Ca. CO 3(s) ® CO 2(g) + H 2 O(l) + Ca 2+(aq) 24

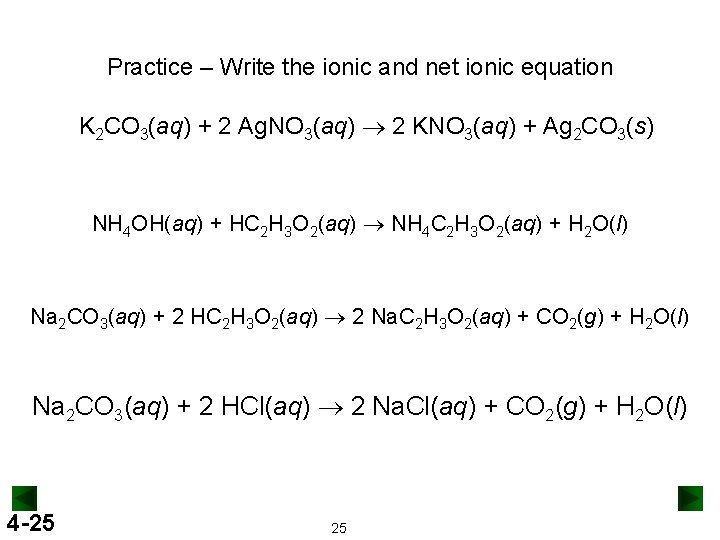
Practice – Write the ionic and net ionic equation K 2 CO 3(aq) + 2 Ag. NO 3(aq) ® 2 KNO 3(aq) + Ag 2 CO 3(s) NH 4 OH(aq) + HC 2 H 3 O 2(aq) ® NH 4 C 2 H 3 O 2(aq) + H 2 O(l) Na 2 CO 3(aq) + 2 HC 2 H 3 O 2(aq) ® 2 Na. C 2 H 3 O 2(aq) + CO 2(g) + H 2 O(l) Na 2 CO 3(aq) + 2 HCl(aq) ® 2 Na. Cl(aq) + CO 2(g) + H 2 O(l) 4 -25 25

Precipitation Reactions • In a precipitation reaction two soluble ionic compounds react to give an insoluble product, called a precipitate. • The precipitate forms through the net removal of ions from solution. • It is possible for more than one precipitate to form in such a reaction. 4 -26

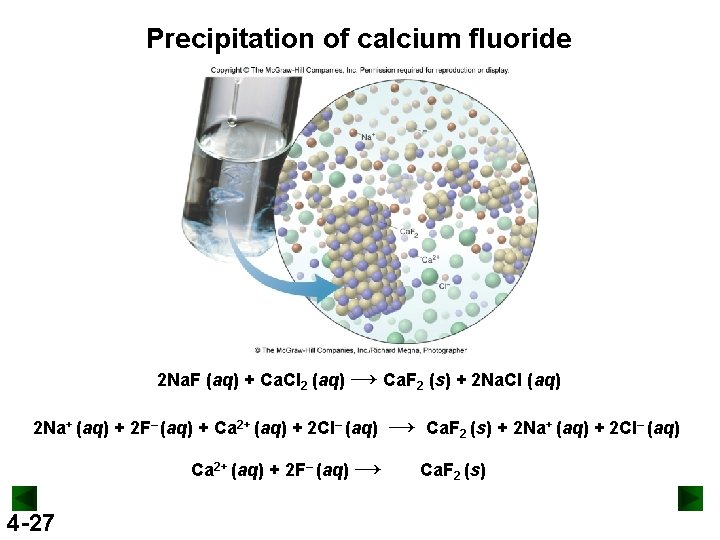
Precipitation of calcium fluoride 2 Na. F (aq) + Ca. Cl 2 (aq) → Ca. F 2 (s) + 2 Na. Cl (aq) 2 Na+ (aq) + 2 F– (aq) + Ca 2+ (aq) + 2 Cl– (aq) Ca 2+ (aq) + 2 F– (aq) → 4 -27 → Ca. F 2 (s) + 2 Na+ (aq) + 2 Cl– (aq) Ca. F 2 (s)

Predicting Whether a Precipitate Will Form • Note the ions present in the reactants. • Consider all possible cation-anion combinations. • Use the solubility rules to decide whether any of the ion combinations is insoluble. – Any insoluble combination identifies a precipitate that will form. 4 -28

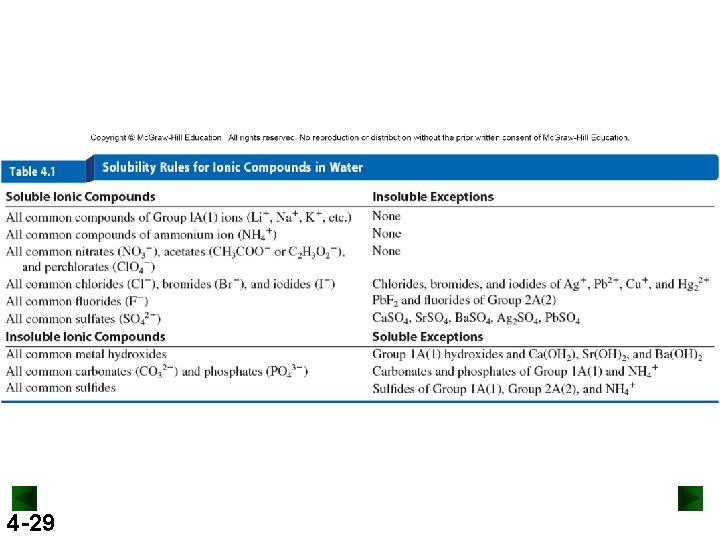
4 -29

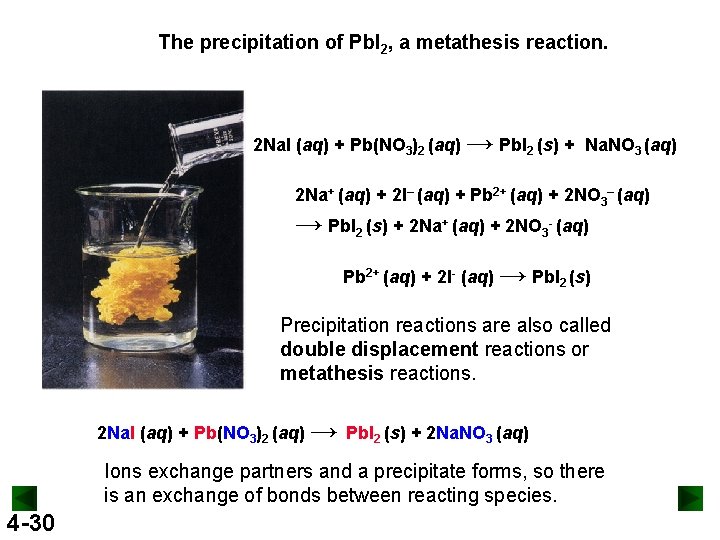
The precipitation of Pb. I 2, a metathesis reaction. 2 Na. I (aq) + Pb(NO 3)2 (aq) → Pb. I 2 (s) + Na. NO 3 (aq) 2 Na+ (aq) + 2 I– (aq) + Pb 2+ (aq) + 2 NO 3– (aq) → Pb. I 2 (s) + 2 Na+ (aq) + 2 NO 3 - (aq) Pb 2+ (aq) + 2 I- (aq) → Pb. I 2 (s) Precipitation reactions are also called double displacement reactions or metathesis reactions. 2 Na. I (aq) + Pb(NO 3)2 (aq) → Pb. I 2 (s) + 2 Na. NO 3 (aq) Ions exchange partners and a precipitate forms, so there is an exchange of bonds between reacting species. 4 -30

Predicting Whether a Precipitation Reaction Occurs; Writing Ionic Equations Predict whether or not a reaction occurs when each of the following pairs of solutions are mixed. If a reaction does occur, write balanced molecular, total ionic, and net ionic equations, and identify the spectator ions. (a) potassium fluoride (aq) + strontium nitrate (aq) → (b) ammonium perchlorate (aq) + sodium bromide (aq) → (a) Net ionic equation: 4 -31 Sr 2+ (aq) + 2 F- (aq) → Sr. F 2 (s) (b) No reaction

