OxidationReduction Reactions Redox LEO SAYS GER Oxidation and


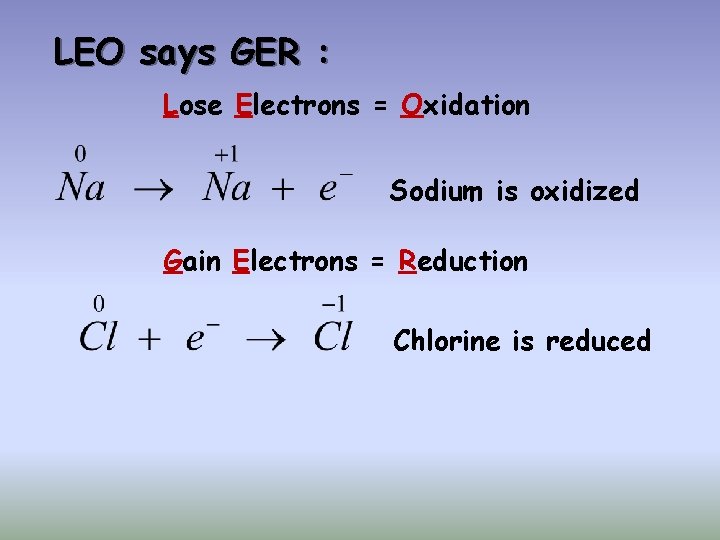





- Slides: 14

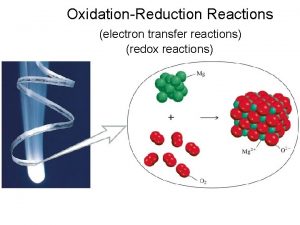
Oxidation-Reduction Reactions “Redox” LEO SAYS GER

Oxidation and Reduction (Redox) q Electrons are transferred q Spontaneous redox rxns can transfer energy q Electrons (electricity) q Heat q Non-spontaneous redox rxns can be made to happen with electricity

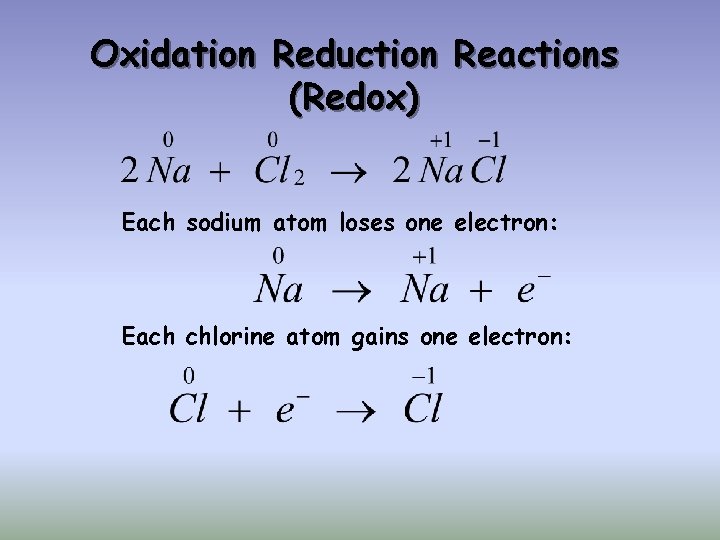
Oxidation Reduction Reactions (Redox) Each sodium atom loses one electron: Each chlorine atom gains one electron:
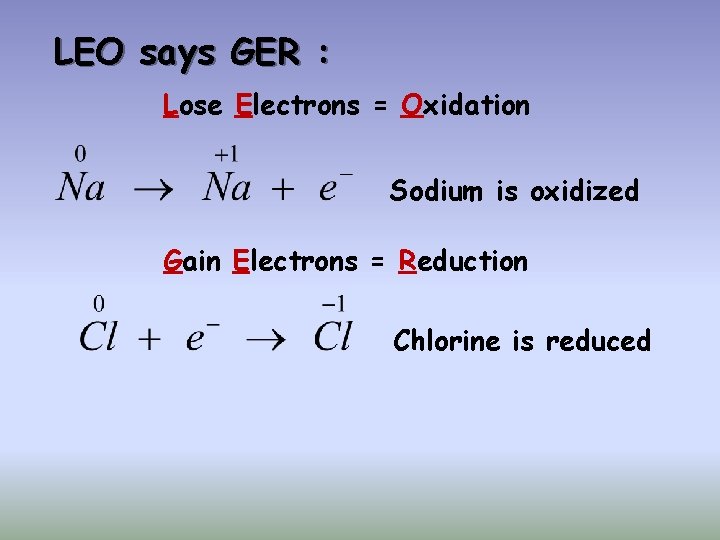
LEO says GER : Lose Electrons = Oxidation Sodium is oxidized Gain Electrons = Reduction Chlorine is reduced

