Topic Redox Half reactions Do Now Quiz assign

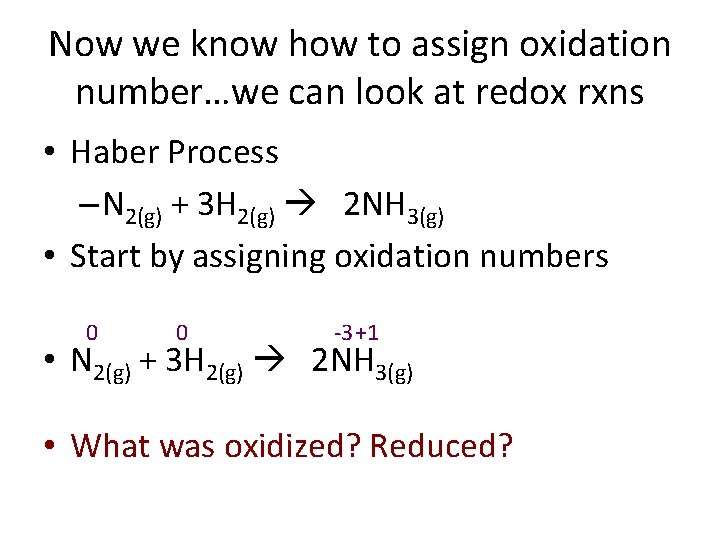
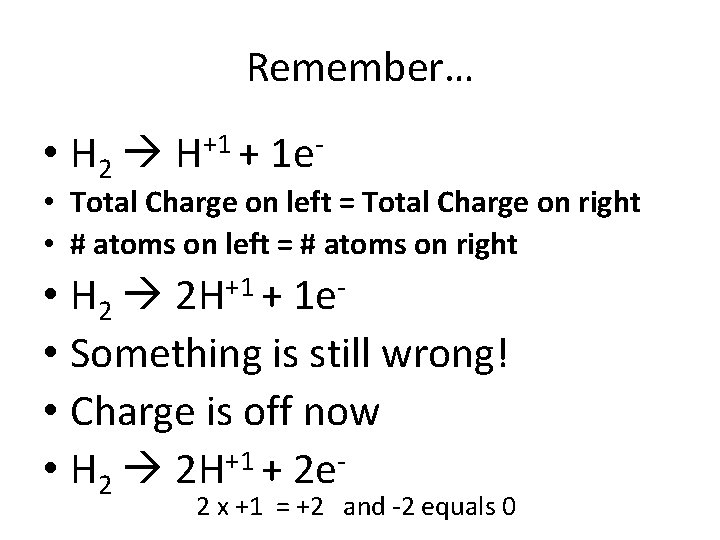