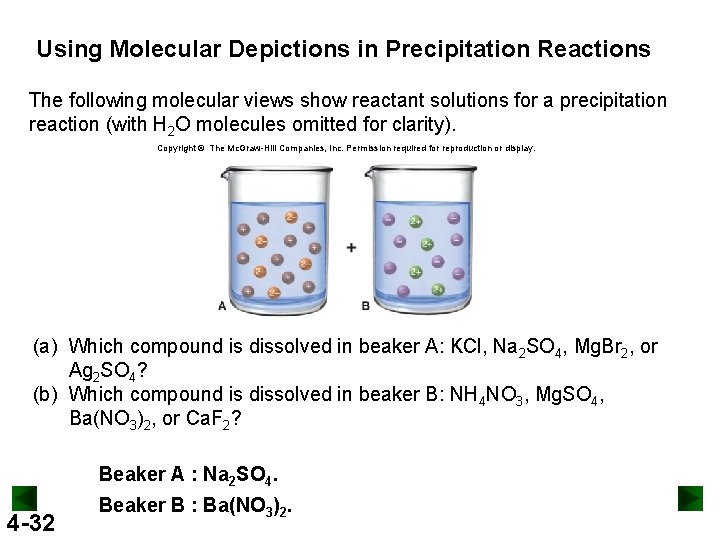
Using Molecular Depictions in Precipitation Reactions The following molecular views show reactant solutions for a precipitation reaction (with H 2 O molecules omitted for clarity). Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. (a) Which compound is dissolved in beaker A: KCl, Na 2 SO 4, Mg. Br 2, or Ag 2 SO 4? (b) Which compound is dissolved in beaker B: NH 4 NO 3, Mg. SO 4, Ba(NO 3)2, or Ca. F 2? Beaker A : Na 2 SO 4. 4 -32 Beaker B : Ba(NO 3)2.

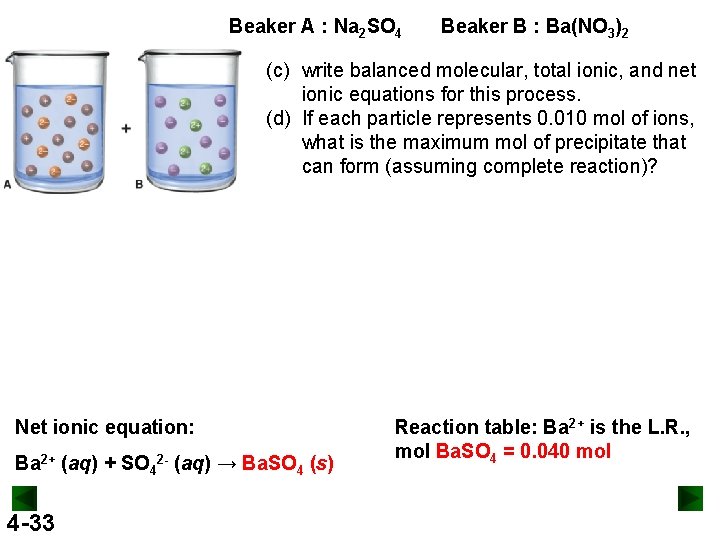
Beaker A : Na 2 SO 4 Beaker B : Ba(NO 3)2 (c) write balanced molecular, total ionic, and net ionic equations for this process. (d) If each particle represents 0. 010 mol of ions, what is the maximum mol of precipitate that can form (assuming complete reaction)? Net ionic equation: Ba 2+ (aq) + SO 42 - (aq) → Ba. SO 4 (s) 4 -33 Reaction table: Ba 2+ is the L. R. , mol Ba. SO 4 = 0. 040 mol

Calculating Amounts of Reactants and Products in a Precipitation Reaction Magnesium is abundant in sea water. The first step in its industrial extraction involves the reaction of Mg 2+ with Ca(OH)2 to precipitate Mg(OH)2. What mass of Mg(OH)2 is formed when 0. 180 L of 0. 0155 M Mg. Cl 2 reacts with excess Ca(OH)2? 0. 00279 mol Mg(OH)2 4 -34 0. 163 g Mg(OH)2

Summary of amount-mass-number relationships for a chemical reaction in solution. 4 -35

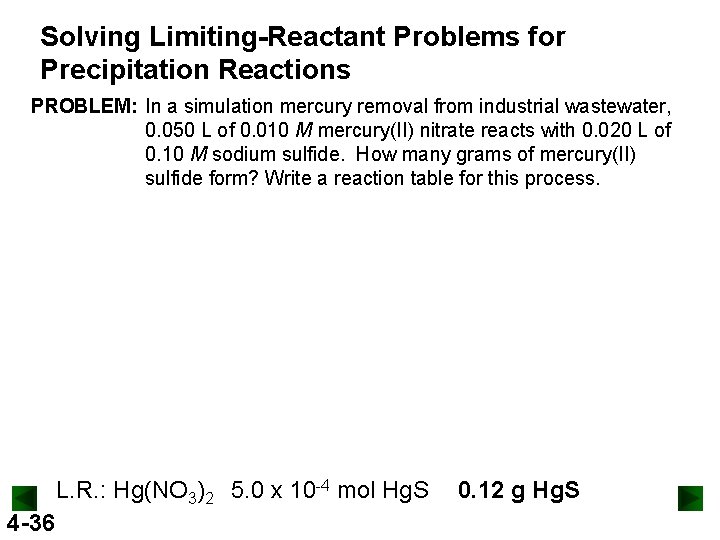
Solving Limiting-Reactant Problems for Precipitation Reactions PROBLEM: In a simulation mercury removal from industrial wastewater, 0. 050 L of 0. 010 M mercury(II) nitrate reacts with 0. 020 L of 0. 10 M sodium sulfide. How many grams of mercury(II) sulfide form? Write a reaction table for this process. L. R. : Hg(NO 3)2 5. 0 x 10 -4 mol Hg. S 4 -36 0. 12 g Hg. S

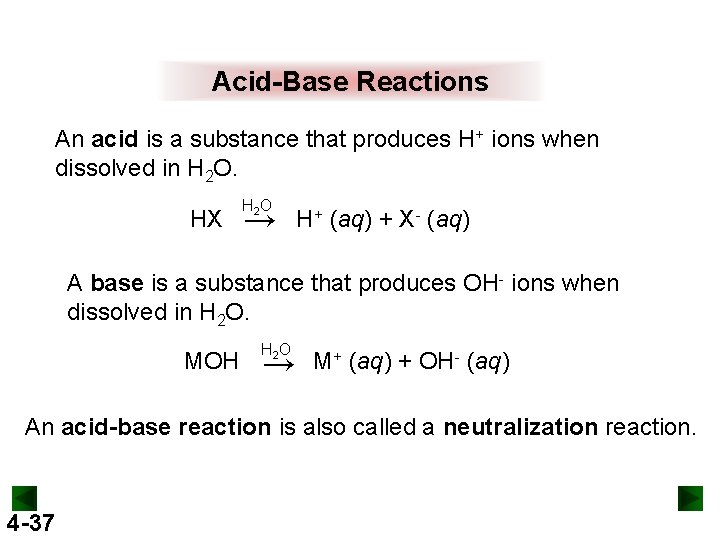
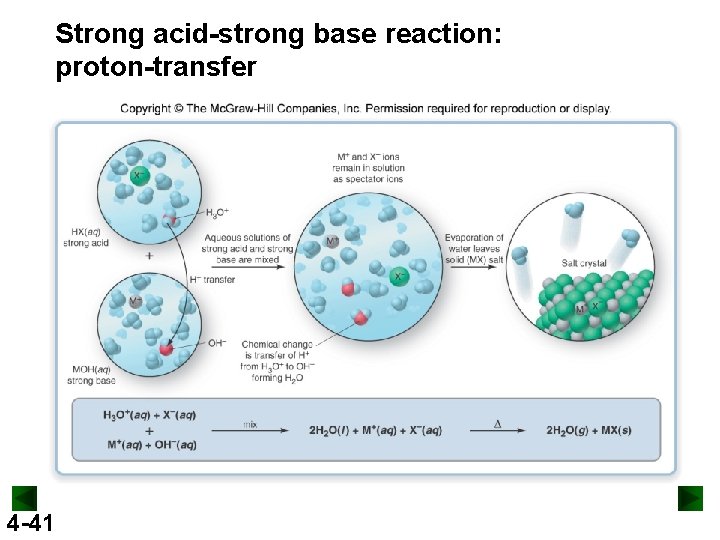
Acid-Base Reactions An acid is a substance that produces H+ ions when dissolved in H 2 O. HX H 2 O → H+ (aq) + X- (aq) A base is a substance that produces OH- ions when dissolved in H 2 O. MOH H 2 O → M+ (aq) + OH- (aq) An acid-base reaction is also called a neutralization reaction. 4 -37

