Molecules and Compounds Display Constant Composition Elements combine
Molecules and Compounds Display Constant Composition • Elements combine in fixed Proportions • Law of Constant Composition – H 2 O – CO 2
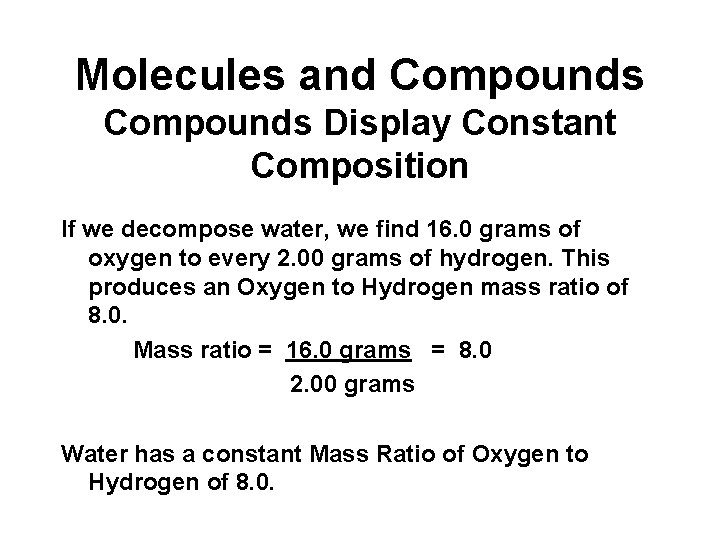
Molecules and Compounds Display Constant Composition If we decompose water, we find 16. 0 grams of oxygen to every 2. 00 grams of hydrogen. This produces an Oxygen to Hydrogen mass ratio of 8. 0. Mass ratio = 16. 0 grams = 8. 0 2. 00 grams Water has a constant Mass Ratio of Oxygen to Hydrogen of 8. 0.
Molecules and Compounds Chemical Formulas: How to Represent Compounds • Chemical Formula – Elements present – Relative numbers • Subscripts – Part of the definition – Changing subscript changes compound • Metals – Left-hand side of Periodic Table – Listed first • Nonmetals – Right-hand side of the Periodic Table – Those to the left– More Metal-like – Listed first
Significance of Chemical Formulas • A chemical formula indicates the relative number of atoms of each kind in a chemical compound. • For a molecular compound, the chemical formula reveals the number of atoms of each element contained in a single molecule of the compound. – example: octane — C 8 H 18
Molecules and Compounds Writing Chemical Formulas • Compound containing 2 aluminum atoms to every 3 oxygen atoms – Al 2 O 3 • Compound containing 3 oxygen atoms to every 1 sulfur atom – SO 3
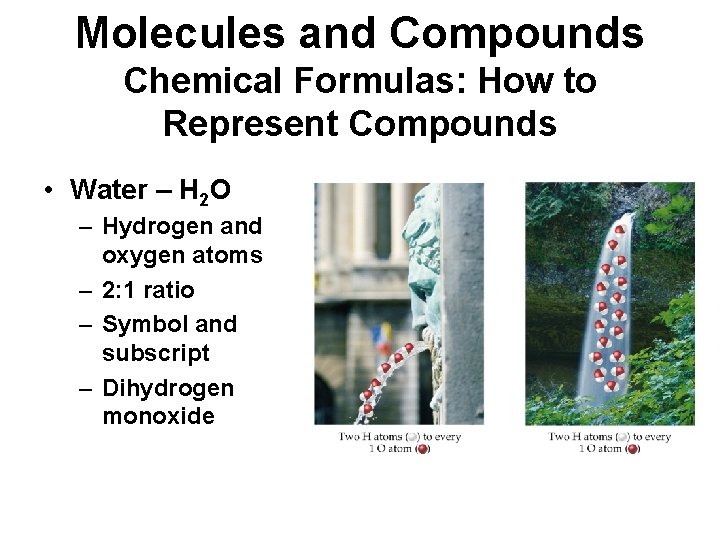
Molecules and Compounds Chemical Formulas: How to Represent Compounds • Water – H 2 O – Hydrogen and oxygen atoms – 2: 1 ratio – Symbol and subscript – Dihydrogen monoxide
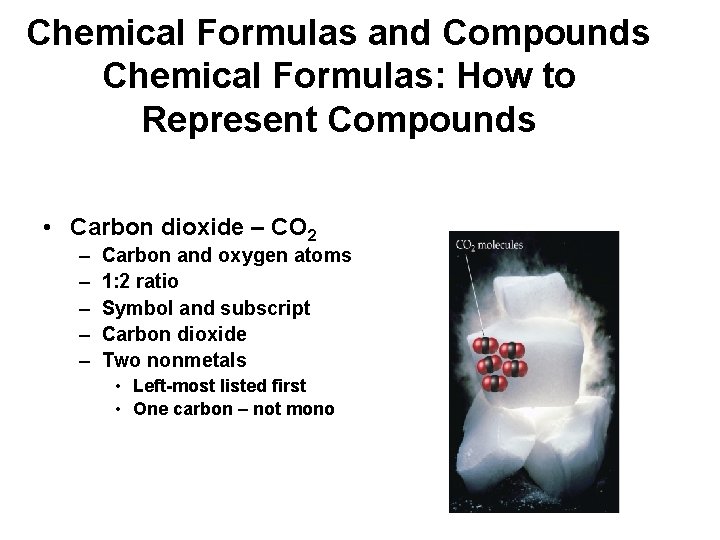
Chemical Formulas and Compounds Chemical Formulas: How to Represent Compounds • Carbon dioxide – CO 2 – – – Carbon and oxygen atoms 1: 2 ratio Symbol and subscript Carbon dioxide Two nonmetals • Left-most listed first • One carbon – not mono