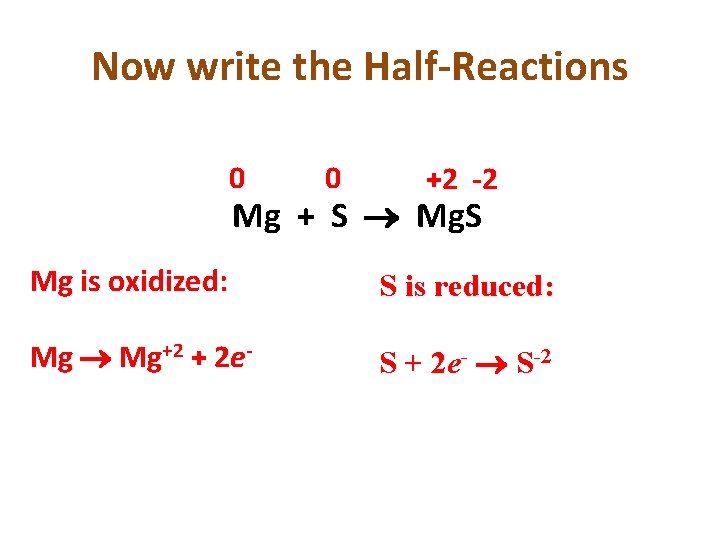



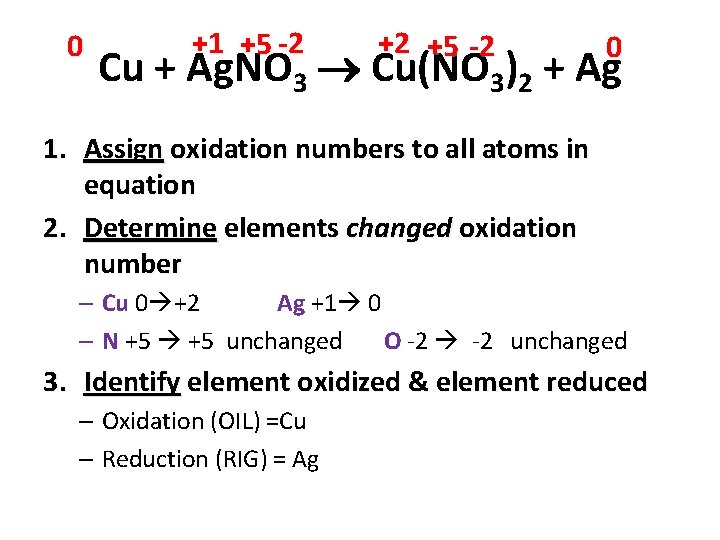
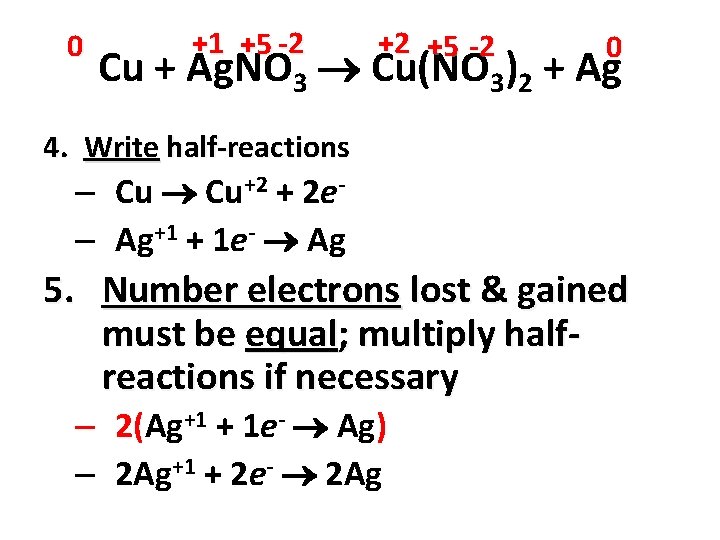
- Slides: 19

Topic: Redox – Half reactions Do Now: Quiz assign oxidation numbers
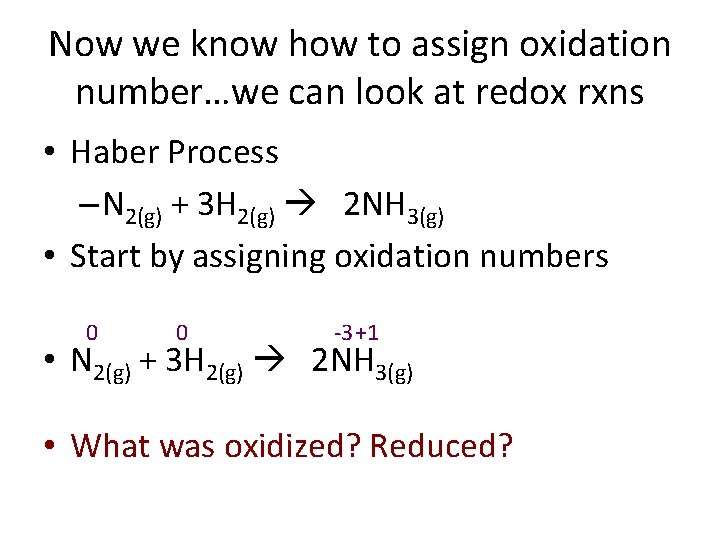
Now we know how to assign oxidation number…we can look at redox rxns • Haber Process – N 2(g) + 3 H 2(g) 2 NH 3(g) • Start by assigning oxidation numbers 0 0 -3 +1 • N 2(g) + 3 H 2(g) 2 NH 3(g) • What was oxidized? Reduced?