Strong acids and strong bases dissociate completely into ions in aqueous solution. 4 -38 Weak acids and weak bases dissociate very little into ions in aqueous solution

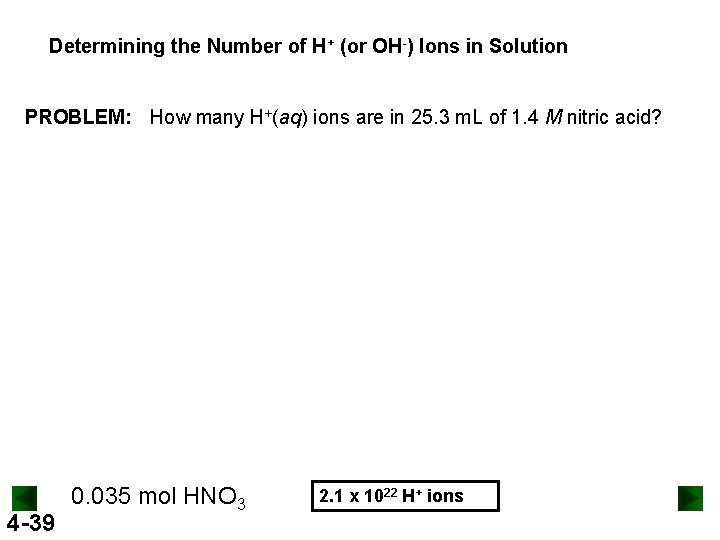
Determining the Number of H+ (or OH-) Ions in Solution PROBLEM: How many H+(aq) ions are in 25. 3 m. L of 1. 4 M nitric acid? 4 -39 0. 035 mol HNO 3 2. 1 x 1022 H+ ions

Writing Ionic Equations for Acid-Base Reactions PROBLEM: Write balanced molecular, total ionic, and net ionic equations for the following acid-base reactions and identify the spectator ions. (a) strontium hydroxide (aq) + perchloric acid (aq) → (b) barium hydroxide (aq) + sulfuric acid (aq) → Note: 4 -40 Only strong electrolytes completely dissociate into ions. Label the states of each compound/ion

Strong acid-strong base reaction: proton-transfer 4 -41

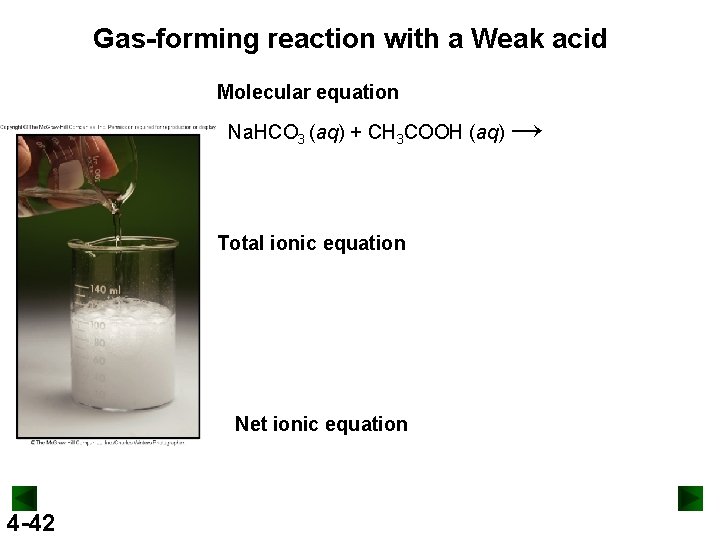
Gas-forming reaction with a Weak acid Molecular equation Na. HCO 3 (aq) + CH 3 COOH (aq) Total ionic equation Net ionic equation 4 -42 →

Writing Proton-Transfer Equations for Acid. Base Reactions Write balanced total and net ionic equations for the following reactions (a) hydroiodic acid (aq) + magnesium hydroxide (aq) → Give the name and formula of the salt present when the water evaporates. (b) potassium hydroxide (aq) + hydrofluoric acid (aq) → Note that propionic acid is a weak acid. Be sure to identify the spectator ions in this reaction. 4 -43

Acid-Base Titrations • Purpose: the concentration of one solution is used to determine the concentration of another. • In an acid-base titration, a standard solution of base is usually added to a sample of acid of unknown molarity. • An acid-base indicator has different colors in acid and base, and is used to monitor the reaction progress. • At the equivalence point, the mol of H+ from the acid equals the mol of OH- ion produced by the base. – Amount of H+ ion in flask = amount of OH- ion added • The end point occurs when there is a slight excess of base and the indicator changes color permanently. 4 -44

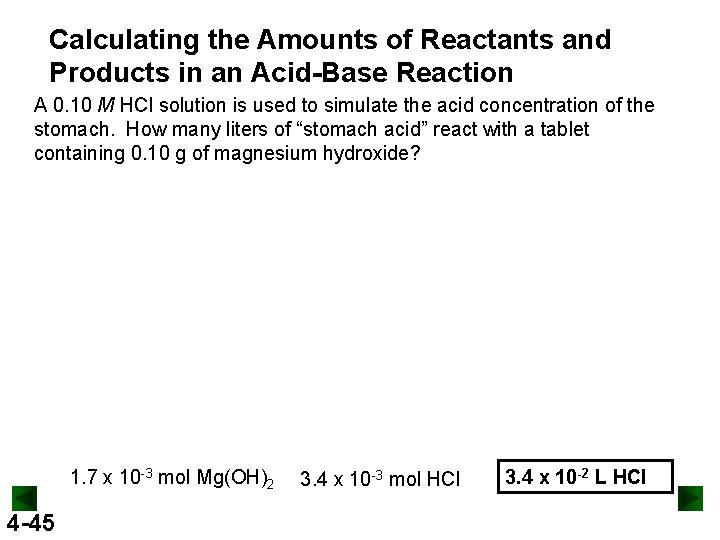
Calculating the Amounts of Reactants and Products in an Acid-Base Reaction A 0. 10 M HCl solution is used to simulate the acid concentration of the stomach. How many liters of “stomach acid” react with a tablet containing 0. 10 g of magnesium hydroxide? 1. 7 x 10 -3 mol Mg(OH)2 4 -45 3. 4 x 10 -3 mol HCl 3. 4 x 10 -2 L HCl

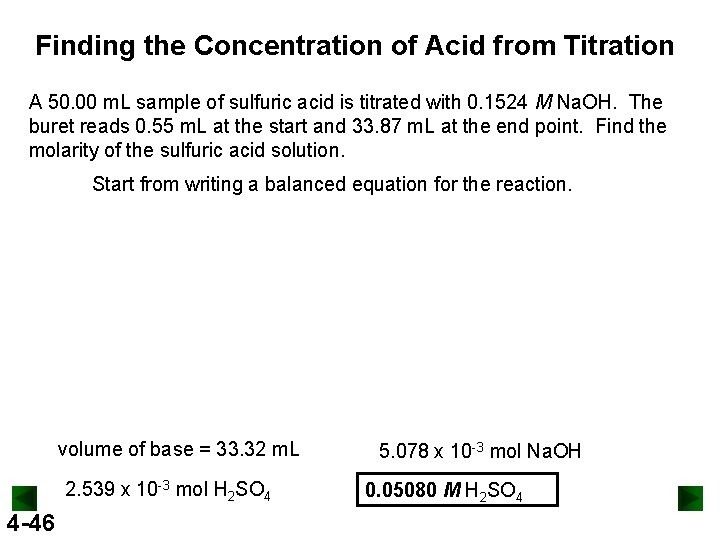
Finding the Concentration of Acid from Titration A 50. 00 m. L sample of sulfuric acid is titrated with 0. 1524 M Na. OH. The buret reads 0. 55 m. L at the start and 33. 87 m. L at the end point. Find the molarity of the sulfuric acid solution. Start from writing a balanced equation for the reaction. volume of base = 33. 32 m. L 2. 539 x 10 -3 mol H 2 SO 4 4 -46 5. 078 x 10 -3 mol Na. OH 0. 05080 M H 2 SO 4