Rules for Assigning Oxidation Numbers Rules 1 & 2 1. The oxidation number of any uncombined element is zero 2. The oxidation number of a monatomic ion equals its charge

Rules for Assigning Oxidation Numbers Rules 3 & 4 3. The oxidation number of oxygen in compounds is -2 4. The oxidation number of hydrogen in compounds is +1

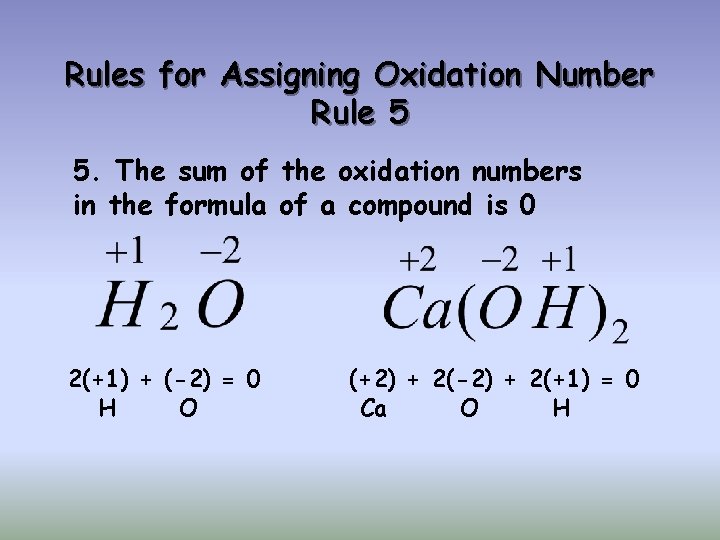
Rules for Assigning Oxidation Number Rule 5 5. The sum of the oxidation numbers in the formula of a compound is 0 2(+1) + (-2) = 0 H O (+2) + 2(-2) + 2(+1) = 0 Ca O H

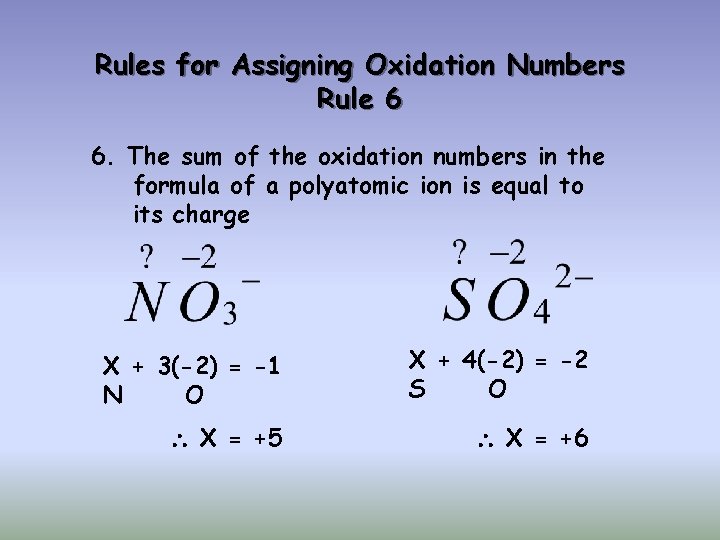
Rules for Assigning Oxidation Numbers Rule 6 6. The sum of the oxidation numbers in the formula of a polyatomic ion is equal to its charge X + 3(-2) = -1 N O X + 4(-2) = -2 S O X = +5 X = +6

The Oxidation Number Rules SIMPLIFIED 1. The sum of the oxidation numbers in ANYTHING is equal to its charge 2. Hydrogen in compounds is +1 3. Oxygen in compounds is -2

Not All Reactions are Redox Reactions in which there has been no change in oxidation number are not redox rxns. Examples:

This slide refers to vocabulary that has been excluded from AP Chemistry by the College Board, and will not be tested. Reducing Agents and Oxidizing Agents q The substance reduced is the oxidizing agent q The substance oxidized is the reducing agent Sodium is oxidized – it is the reducing agent Chlorine is reduced – it is the oxidizing agent