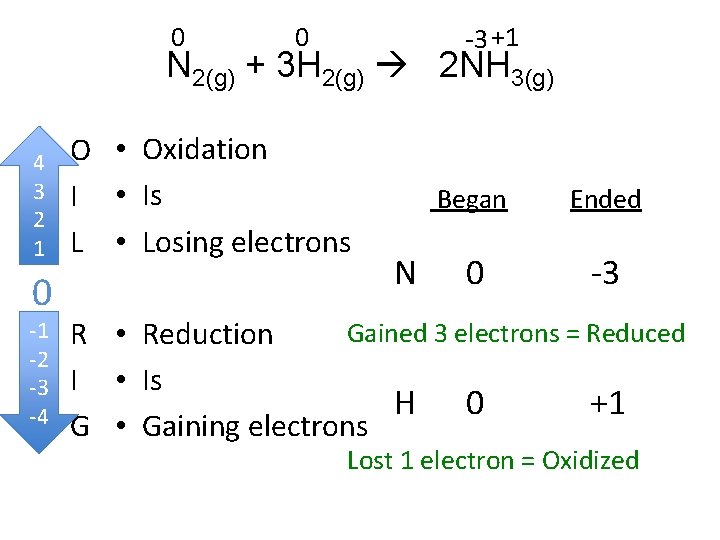
0 0 -3 +1 N 2(g) + 3 H 2(g) 2 NH 3(g) 4 • 3 • 2 1 • 0 -1 • -2 -3 • -4 • O • Oxidation I • Is L • Losing electrons N Began Ended 0 -3 Gained 3 electrons = Reduced R • Reduction I • Is H 0 +1 G • Gaining electrons Lost 1 electron = Oxidized

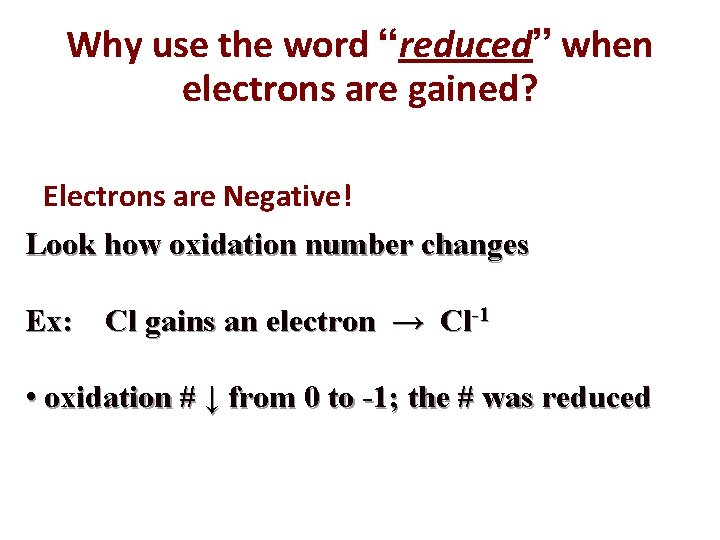
Why use the word “reduced” when electrons are gained? Electrons are Negative! Look how oxidation number changes Ex: Cl gains an electron → Cl-1 • oxidation # ↓ from 0 to -1; the # was reduced