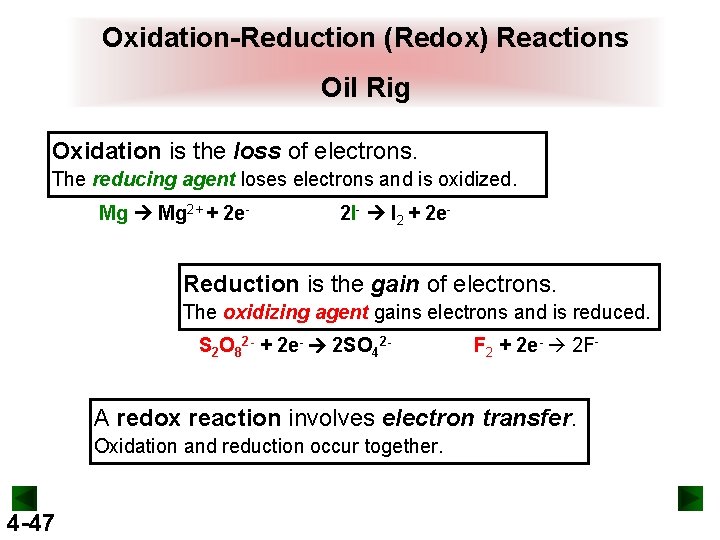
Oxidation-Reduction (Redox) Reactions Oil Rig Oxidation is the loss of electrons. The reducing agent loses electrons and is oxidized. Mg 2+ + 2 e- 2 I- I 2 + 2 e- Reduction is the gain of electrons. The oxidizing agent gains electrons and is reduced. S 2 O 82 - + 2 e- 2 SO 42 - F 2 + 2 e- 2 F- A redox reaction involves electron transfer. Oxidation and reduction occur together. 4 -47

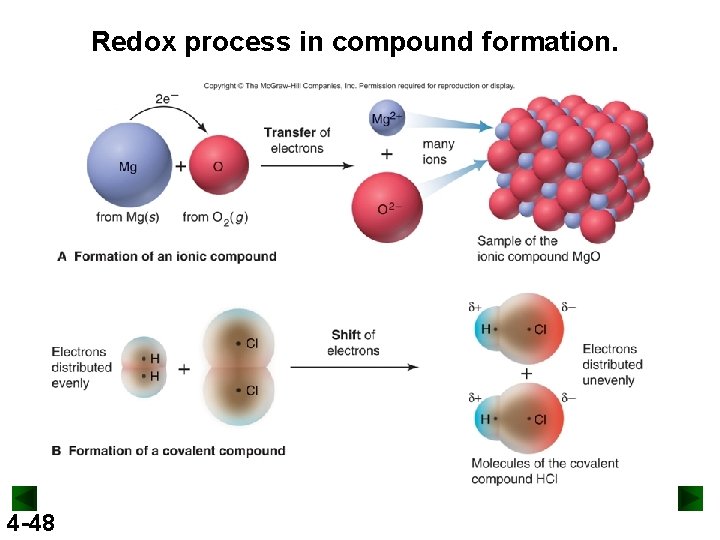
Redox process in compound formation. 4 -48

Rules for Assigning an Oxidation Number (O. N. ) General rules 1. For an atom in its elemental form (Na, O 2, Cl 2, etc. ): O. N. = 0 2. For a monoatomic ion: O. N. = ion charge 3. The sum of O. N. values for the atoms in a compound equals zero. The sum of O. N. values for the atoms in a polyatomic ion equals the ion’s charge. Rules for Specific Atoms or Periodic Table Groups 1. For Group 1 A(1): O. N. = +1 in all compounds 2. For Group 2 A(2): O. N. = +2 in all compounds 3. For hydrogen: O. N. = +1 in combination with nonmetals O. N. = -1 in combination with metals and boron O. N. = -1 in all compounds O. N. = -1 in peroxides O. N. = -2 in all other compounds(except with F) O. N. = -1 in combination with metals, nonmetals (except O), and other halogens lower in the group 4. For fluorine: 5. For oxygen: 6. For Group 7 A(17): 4 -49

Determining the Oxidation Number of Each Element in a Compound (or Ion) (a) zinc chloride (d) C 6 H 12 O 6 (a) Zn = +2, Cl = -1 (d) C = 0, H = +1, O = -2 4 -50 (b) sulfur trioxide (e) Na. H (b) S = +6, O = -2 (e) Na = +1, H = -1 (c) nitric acid (f) S 2 O 82 - (c) H = +1, N = +5, O = -2 (f) S = +7, O = -2

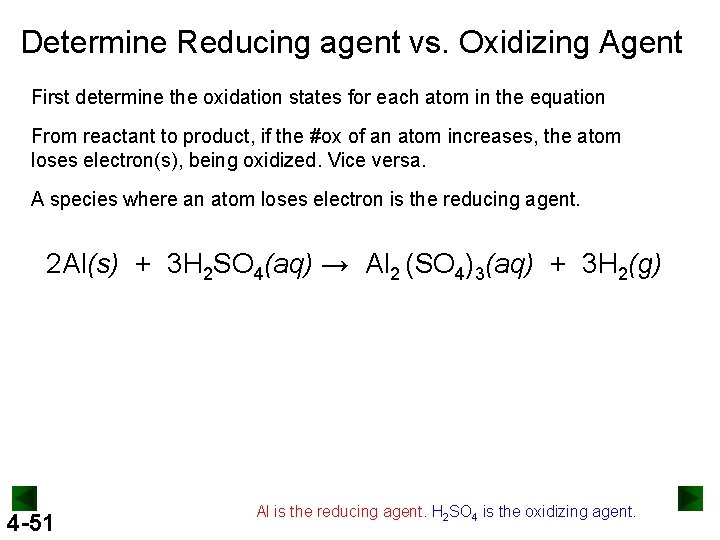
Determine Reducing agent vs. Oxidizing Agent First determine the oxidation states for each atom in the equation From reactant to product, if the #ox of an atom increases, the atom loses electron(s), being oxidized. Vice versa. A species where an atom loses electron is the reducing agent. 2 Al(s) + 3 H 2 SO 4(aq) → Al 2 (SO 4)3(aq) + 3 H 2(g) 4 -51 Al is the reducing agent. H 2 SO 4 is the oxidizing agent.

Oxidizing Agent is Reduced Reducing Agent is Oxidized 4 -52

Balancing Redox Reactions by the Half. Reaction Method (WPE) 4 -53 • The reaction is broken down into two half-reactions, based on the elements (except Oxygen or Hydrogen, why? ) • One of the half reactions for oxidation and another for reduction. • Each half-reaction includes electrons. – Electrons go on the product side of the oxidation half-reaction—loss of electrons. – Electrons go on the reactant side of the reduction half-reaction—gain of electrons. • Each half-reaction is balanced for its atoms. • Then the two half-reactions are adjusted so that the 53 electrons lost and gained will be equal when added. 53

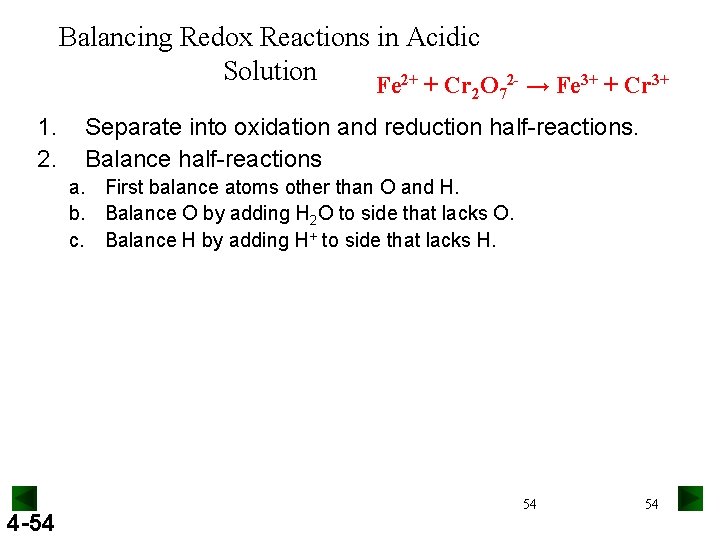
Balancing Redox Reactions in Acidic Solution Fe 2+ + Cr O 2 - → Fe 3+ + Cr 3+ 2 1. 2. 7 Separate into oxidation and reduction half-reactions. Balance half-reactions a. First balance atoms other than O and H. b. Balance O by adding H 2 O to side that lacks O. c. Balance H by adding H+ to side that lacks H. 4 -54 54 54