• The chemical formula for an ionic compound represents one formula unit— the simplest ratio of the compound’s positive ions (cations) and its negative ions (anions). – example: aluminum sulfate — Al 2(SO 4)3 – Parentheses surround the polyatomic ion a unit. The subscript 3 refers to the unit. SO 42 - to identify it as

Molecules and Compounds Writing Chemical Formulas Polyatomic Ions • Contain several identical groupings of atoms • Polyatomic Ions • Parenthesis • Mg(NO 3)2 • Magnesium nitrate
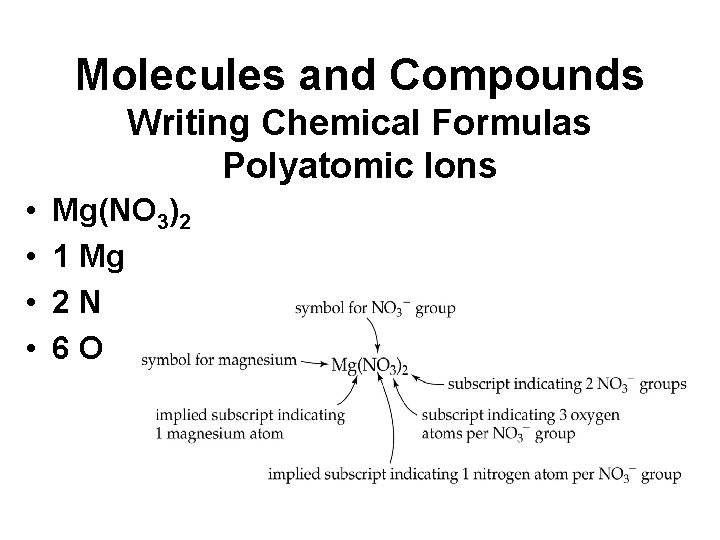
Molecules and Compounds Writing Chemical Formulas Polyatomic Ions • • Mg(NO 3)2 1 Mg 2 N 6 O
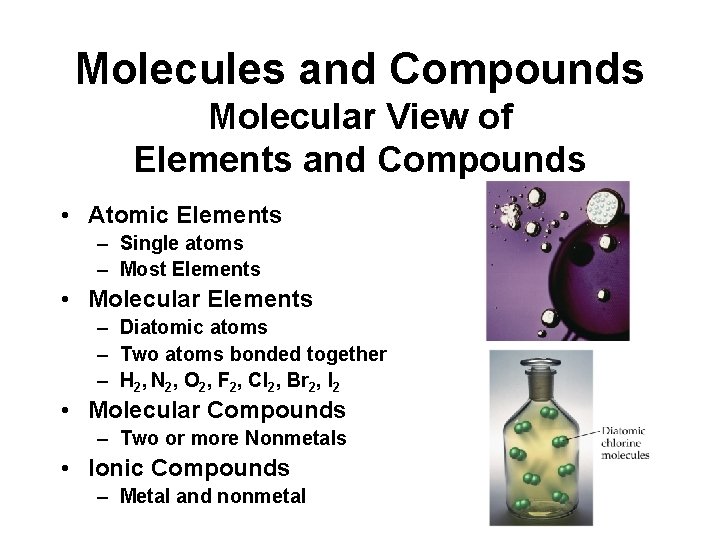
Molecules and Compounds Molecular View of Elements and Compounds • Atomic Elements – Single atoms – Most Elements • Molecular Elements – Diatomic atoms – Two atoms bonded together – H 2, N 2, O 2, F 2, Cl 2, Br 2, I 2 • Molecular Compounds – Two or more Nonmetals • Ionic Compounds – Metal and nonmetal
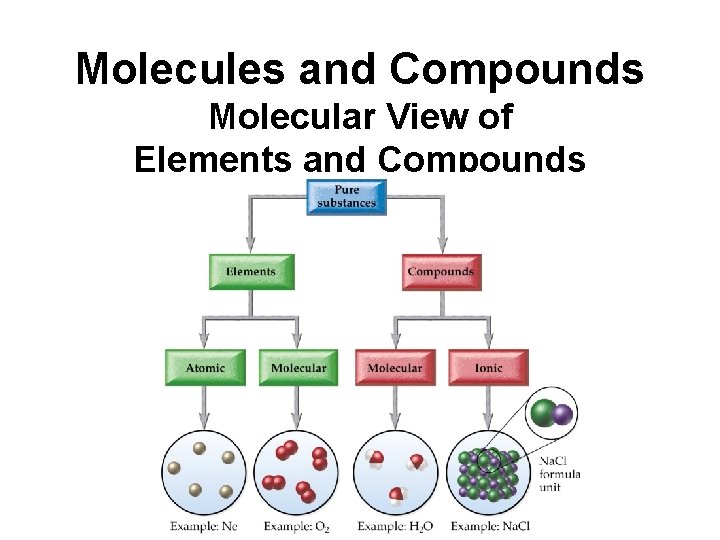
Molecules and Compounds Molecular View of Elements and Compounds
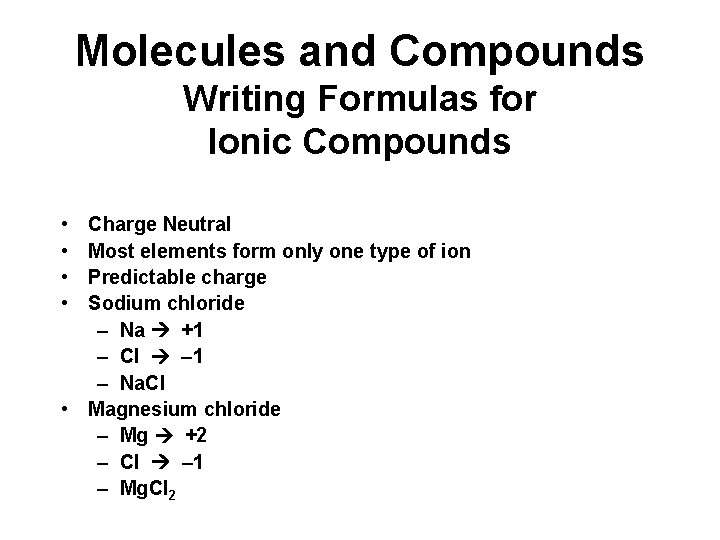
Molecules and Compounds Writing Formulas for Ionic Compounds • • Charge Neutral Most elements form only one type of ion Predictable charge Sodium chloride – Na +1 – Cl – 1 – Na. Cl • Magnesium chloride – Mg +2 – Cl – 1 – Mg. Cl 2
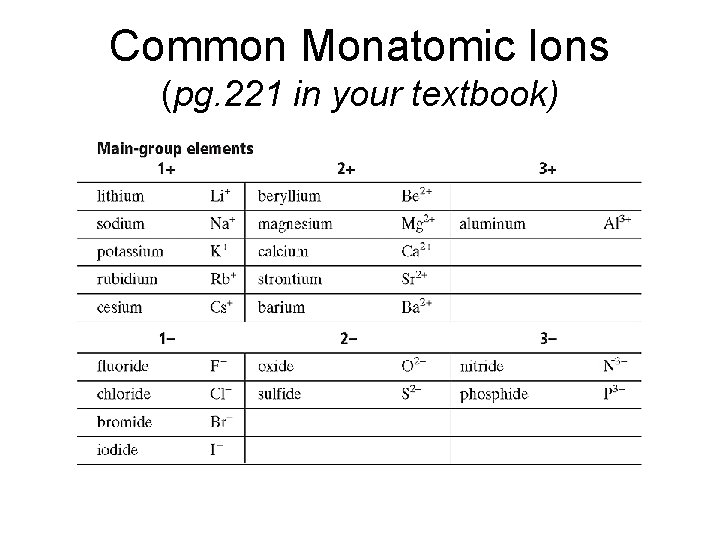
Common Monatomic Ions (pg. 221 in your textbook)
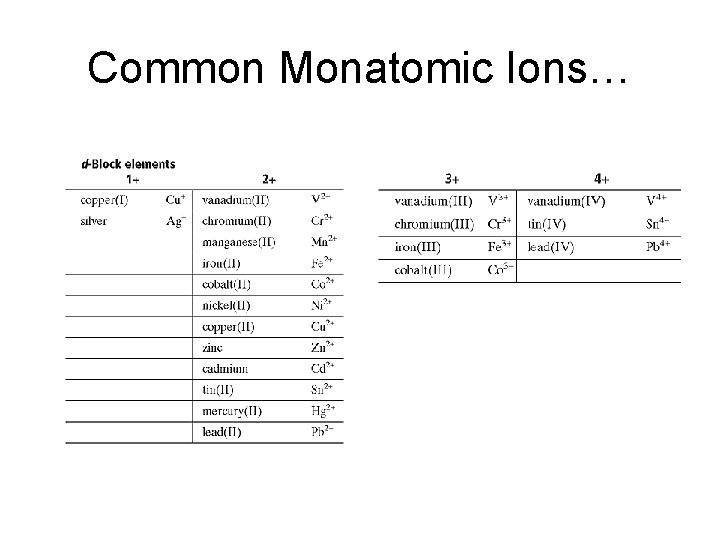
Common Monatomic Ions…
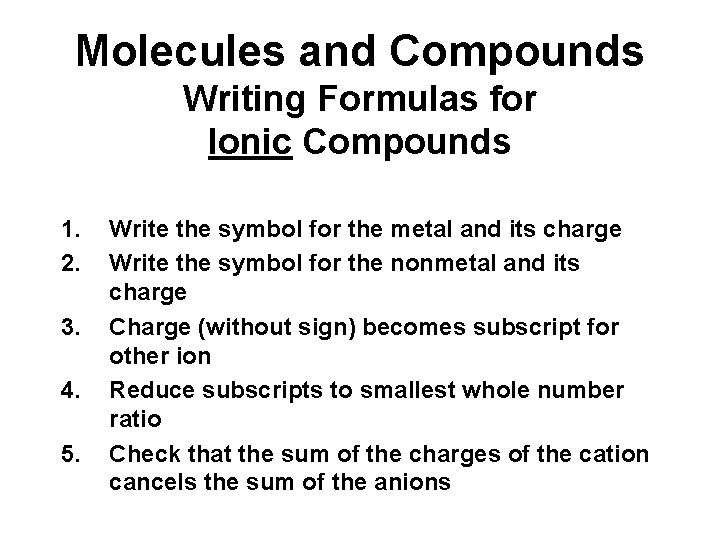
Molecules and Compounds Writing Formulas for Ionic Compounds 1. 2. 3. 4. 5. Write the symbol for the metal and its charge Write the symbol for the nonmetal and its charge Charge (without sign) becomes subscript for other ion Reduce subscripts to smallest whole number ratio Check that the sum of the charges of the cation cancels the sum of the anions