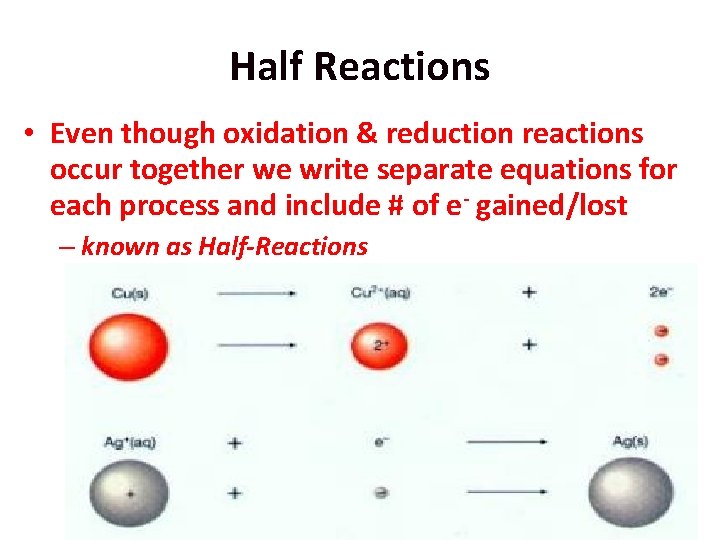
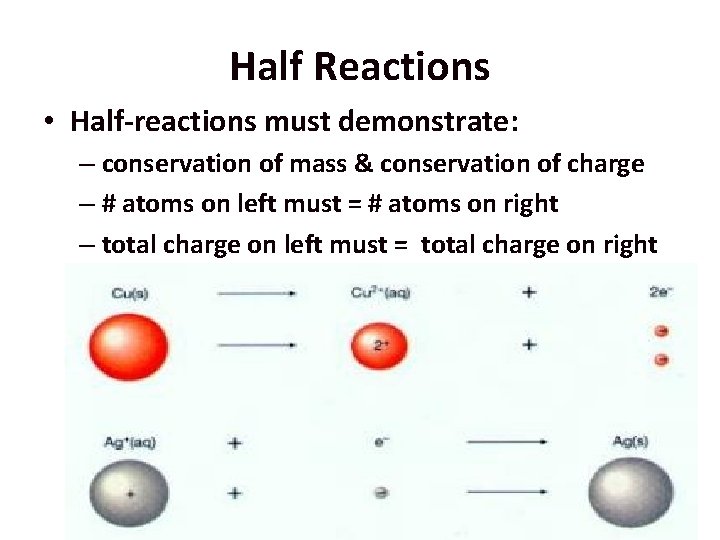
Half Reactions • Even though oxidation & reduction reactions occur together we write separate equations for each process and include # of e- gained/lost – known as Half-Reactions

Half Reactions • Half-reactions must demonstrate: – conservation of mass & conservation of charge – # atoms on left must = # atoms on right – total charge on left must = total charge on right

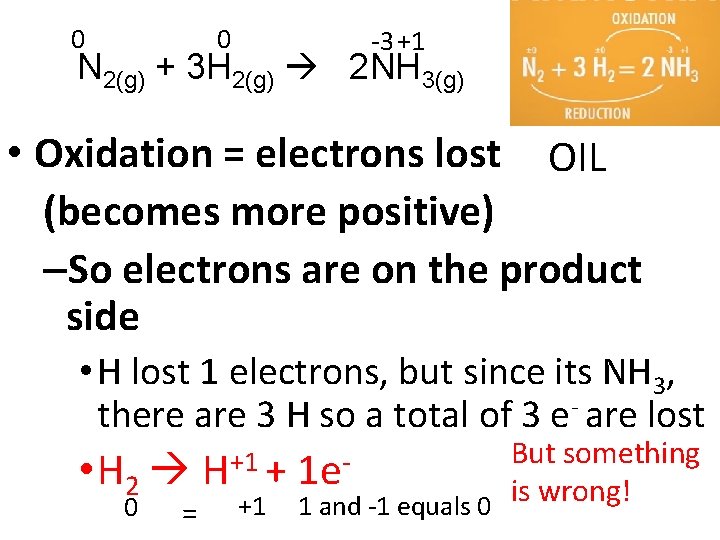
0 0 -3 +1 N 2(g) + 3 H 2(g) 2 NH 3(g) • Oxidation = electrons lost OIL (becomes more positive) –So electrons are on the product side • H lost 1 electrons, but since its NH 3, there are 3 H so a total of 3 e- are lost But something +1 • H 2 H + 1 e is wrong! +1 1 and -1 equals 0 0 =
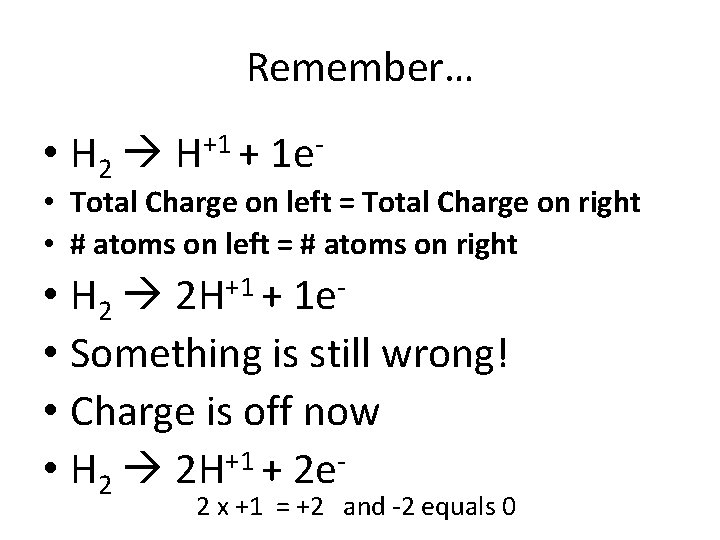
Remember… • H 2 H+1 + 1 e- • Total Charge on left = Total Charge on right • # atoms on left = # atoms on right • H 2 2 H+1 + 1 e • Something is still wrong! • Charge is off now • H 2 2 H+1 + 2 e- 2 x +1 = +2 and -2 equals 0