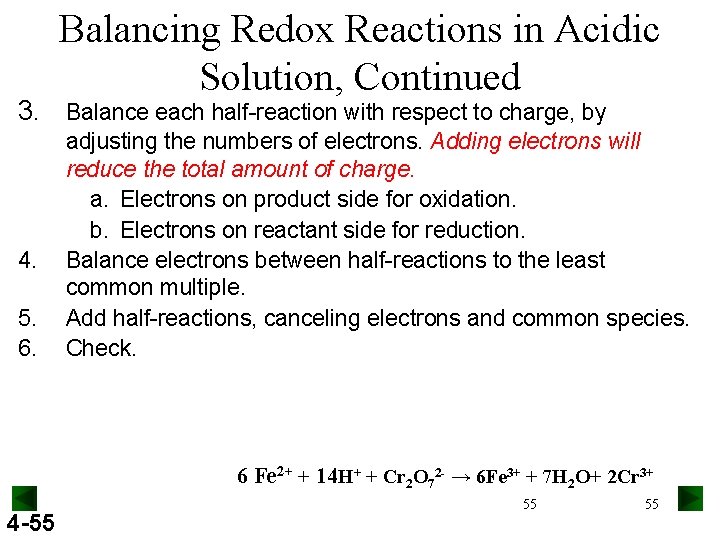
Balancing Redox Reactions in Acidic Solution, Continued 3. Balance each half-reaction with respect to charge, by 4. 5. 6. adjusting the numbers of electrons. Adding electrons will reduce the total amount of charge. a. Electrons on product side for oxidation. b. Electrons on reactant side for reduction. Balance electrons between half-reactions to the least common multiple. Add half-reactions, canceling electrons and common species. Check. 6 Fe 2+ + 14 H+ + Cr 2 O 72 - → 6 Fe 3+ + 7 H 2 O+ 2 Cr 3+ 4 -55 55 55

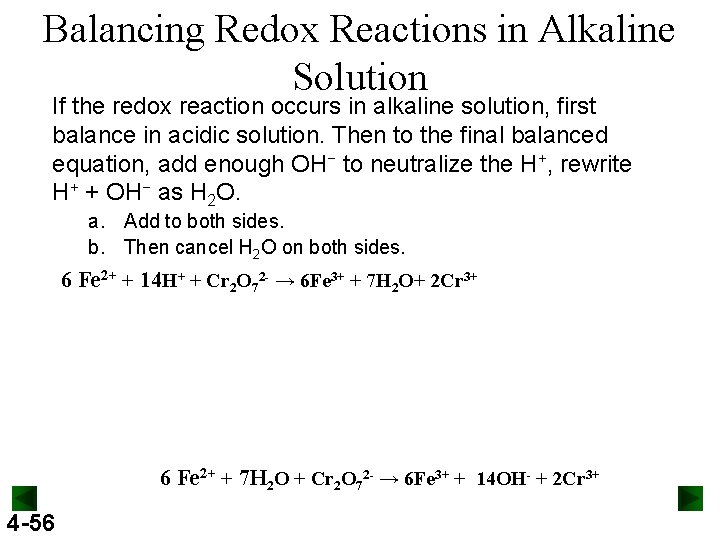
Balancing Redox Reactions in Alkaline Solution If the redox reaction occurs in alkaline solution, first balance in acidic solution. Then to the final balanced equation, add enough OH− to neutralize the H+, rewrite H+ + OH− as H 2 O. a. Add to both sides. b. Then cancel H 2 O on both sides. 6 Fe 2+ + 14 H+ + Cr 2 O 72 - → 6 Fe 3+ + 7 H 2 O+ 2 Cr 3+ 6 Fe 2+ + 7 H 2 O + Cr 2 O 72 - → 6 Fe 3+ + 14 OH- + 2 Cr 3+ 4 -56

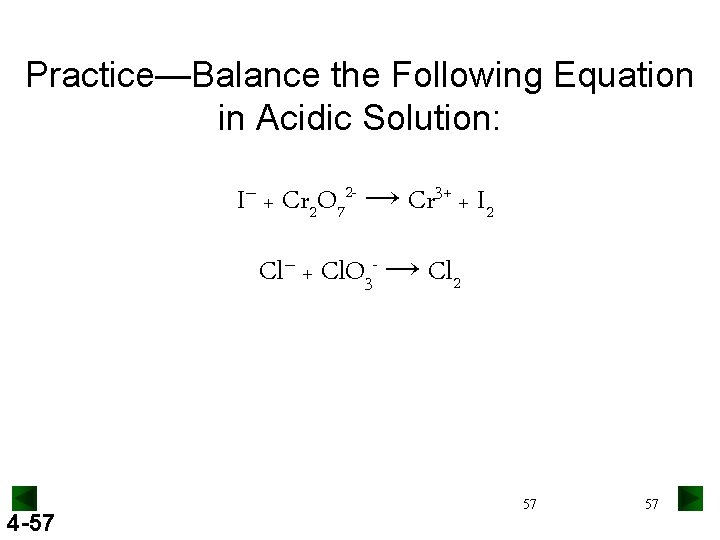
Practice—Balance the Following Equation in Acidic Solution: I– + Cr 2 O 72 - → Cr 3+ + I 2 Cl– + Cl. O 3 - → Cl 2 4 -57 57 57

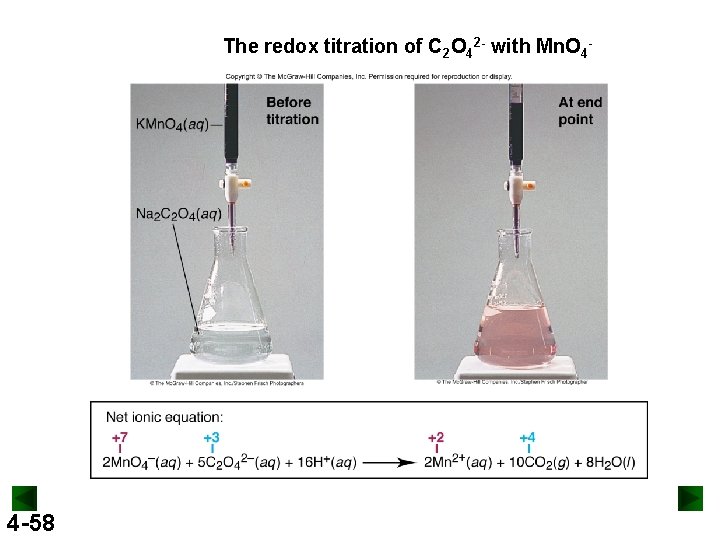
The redox titration of C 2 O 42 - with Mn. O 4 - 4 -58

Finding the Amount of Reducing Agent by Titration To measure the Ca 2+ concentration in human blood, 1. 00 m. L of blood was treated with Na 2 C 2 O 4 solution to precipitate the Ca 2+ as Ca. C 2 O 4. The precipitate was filtered and dissolved in dilute H 2 SO 4 to release C 2 O 42 -, which was titrated with KMn. O 4 solution. The solution required 2. 05 m. L of 4. 88 x 10 -4 M KMn. O 4 to reach the end point. The balanced equation is 2 KMn. O 4 (aq) + 5 Ca. C 2 O 4 (s) + 8 H 2 SO 4 (aq) → 2 Mn. SO 4 (aq) + K 2 SO 4 (aq) + 5 Ca. SO 4 (s) + 10 CO 2 (g) + 8 H 2 O (l) Calculate the amount (mol) of Ca 2+ in 1. 00 m. L of blood. 1. 00 x 10 -6 mol KMn. O 4 4 -59 2. 50 x 10 -6 mol Ca. C 2 O 4 2. 50 x 10 -6 mol Ca+2

Elements in Redox Reactions Types of Reactions • Combination Reactions – Two or more reactants combine to form a new compound: – X+Y→Z • Decomposition Reactions – A single compound decomposes to form two or more products: – Z→X+Y • Displacement Reactions – double diplacement: AB + CD → AD + CB – single displacement: X + YZ → XZ + Y • Combustion – the process of combining with O 2 4 -60

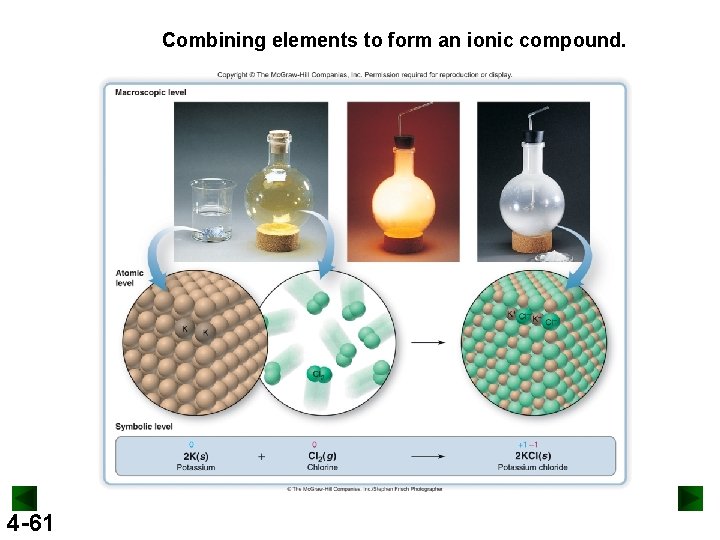
Combining elements to form an ionic compound. 4 -61