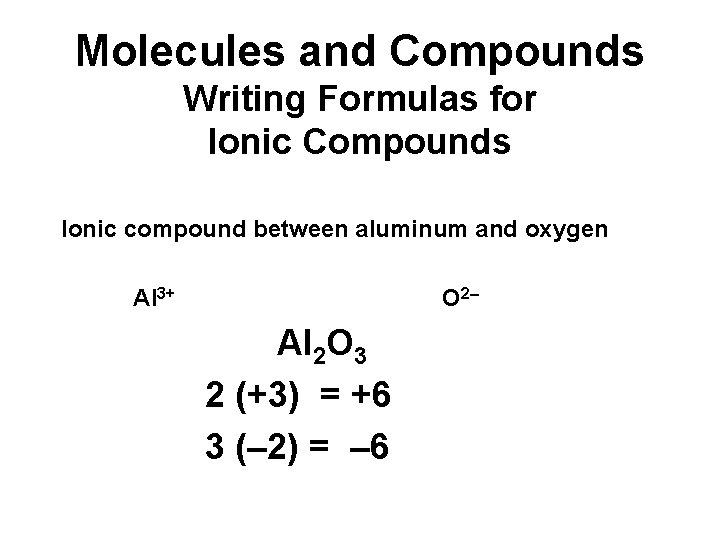
Molecules and Compounds Writing Formulas for Ionic Compounds Ionic compound between aluminum and oxygen Al 3+ O 2– Al 2 O 3 2 (+3) = +6 3 (– 2) = – 6
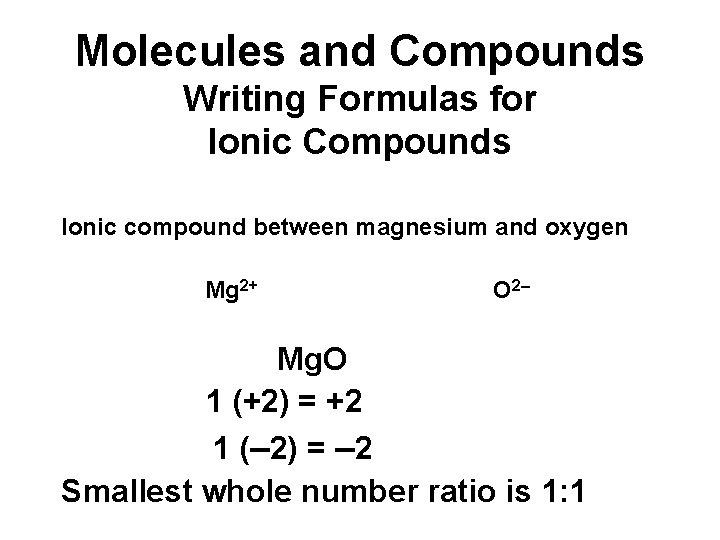
Molecules and Compounds Writing Formulas for Ionic Compounds Ionic compound between magnesium and oxygen Mg 2+ O 2– Mg. O 1 (+2) = +2 1 (– 2) = – 2 Smallest whole number ratio is 1: 1
Molecules and Compounds Naming Ionic Compounds • Metal and Nonmetal • Two Types – Type I • Metal has constant predictable charge • Inferred from group number in Periodic Table – Type II • Charge NOT always the same • Transition Metals
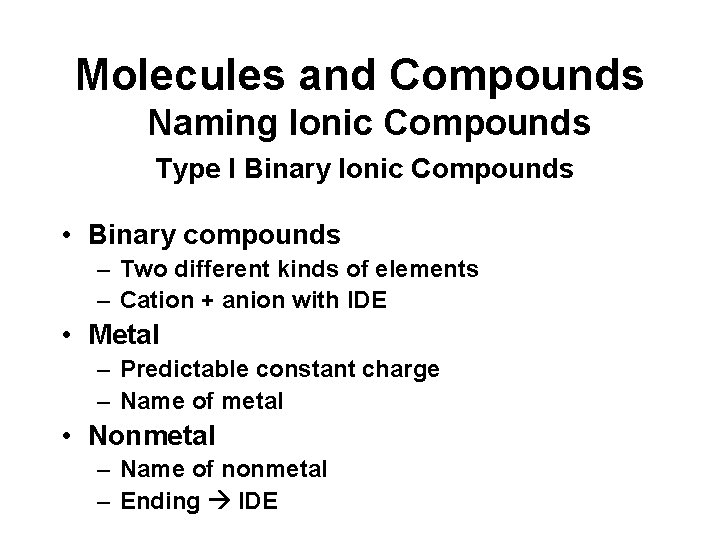
Molecules and Compounds Naming Ionic Compounds Type I Binary Ionic Compounds • Binary compounds – Two different kinds of elements – Cation + anion with IDE • Metal – Predictable constant charge – Name of metal • Nonmetal – Name of nonmetal – Ending IDE
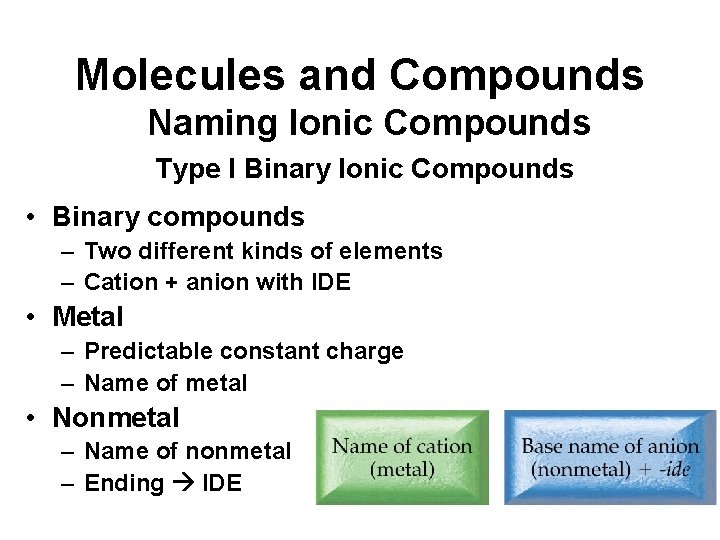
Molecules and Compounds Naming Ionic Compounds Type I Binary Ionic Compounds • Binary compounds – Two different kinds of elements – Cation + anion with IDE • Metal – Predictable constant charge – Name of metal • Nonmetal – Name of nonmetal – Ending IDE
Molecules and Compounds Naming Ionic Compounds Type I Binary Ionic Compounds • Mg. F 2 – Metal Magnesium – Nonmetal Fluoride – Magnesium fluoride • KBr – Metal Potassium – Nonmetal Bromine – Potassium bromide
Molecules and Compounds Naming Ionic Compounds Type I Binary Ionic Compounds • Ca. Cl 2 – Metal Calcium – Nonmetal Chlorine – Calcium chloride • Na 2 O – Metal Sodium – Nonmetal Oxygen – Sodium oxide