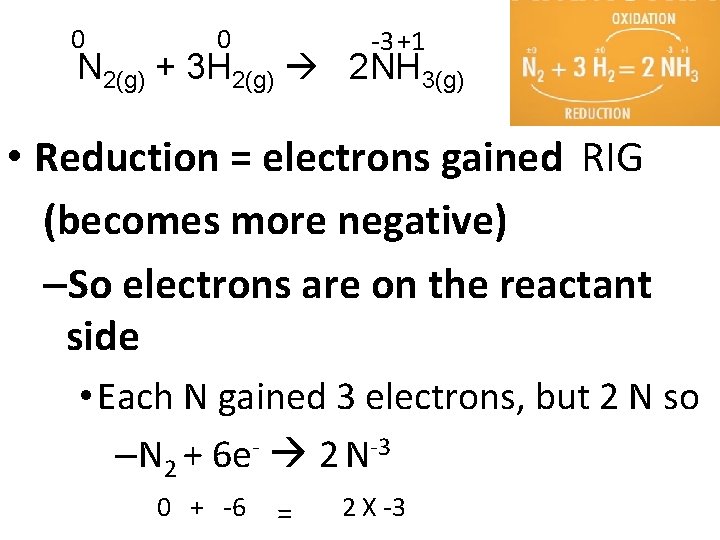
0 0 -3 +1 N 2(g) + 3 H 2(g) 2 NH 3(g) • Reduction = electrons gained RIG (becomes more negative) –So electrons are on the reactant side • Each N gained 3 electrons, but 2 N so –N 2 + 6 e- 2 N-3 0 + -6 = 2 X -3

Oxidizing Agent: Substance being reduced – Accepts electrons – aids oxidation for another species Nitrogen is the Oxidizing Agent Reducing Agent: Substance being oxidized – Loses electrons – aids reduction for another species Hydrogen is the Reducing Agent

You Try: • • 0 0 +2 -2 Mg + S Mg. S What is oxidized? What is reduced? Assign Oxidation Numbers Figure out change in oxidation numbers Mg: 0 to +2 = Oxidation S: 0 to -2 = Reduction
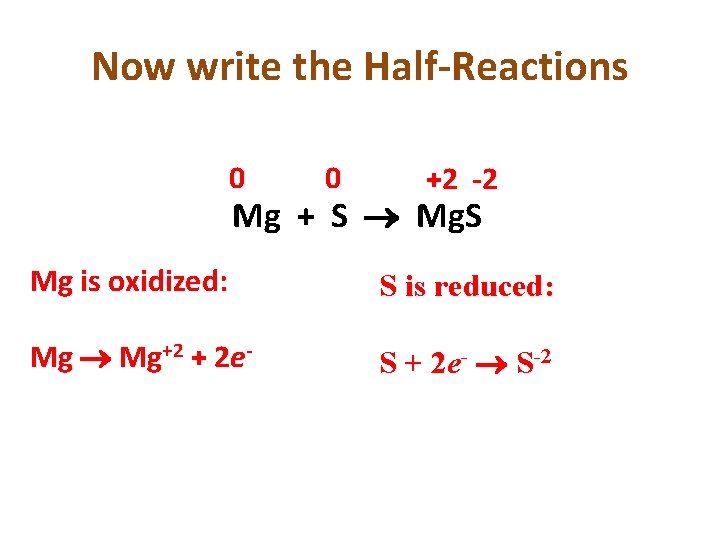
Now write the Half-Reactions 0 0 +2 -2 Mg + S Mg. S Mg is oxidized: S is reduced: Mg+2 + 2 e- S + 2 e- S-2