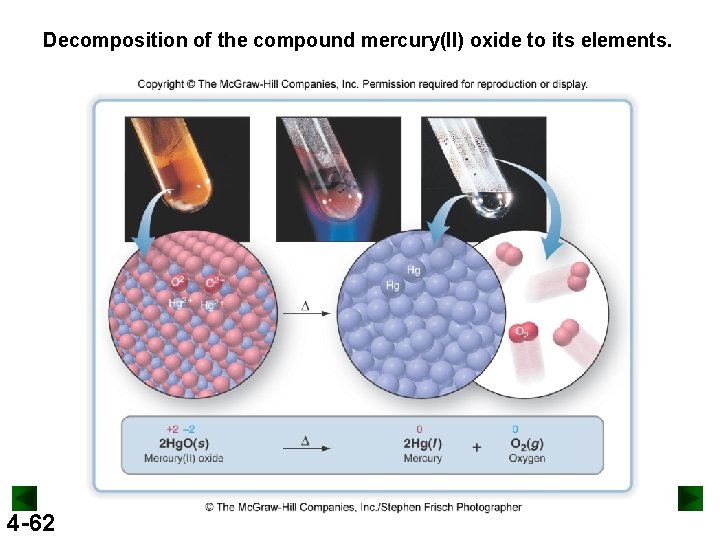
Decomposition of the compound mercury(II) oxide to its elements. 4 -62

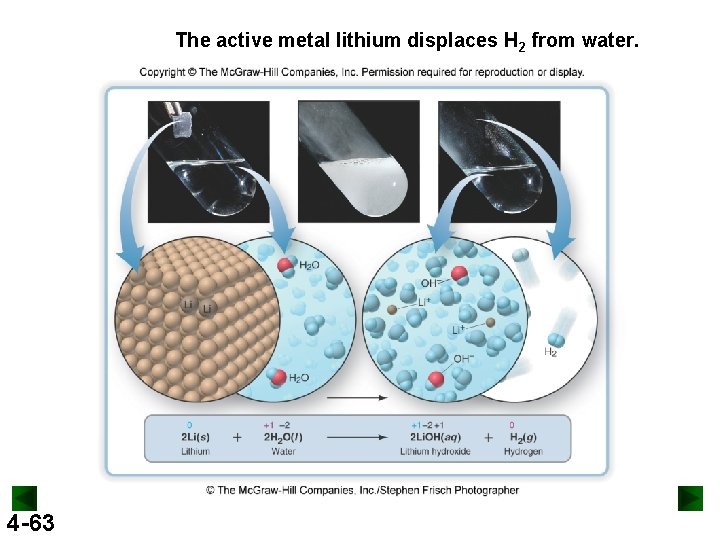
The active metal lithium displaces H 2 from water. 4 -63

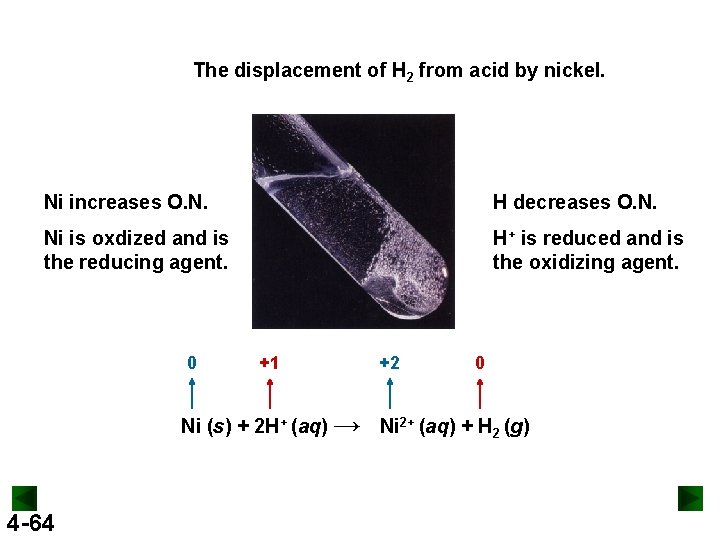
The displacement of H 2 from acid by nickel. Ni increases O. N. H decreases O. N. Ni is oxdized and is the reducing agent. H+ is reduced and is the oxidizing agent. 0 +1 +2 0 Ni (s) + 2 H+ (aq) → Ni 2+ (aq) + H 2 (g) 4 -64

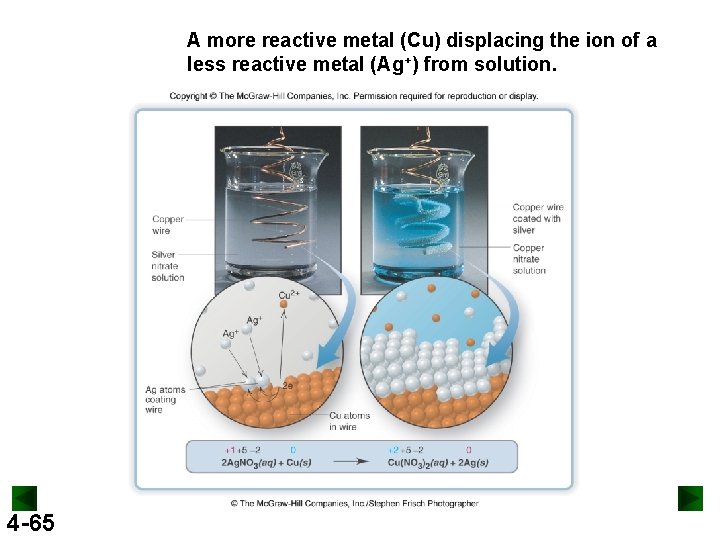
A more reactive metal (Cu) displacing the ion of a less reactive metal (Ag+) from solution. 4 -65

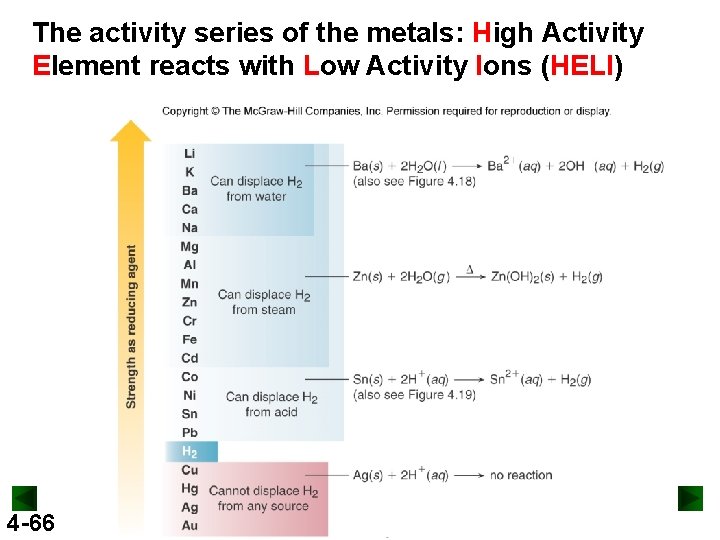
The activity series of the metals: High Activity Element reacts with Low Activity Ions (HELI) 4 -66

Identifying the Type of Redox Reaction PROBLEM: Classify each of the following redox reactions as a combination, decomposition, or displacement reaction. Write a balanced molecular equation for each, as well as total and net ionic equations for part (c), and identify the oxidizing and reducing agents: (a) magnesium (s) + nitrogen (g) → magnesium nitride (s) (b) hydrogen peroxide (l) → water (l) + oxygen gas (c) aluminum (s) + lead(II) nitrate (aq) → aluminum nitrate (aq) + lead (s) 4 -67