Molecules and Compounds Naming Ionic Compounds Type II Binary Ionic Compounds • Binary compounds – Two different kinds of elements – Cation + anion with IDE • Metal – Charge NOT always the same – Transition metals and roman numeral for charge – Name of metal • Nonmetal – Name of nonmetal – Ending IDE
Molecules and Compounds Naming Ionic Compounds Type II Binary Ionic Compounds • Fe. Cl 3 – – Metal Iron Nonmetal Chlorine Charge on Iron must be +3 Iron(III) chloride • Cr. O – – Metal Chromium Nonmetal Oxygen Charge on Chromium must be +2 Chromium(II) oxide
Molecules and Compounds Naming Ionic Compounds Type II Binary Ionic Compounds • Pb. Cl 4 – Metal Lead – Nonmetal Chlorine – Charge on Lead must be +4 – Lead(IV) chloride • Must determine charge of Cation from the Formula
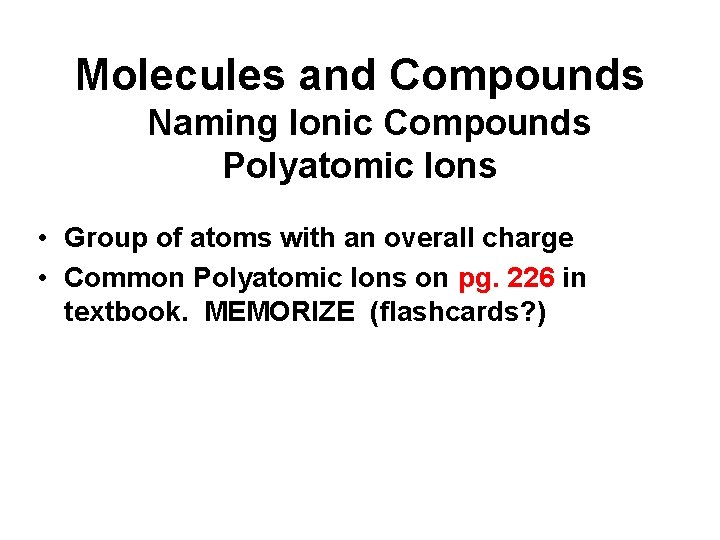
Molecules and Compounds Naming Ionic Compounds Polyatomic Ions • Group of atoms with an overall charge • Common Polyatomic Ions on pg. 226 in textbook. MEMORIZE (flashcards? )
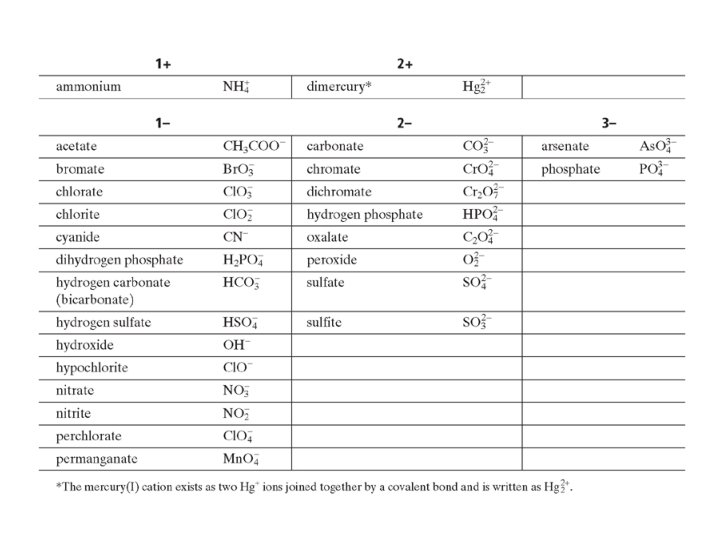
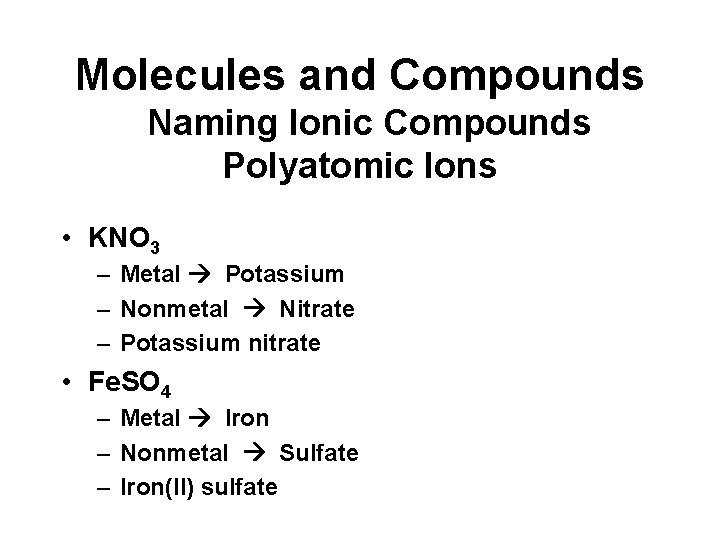
Molecules and Compounds Naming Ionic Compounds Polyatomic Ions • KNO 3 – Metal Potassium – Nonmetal Nitrate – Potassium nitrate • Fe. SO 4 – Metal Iron – Nonmetal Sulfate – Iron(II) sulfate

Ionic Compounds Containing Polyatomic Ions…. • Write the formula for tin(IV) sulfate • Sn(SO 4)2
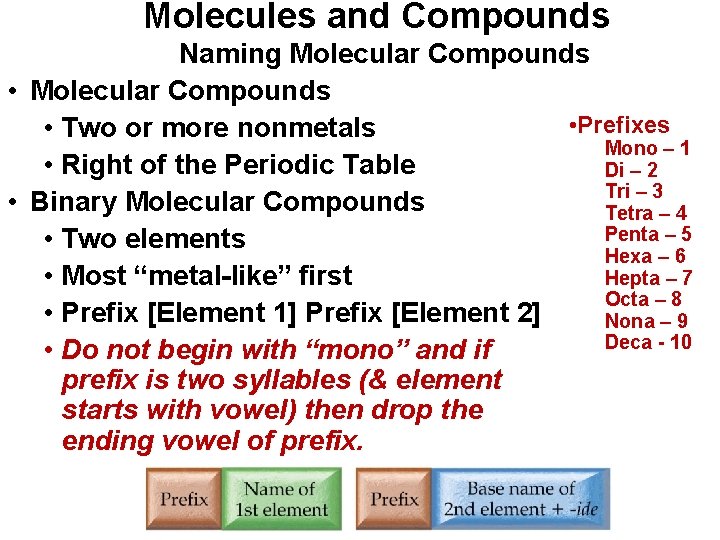
Molecules and Compounds Naming Molecular Compounds • Prefixes • Two or more nonmetals Mono – 1 • Right of the Periodic Table Di – 2 Tri – 3 • Binary Molecular Compounds Tetra – 4 Penta – 5 • Two elements Hexa – 6 Hepta – 7 • Most “metal-like” first Octa – 8 • Prefix [Element 1] Prefix [Element 2] Nona – 9 Deca - 10 • Do not begin with “mono” and if prefix is two syllables (& element starts with vowel) then drop the ending vowel of prefix.