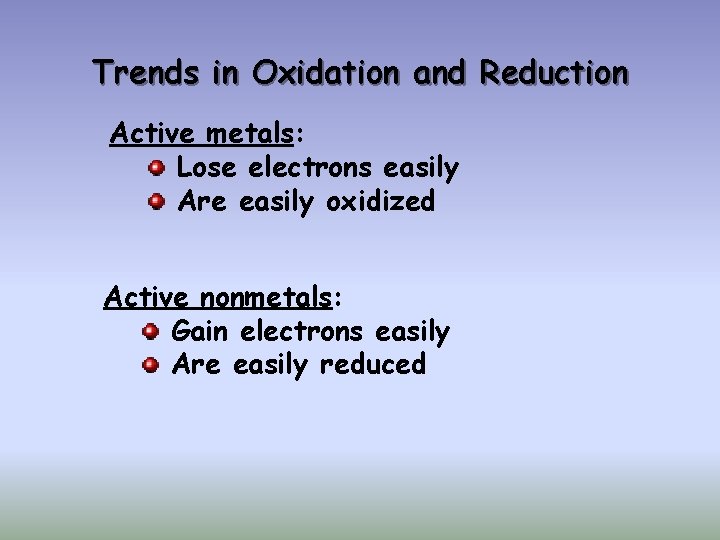
Trends in Oxidation and Reduction Active metals: Lose electrons easily Are easily oxidized Active nonmetals: Gain electrons easily Are easily reduced

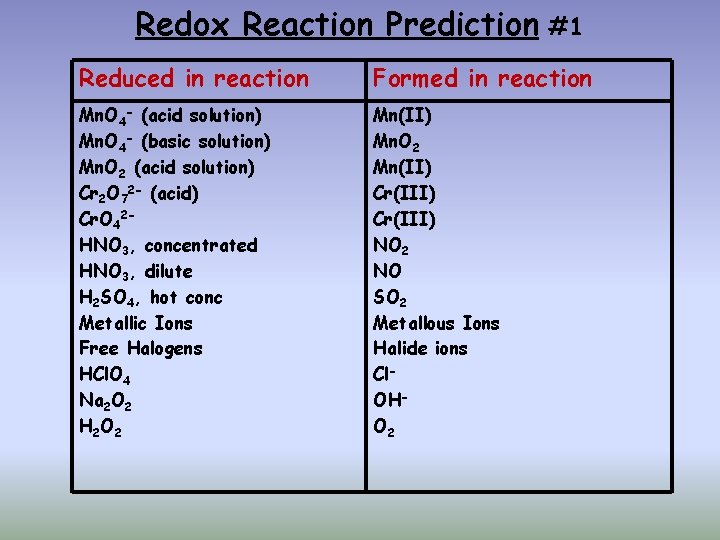
Redox Reaction Prediction #1 Reduced in reaction Formed in reaction Mn. O 4 - (acid solution) Mn. O 4 - (basic solution) Mn. O 2 (acid solution) Cr 2 O 72 - (acid) Cr. O 42 HNO 3, concentrated HNO 3, dilute H 2 SO 4, hot conc Metallic Ions Free Halogens HCl. O 4 Na 2 O 2 H 2 O 2 Mn(II) Mn. O 2 Mn(II) Cr(III) NO 2 NO SO 2 Metallous Ions Halide ions Cl. OHO 2

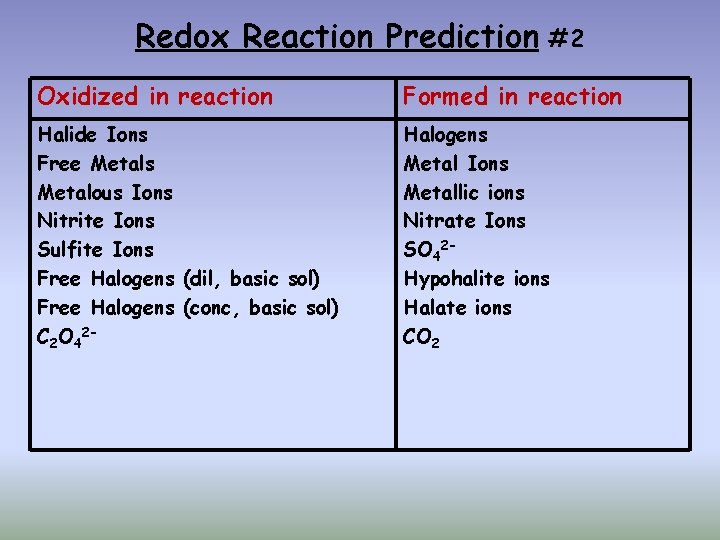
Redox Reaction Prediction #2 Oxidized in reaction Formed in reaction Halide Ions Free Metals Metalous Ions Nitrite Ions Sulfite Ions Free Halogens (dil, basic sol) Free Halogens (conc, basic sol) C 2 O 42 - Halogens Metal Ions Metallic ions Nitrate Ions SO 42 Hypohalite ions Halate ions CO 2