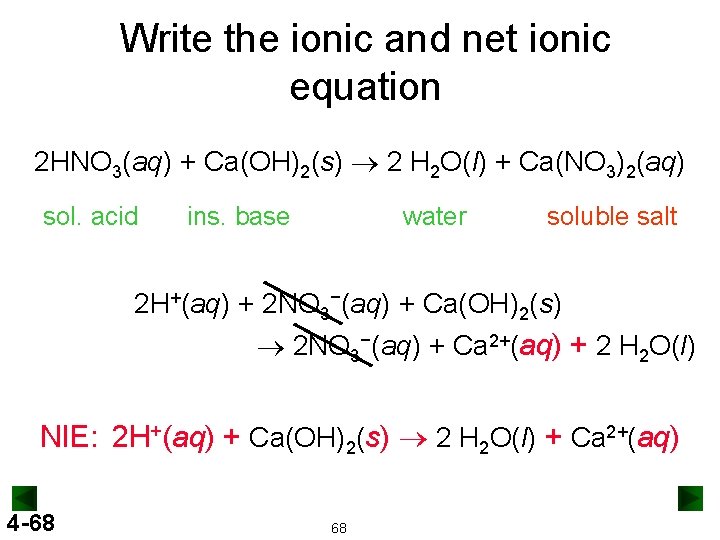
Write the ionic and net ionic equation 2 HNO 3(aq) + Ca(OH)2(s) ® 2 H 2 O(l) + Ca(NO 3)2(aq) sol. acid ins. base water soluble salt 2 H+(aq) + 2 NO 3−(aq) + Ca(OH)2(s) ® 2 NO 3−(aq) + Ca 2+(aq) + 2 H 2 O(l) NIE: 2 H+(aq) + Ca(OH)2(s) ® 2 H 2 O(l) + Ca 2+(aq) 4 -68 68

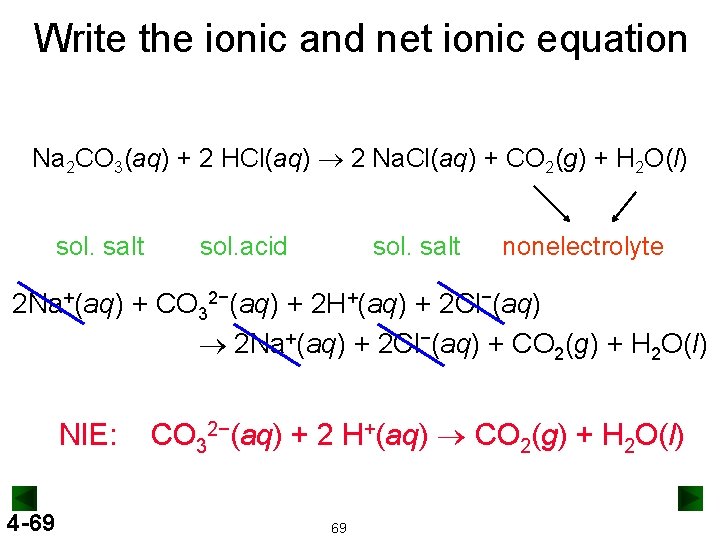
Write the ionic and net ionic equation Na 2 CO 3(aq) + 2 HCl(aq) ® 2 Na. Cl(aq) + CO 2(g) + H 2 O(l) sol. salt sol. acid sol. salt nonelectrolyte 2 Na+(aq) + CO 32−(aq) + 2 H+(aq) + 2 Cl−(aq) ® 2 Na+(aq) + 2 Cl−(aq) + CO 2(g) + H 2 O(l) NIE: 4 -69 CO 32−(aq) + 2 H+(aq) ® CO 2(g) + H 2 O(l) 69

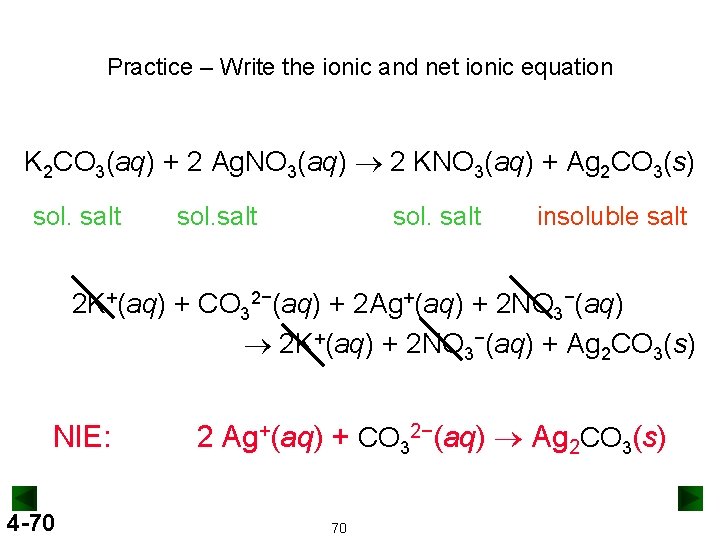
Practice – Write the ionic and net ionic equation K 2 CO 3(aq) + 2 Ag. NO 3(aq) ® 2 KNO 3(aq) + Ag 2 CO 3(s) sol. salt insoluble salt 2 K+(aq) + CO 32−(aq) + 2 Ag+(aq) + 2 NO 3−(aq) ® 2 K+(aq) + 2 NO 3−(aq) + Ag 2 CO 3(s) NIE: 4 -70 2 Ag+(aq) + CO 32−(aq) ® Ag 2 CO 3(s) 70

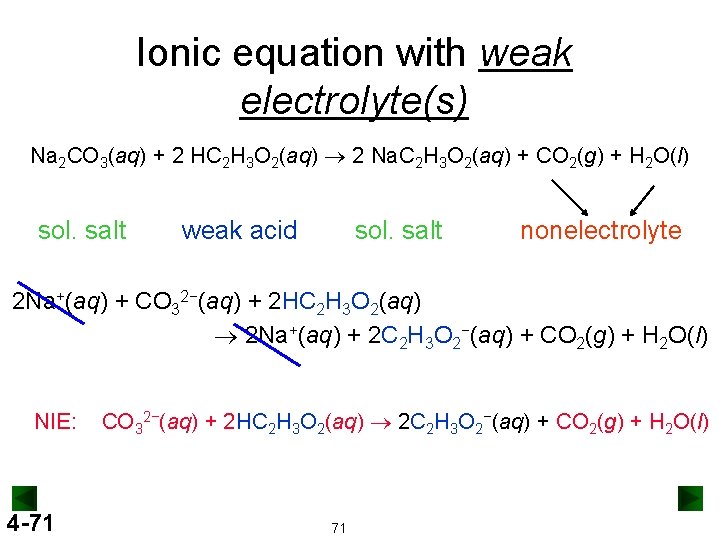
Ionic equation with weak electrolyte(s) Na 2 CO 3(aq) + 2 HC 2 H 3 O 2(aq) ® 2 Na. C 2 H 3 O 2(aq) + CO 2(g) + H 2 O(l) sol. salt weak acid sol. salt nonelectrolyte 2 Na+(aq) + CO 32−(aq) + 2 HC 2 H 3 O 2(aq) ® 2 Na+(aq) + 2 C 2 H 3 O 2−(aq) + CO 2(g) + H 2 O(l) NIE: 4 -71 CO 32−(aq) + 2 HC 2 H 3 O 2(aq) ® 2 C 2 H 3 O 2−(aq) + CO 2(g) + H 2 O(l) 71

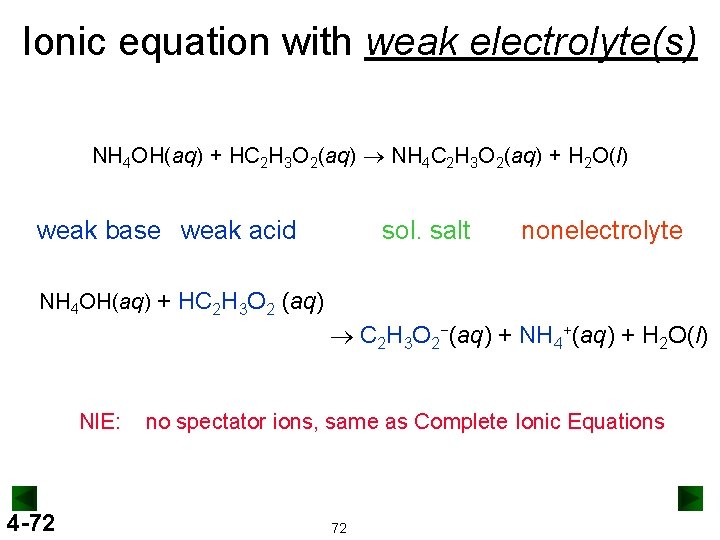
Ionic equation with weak electrolyte(s) NH 4 OH(aq) + HC 2 H 3 O 2(aq) ® NH 4 C 2 H 3 O 2(aq) + H 2 O(l) weak base weak acid sol. salt nonelectrolyte NH 4 OH(aq) + HC 2 H 3 O 2 (aq) ® C 2 H 3 O 2−(aq) + NH 4+(aq) + H 2 O(l) NIE: 4 -72 no spectator ions, same as Complete Ionic Equations 72