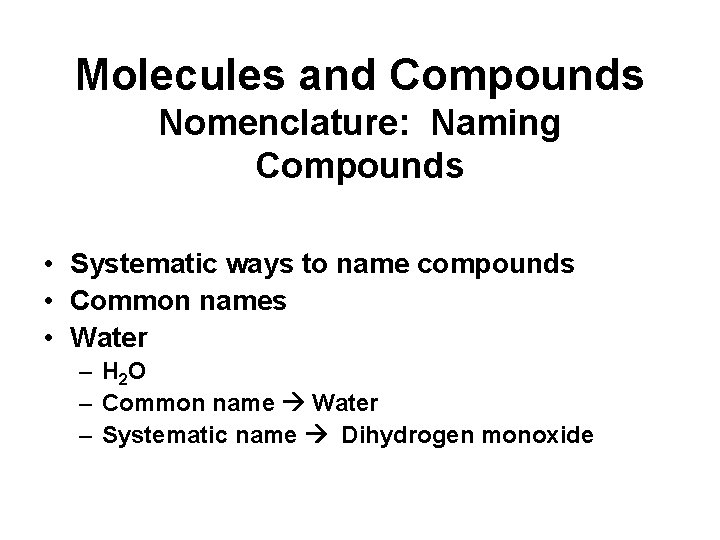
Molecules and Compounds Nomenclature: Naming Compounds • Systematic ways to name compounds • Common names • Water – H 2 O – Common name Water – Systematic name Dihydrogen monoxide
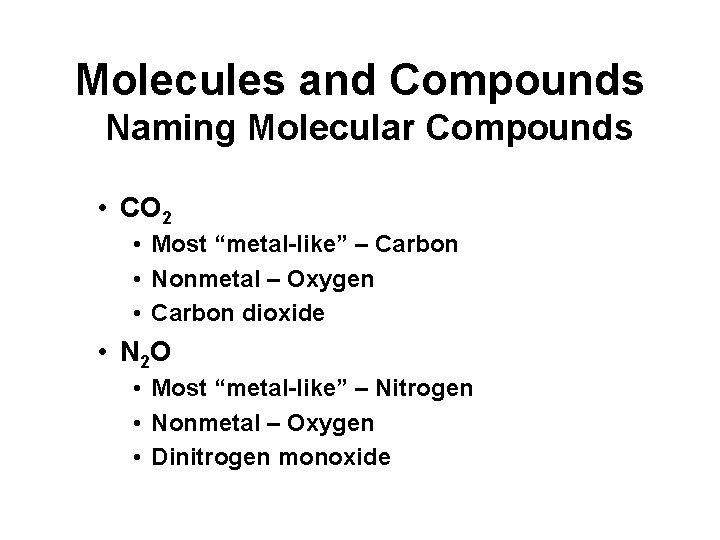
Molecules and Compounds Naming Molecular Compounds • CO 2 • Most “metal-like” – Carbon • Nonmetal – Oxygen • Carbon dioxide • N 2 O • Most “metal-like” – Nitrogen • Nonmetal – Oxygen • Dinitrogen monoxide

• Give name for As 2 O 5: • Write formula for oxygen difluoride:
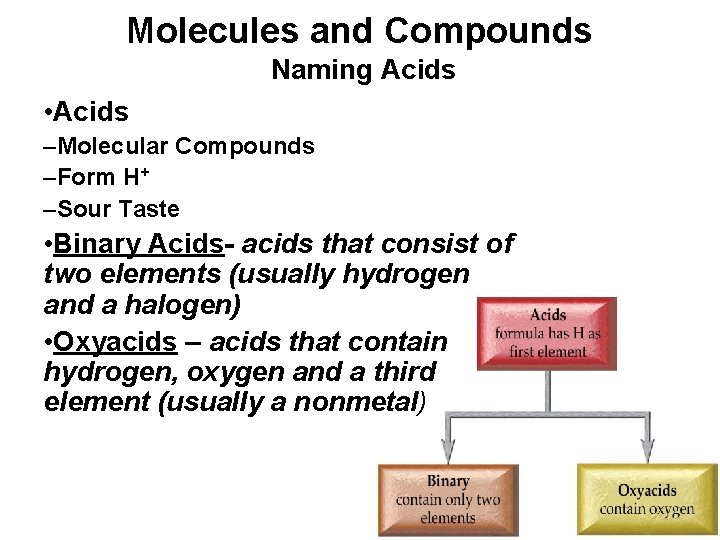
Molecules and Compounds Naming Acids • Acids –Molecular Compounds –Form H+ –Sour Taste • Binary Acids- acids that consist of two elements (usually hydrogen and a halogen) • Oxyacids – acids that contain hydrogen, oxygen and a third element (usually a nonmetal)

Binary Acids… If anion ends in “ide” then acid name is “hydro—ic” • HCl hydrochloric acid • HBr hydrobromic acid • HI hydroiodic acid

Naming Oxyacids • No “hydro” in name • If anion ends in “ate” then acid name is “— ic” • If anion ends in “ite” then acid name is “— ous”
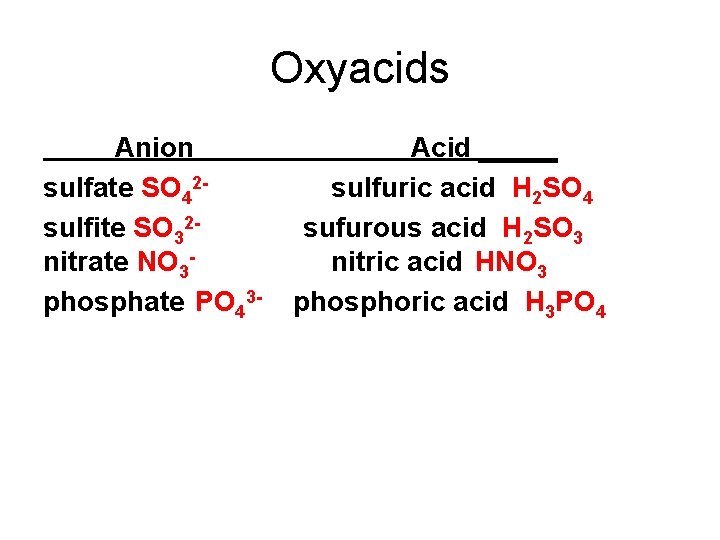
Oxyacids Anion sulfate SO 42 sulfite SO 32 nitrate NO 3 phosphate PO 43 - Acid _____ sulfuric acid H 2 SO 4 sufurous acid H 2 SO 3 nitric acid HNO 3 phosphoric acid H 3 PO 4

Salts • An ionic compound composed of a cation and the anion from an acid is often referred to as a salt. – examples: • Table salt, Na. Cl, contains the anion from hydrochloric acid, HCl. • Calcium sulfate, Ca. SO 4, is a salt containing the anion from sulfuric acid, H 2 SO 4.
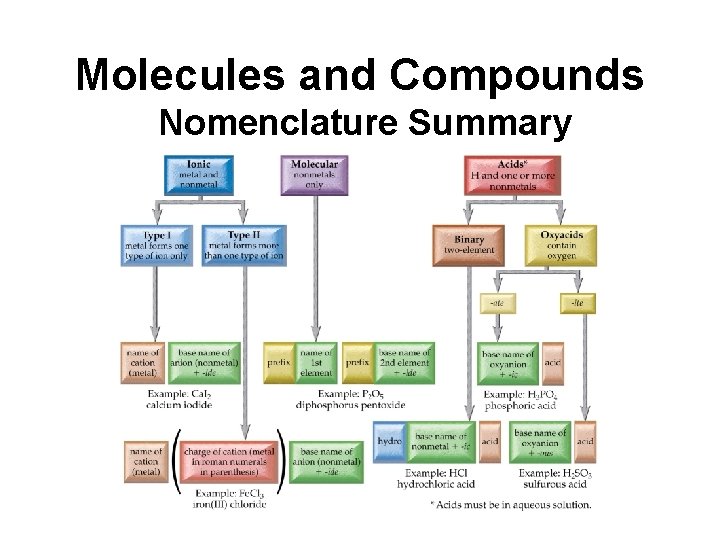
Molecules and Compounds Nomenclature Summary
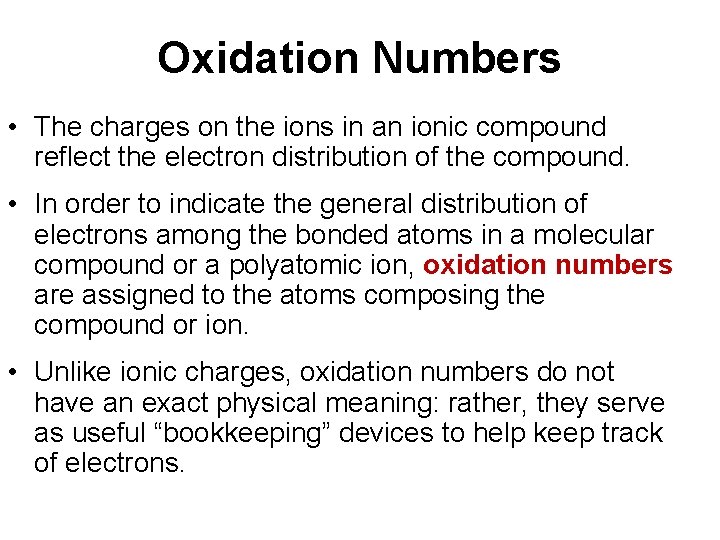
Oxidation Numbers • The charges on the ions in an ionic compound reflect the electron distribution of the compound. • In order to indicate the general distribution of electrons among the bonded atoms in a molecular compound or a polyatomic ion, oxidation numbers are assigned to the atoms composing the compound or ion. • Unlike ionic charges, oxidation numbers do not have an exact physical meaning: rather, they serve as useful “bookkeeping” devices to help keep track of electrons.