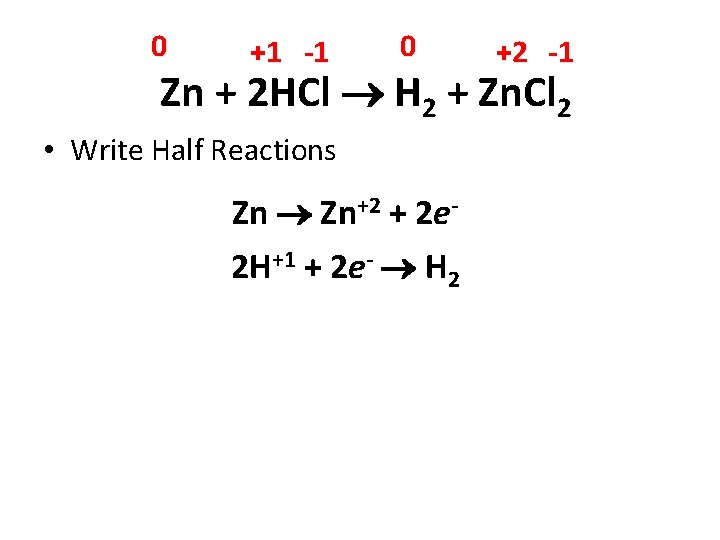
0 +1 -1 0 +2 -1 Zn + 2 HCl H 2 + Zn. Cl 2 What is oxidized? Reduced? • Zn goes from 0 to +2 = oxidation • H goes from +1 to 0 = reduction • Cl goes from -1 to -1; No change

0 +1 -1 0 +2 -1 Zn + 2 HCl H 2 + Zn. Cl 2 • Write Half Reactions Zn Zn+2 + 2 e 2 H+1 + 2 e- H 2