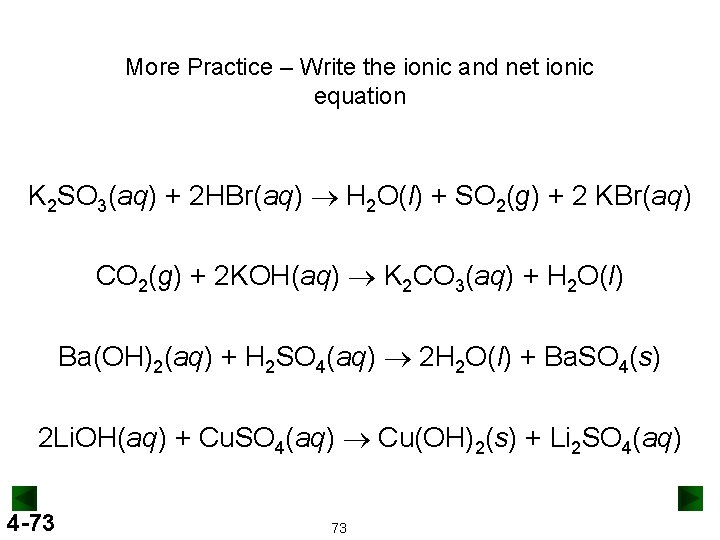
More Practice – Write the ionic and net ionic equation K 2 SO 3(aq) + 2 HBr(aq) ® H 2 O(l) + SO 2(g) + 2 KBr(aq) CO 2(g) + 2 KOH(aq) ® K 2 CO 3(aq) + H 2 O(l) Ba(OH)2(aq) + H 2 SO 4(aq) ® 2 H 2 O(l) + Ba. SO 4(s) 2 Li. OH(aq) + Cu. SO 4(aq) ® Cu(OH)2(s) + Li 2 SO 4(aq) 4 -73 73

More Practice – Convert and Complete, then Write the ionic and net ionic equation chloric acid + ammonium sulfite silver acetate + iron(III) bromide zinc hydroxide + sulfuric acid lithium sulfite + manganese(II) chloride 4 -74 74

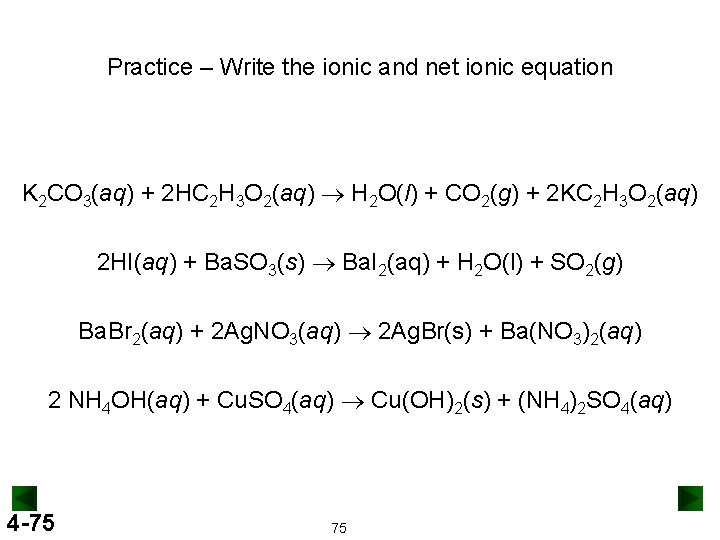
Practice – Write the ionic and net ionic equation K 2 CO 3(aq) + 2 HC 2 H 3 O 2(aq) ® H 2 O(l) + CO 2(g) + 2 KC 2 H 3 O 2(aq) 2 HI(aq) + Ba. SO 3(s) ® Ba. I 2(aq) + H 2 O(l) + SO 2(g) Ba. Br 2(aq) + 2 Ag. NO 3(aq) ® 2 Ag. Br(s) + Ba(NO 3)2(aq) 2 NH 4 OH(aq) + Cu. SO 4(aq) ® Cu(OH)2(s) + (NH 4)2 SO 4(aq) 4 -75 75

More Practice – Convert and Complete, then Write the ionic and net ionic equation sulfuric acid + ammonium hydroxide aluminum acetate + ammonium hydroxide iron(II) sulfite + acetic acid scandium(III) hydroxide + acetic acid 4 -76 76

Write and Balance the Ionic and Net Ionic Equation in aqueous solution • hydrobromic acid + zinc metal zinc bromide + hydrogen gas • chlorine gas + calcium hydroxide(aq) calcium chloride + calcium hypochlorite(aq) + water • Potassium hydroxide + iron(III) sulfate iron(III) hydroxide + potassium sulfate • acetic acid + magnesium metal magnesium acetate + hydrogen gas 4 -77 77
 Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Reactions in aqueous solutions
Reactions in aqueous solutions Calculate the number of grams of al in 371g of al2o3
Calculate the number of grams of al in 371g of al2o3 Chapter 4 reactions in aqueous solutions
Chapter 4 reactions in aqueous solutions Chapter 4 reactions in aqueous solutions
Chapter 4 reactions in aqueous solutions Chapter 4 reactions in aqueous solutions worksheet answers
Chapter 4 reactions in aqueous solutions worksheet answers Dilute solution
Dilute solution Aqueous reactions and solution stoichiometry
Aqueous reactions and solution stoichiometry Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Reactions that produce gas
Reactions that produce gas Modern chemistry chapter 13
Modern chemistry chapter 13 Aqueous solutions module
Aqueous solutions module Freezing point chapter 13
Freezing point chapter 13 General properties of aqueous solutions
General properties of aqueous solutions Electrical conductivity of aqueous solutions
Electrical conductivity of aqueous solutions Are all aqueous solutions homogeneous
Are all aqueous solutions homogeneous Are aqueous solutions homogeneous mixtures
Are aqueous solutions homogeneous mixtures Solubility guidelines for aqueous solutions
Solubility guidelines for aqueous solutions Balance redox half reactions
Balance redox half reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Proportional relationships in chemical reactions
Proportional relationships in chemical reactions Balancing chemical equations definition
Balancing chemical equations definition Types of chemical reactions redox
Types of chemical reactions redox How to identify types of chemical reactions
How to identify types of chemical reactions Types of reactions chemistry
Types of reactions chemistry Types of reactions
Types of reactions Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions Chapter 10 chemical reactions answer key
Chapter 10 chemical reactions answer key The calculation of quantities in chemical reactions
The calculation of quantities in chemical reactions Principles of immuno chemical reactions
Principles of immuno chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions Predicting products synthesis
Predicting products synthesis Section 3 predicting the products of chemical reactions
Section 3 predicting the products of chemical reactions Unit 11 chemical reactions
Unit 11 chemical reactions Toxic reactions chemical equations answer key
Toxic reactions chemical equations answer key Four types of chemical reactions
Four types of chemical reactions Biology-roots.com
Biology-roots.com Describing chemical reactions
Describing chemical reactions Chemical reactions classification
Chemical reactions classification Examples of chemical reactions in everyday life
Examples of chemical reactions in everyday life What are the five chemical changes
What are the five chemical changes Reactants and products
Reactants and products 5 general types of chemical reactions
5 general types of chemical reactions Solubility rules
Solubility rules Chapter 9 chemical reactions test answers
Chapter 9 chemical reactions test answers Equilibrium occurs when
Equilibrium occurs when Chapter 9 chemical reactions
Chapter 9 chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Chapter 8 review describing chemical reactions
Chapter 8 review describing chemical reactions Four types of chemical reactions
Four types of chemical reactions Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions 5 chemical reactions
5 chemical reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes What is the type of reaction
What is the type of reaction Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Chapter 11 chemical reactions practice problems
Chapter 11 chemical reactions practice problems Physical properties of esters
Physical properties of esters Summary of chemical reactions
Summary of chemical reactions Chemical reaction of bread
Chemical reaction of bread Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Solvent in chemical reactions
Solvent in chemical reactions Three types of chemical reactions
Three types of chemical reactions Mass relationships in chemical reactions
Mass relationships in chemical reactions Indications of a chemical reaction
Indications of a chemical reaction Describing chemical reactions
Describing chemical reactions Rules for chemical reactions
Rules for chemical reactions Rearranging atoms worksheet
Rearranging atoms worksheet Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Chapter 19 chemical reactions answer key
Chapter 19 chemical reactions answer key Classification of chemical reactions worksheet
Classification of chemical reactions worksheet What are the five general types of chemical reactions
What are the five general types of chemical reactions