Assigning Oxidation Numbers In general when assigning oxidation numbers, shared electrons are assumed to “belong” to the more electronegative atom in each bond. More-specific rules are provided by the following guidelines. 1. The atoms in a pure element have an oxidation number of zero. examples: all atoms in sodium, Na, oxygen, O 2, phosphorus, P 4, and sulfur, S 8, have oxidation numbers of zero.

Assigning Oxidation Numbers, continued. . 2. The more-electronegative element in a binary compound is assigned a negative number equal to the charge it would have as an anion. Likewise for the less-electronegative element. 3. Fluorine has an oxidation number of – 1 in all of its compounds because it is the most electronegative element.

Assigning Oxidation Numbers, continued. . 4. Oxygen usually has an oxidation number of – 2. – Exceptions: • In peroxides, such as H 2 O 2, oxygen’s oxidation number is – 1. • In compounds with fluorine, such as OF 2, oxygen’s oxidation number is +2. 5. Hydrogen has an oxidation number of +1 in all compounds containing elements that are more electronegative than it; it has an oxidation number of – 1 with metals.

Assigning Oxidation Numbers, continued. . 6. The algebraic sum of the oxidation numbers of all atoms in an neutral compound is equal to zero. 7. The algebraic sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion. 8. Although rules 1 through 7 apply to covalently bonded atoms, oxidation numbers can also be applied to atoms in ionic compounds similarly.

Assigning Oxidation Numbers, continued. . Assign oxidation numbers to each atom in the following compounds or ions: a. UF 6 b. H 2 SO 4 c. Cl. O 3 -
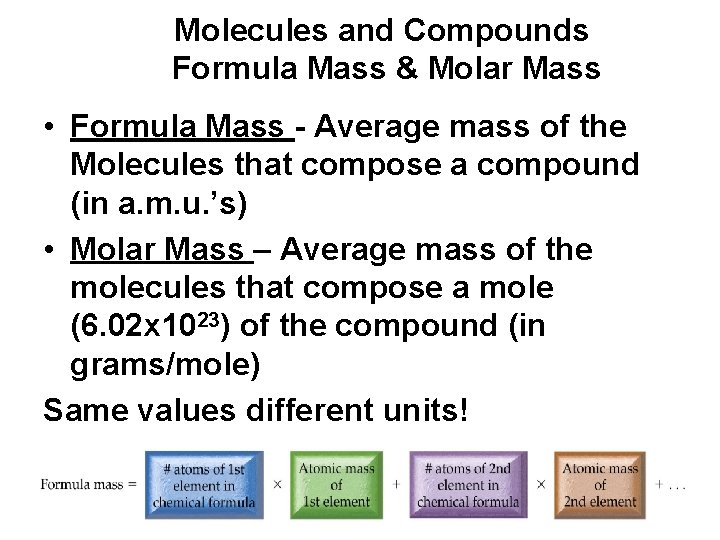
Molecules and Compounds Formula Mass & Molar Mass • Formula Mass - Average mass of the Molecules that compose a compound (in a. m. u. ’s) • Molar Mass – Average mass of the molecules that compose a mole (6. 02 x 1023) of the compound (in grams/mole) Same values different units!
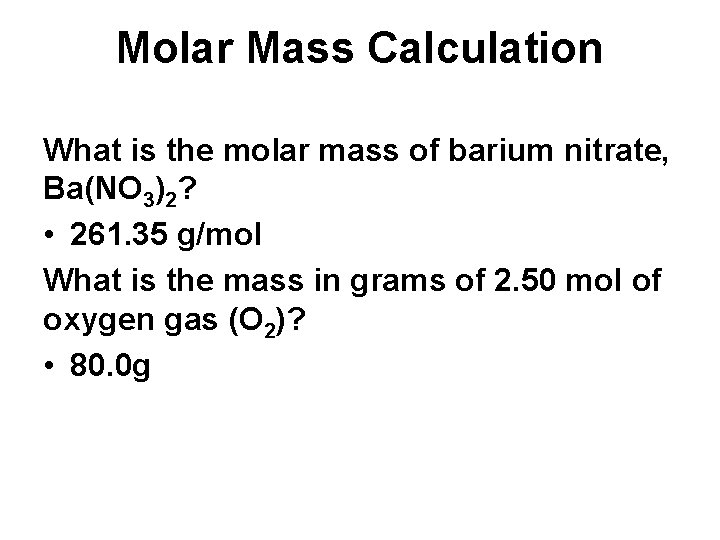
Molar Mass Calculation What is the molar mass of barium nitrate, Ba(NO 3)2? • 261. 35 g/mol What is the mass in grams of 2. 50 mol of oxygen gas (O 2)? • 80. 0 g
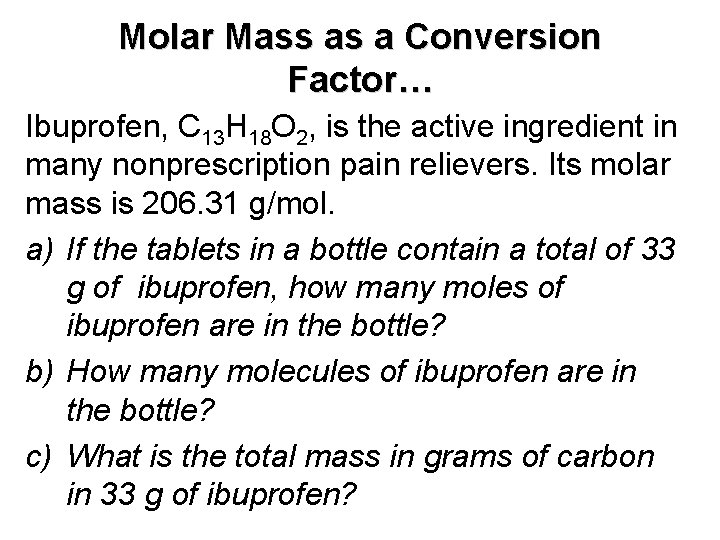
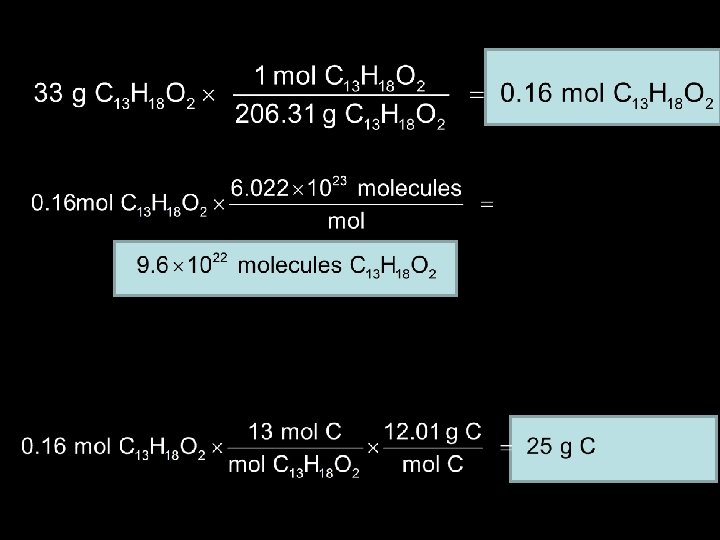
Molar Mass as a Conversion Factor… Ibuprofen, C 13 H 18 O 2, is the active ingredient in many nonprescription pain relievers. Its molar mass is 206. 31 g/mol. a) If the tablets in a bottle contain a total of 33 g of ibuprofen, how many moles of ibuprofen are in the bottle? b) How many molecules of ibuprofen are in the bottle? c) What is the total mass in grams of carbon in 33 g of ibuprofen?
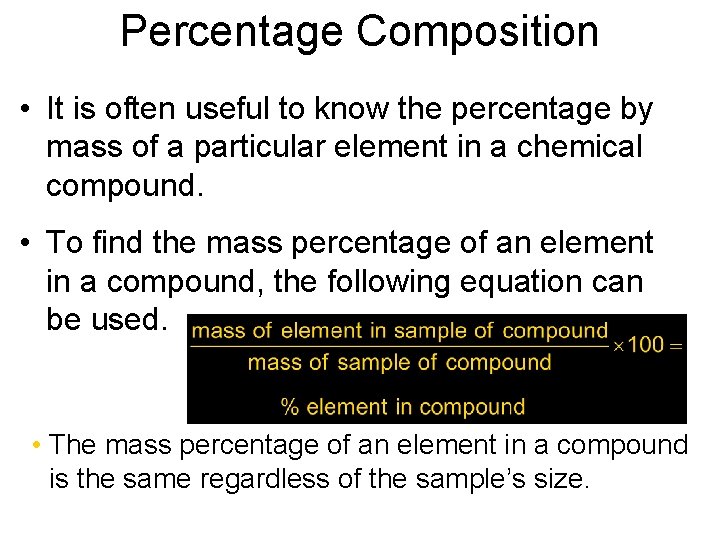
Percentage Chapter 7 Composition • It is often useful to know the percentage by mass of a particular element in a chemical compound. • To find the mass percentage of an element in a compound, the following equation can be used. • The mass percentage of an element in a compound is the same regardless of the sample’s size.
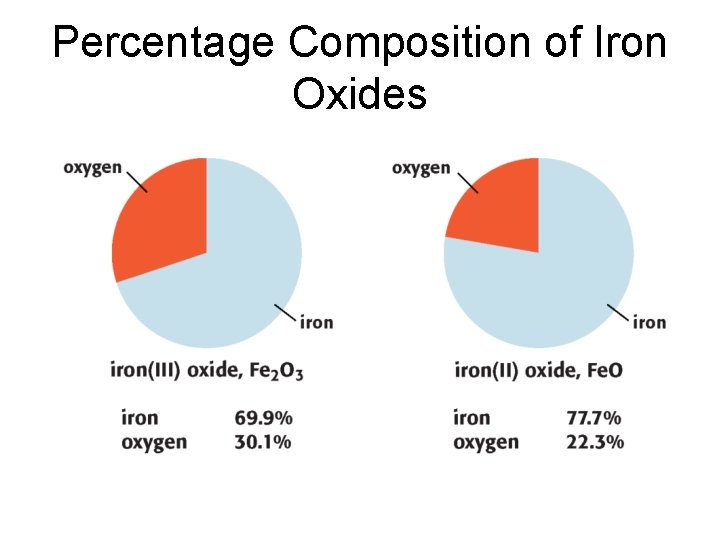
Percentage Composition of Iron Oxides
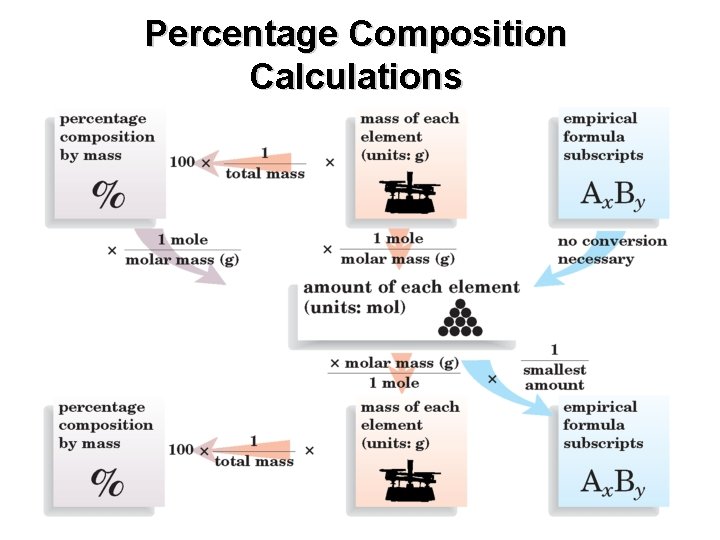
Percentage Composition Calculations

Chapter 7 Percentage Composition, continued
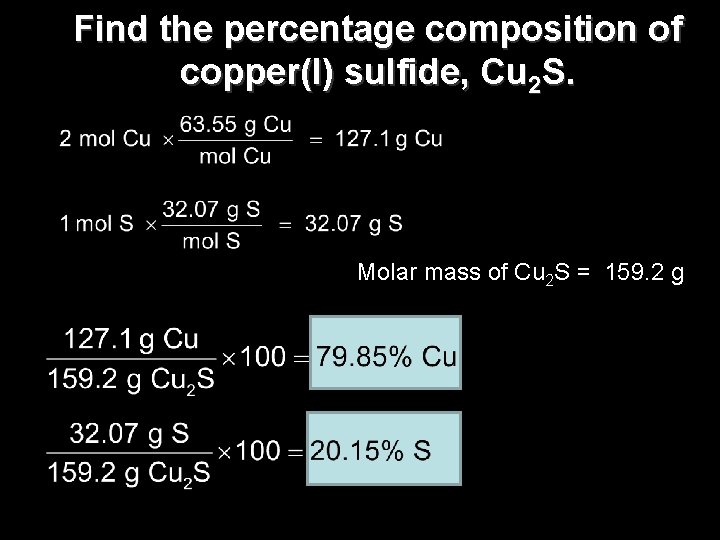
Find the percentage composition of copper(I) sulfide, Cu 2 S. Molar mass of Cu 2 S = 159. 2 g
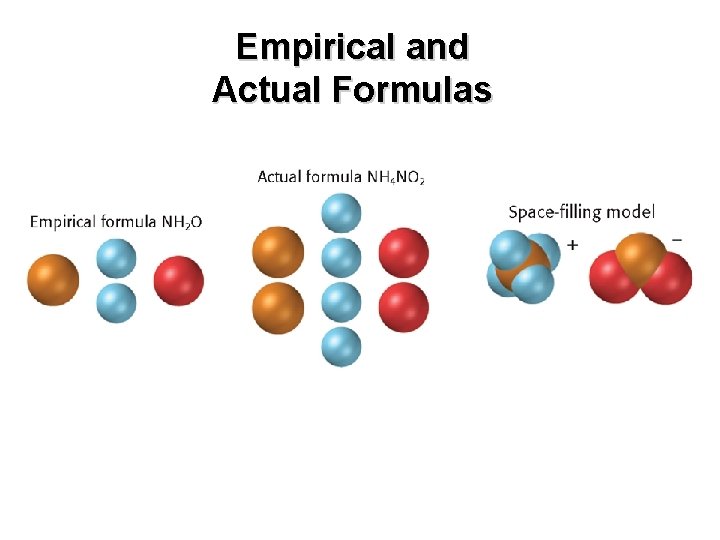
Chapter 7 Empirical and Actual Formulas
• An empirical formula consists of the symbols for the elements combined in a compound, with subscripts showing the smallest whole-number mole ratio of the different atoms in the compound. • For an ionic compound, the formula unit is usually the compound’s empirical formula. • For a molecular compound, however, the empirical formula does not necessarily indicate the actual numbers of atoms present in each molecule. – example: the empirical formula of the gas diborane is BH 3, but the molecular formula is B 2 H 6.
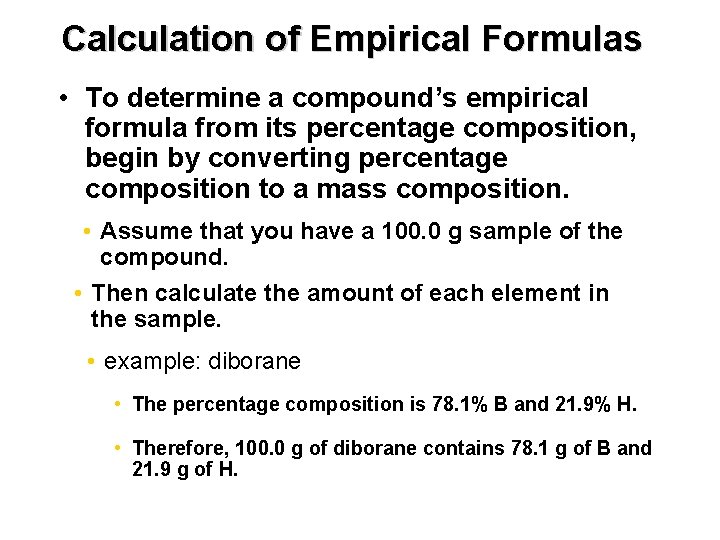
Chapter 7 Calculation of Empirical Formulas • To determine a compound’s empirical formula from its percentage composition, begin by converting percentage composition to a mass composition. • Assume that you have a 100. 0 g sample of the compound. • Then calculate the amount of each element in the sample. • example: diborane • The percentage composition is 78. 1% B and 21. 9% H. • Therefore, 100. 0 g of diborane contains 78. 1 g of B and 21. 9 g of H.
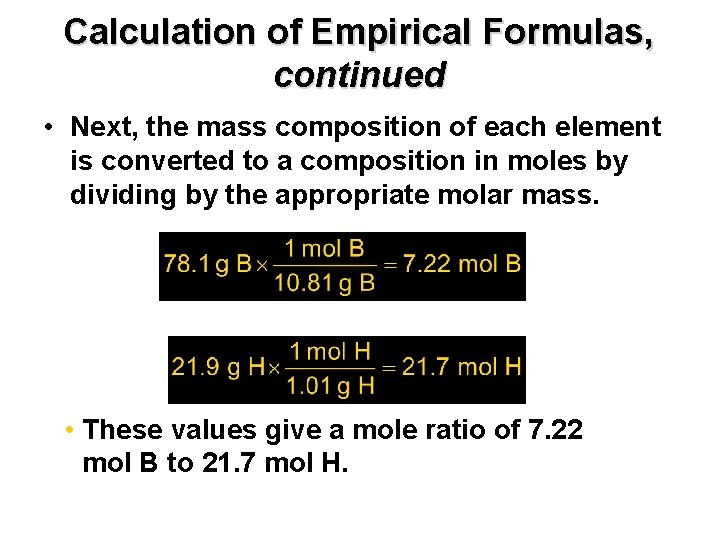
Calculation of Empirical Formulas, Chapter 7 continued • Next, the mass composition of each element is converted to a composition in moles by dividing by the appropriate molar mass. • These values give a mole ratio of 7. 22 mol B to 21. 7 mol H.
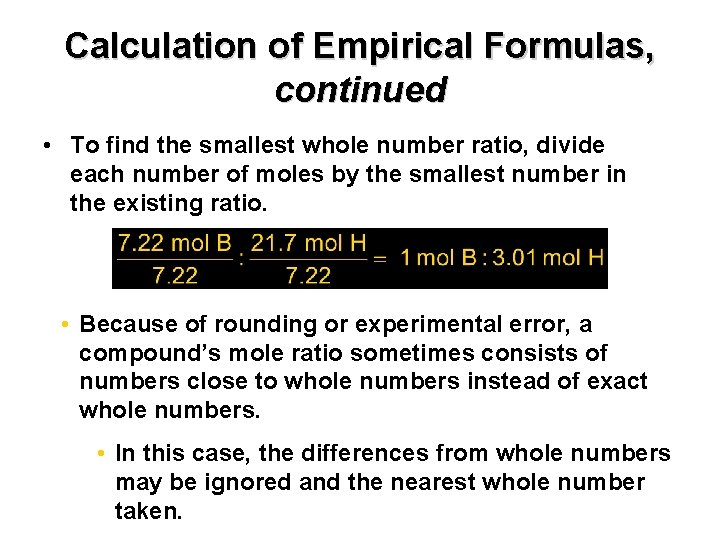
Chapter 7 Calculation of Empirical Formulas, continued • To find the smallest whole number ratio, divide each number of moles by the smallest number in the existing ratio. • Because of rounding or experimental error, a compound’s mole ratio sometimes consists of numbers close to whole numbers instead of exact whole numbers. • In this case, the differences from whole numbers may be ignored and the nearest whole number taken.
Chapter 7 Calculation of Empirical Formulas, continued Quantitative analysis shows that a compound contains 32. 38% sodium, 22. 65% sulfur, and 44. 99% oxygen. Find the empirical formula of this compound.

Chapter 7 of Molecular Formulas Calculation • The empirical formula contains the smallest possible whole numbers that describe the atomic ratio. • The molecular formula is the actual formula of a molecular compound. • An empirical formula may or may not be a correct molecular formula. • The relationship between a compound’s empirical formula and its molecular formula: x(empirical formula) = molecular formula
Chapter 7 Calculation of Molecular Formulas… • The formula masses have a similar relationship. x(empirical formula mass) = molecular formula mass • To determine the molecular formula of a compound, you must know the compound’s formula mass. – Dividing the experimental formula mass by the empirical formula mass gives the value of x. • A compound’s molecular formula mass is numerically equal to its molar mass, so a compound’s molecular formula can also be found given the compound’s empirical formula and its molar mass.
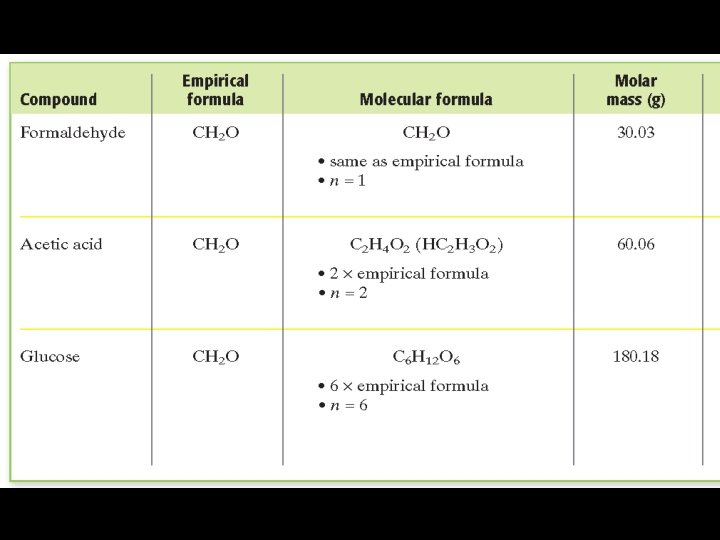
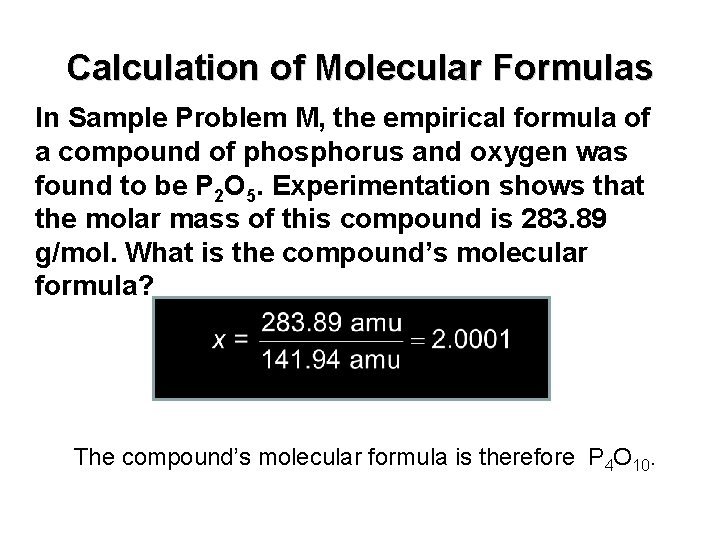
Calculation of Molecular Formulas In Sample Problem M, the empirical formula of a compound of phosphorus and oxygen was found to be P 2 O 5. Experimentation shows that the molar mass of this compound is 283. 89 g/mol. What is the compound’s molecular formula? The compound’s molecular formula is therefore P 4 O 10.
- Slides: 67