Taking it one step further

The steps… 1. 2. 3. 4. Assign oxidation numbers to all atoms in equation Determine elements changed oxidation number Identify element oxidized & element reduced Write half-reactions (diatomics must stay as is, everyone you can write with their oxidation number only – NH 3 = N-3 but Cl 2 stays Cl 2 not Cl) 5. Number electrons lost & gained must be equal; multiply half-reactions if necessary 6. Add half-reactions; Transfer coefficients to skeleton equation 7. Balance rest of equation by counting atoms
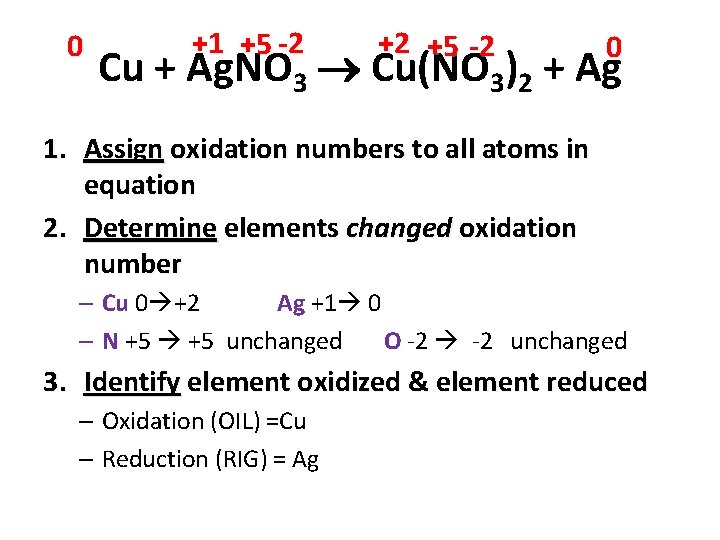
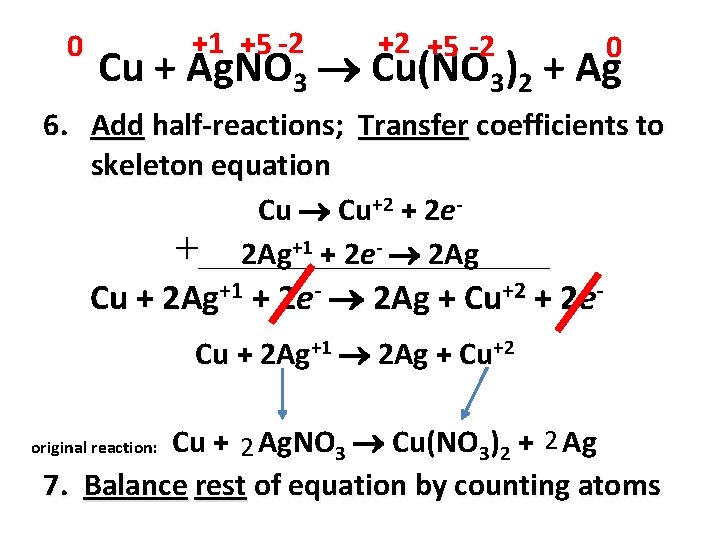
0 +1 +5 -2 +2 +5 -2 0 Cu + Ag. NO 3 Cu(NO 3)2 + Ag 1. Assign oxidation numbers to all atoms in equation 2. Determine elements changed oxidation number – Cu 0 +2 Ag +1 0 – N +5 unchanged O -2 unchanged 3. Identify element oxidized & element reduced – Oxidation (OIL) =Cu – Reduction (RIG) = Ag
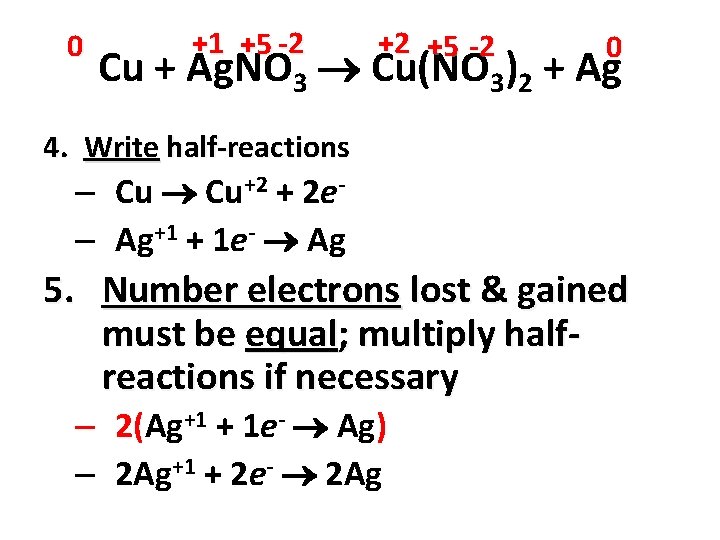
0 +1 +5 -2 +2 +5 -2 0 Cu + Ag. NO 3 Cu(NO 3)2 + Ag 4. Write half-reactions – Cu Cu+2 + 2 e– Ag+1 + 1 e- Ag 5. Number electrons lost & gained must be equal; multiply halfreactions if necessary – 2(Ag+1 + 1 e- Ag) – 2 Ag+1 + 2 e- 2 Ag

0 +1 +5 -2 +2 +5 -2 0 Cu + Ag. NO 3 Cu(NO 3)2 + Ag 6. Add half-reactions; Transfer coefficients to skeleton equation Cu Cu+2 + 2 e+___________ 2 Ag+1 + 2 e- 2 Ag Cu + 2 Ag+1 + 2 e- 2 Ag + Cu+2 + 2 e. Cu + 2 Ag+1 2 Ag + Cu+2 Cu + 2 Ag. NO 3 Cu(NO 3)2 + 2 Ag 7. Balance rest of equation by counting atoms original reaction: