Molecules and Compounds Nomenclature Compounds vs Elements Compound



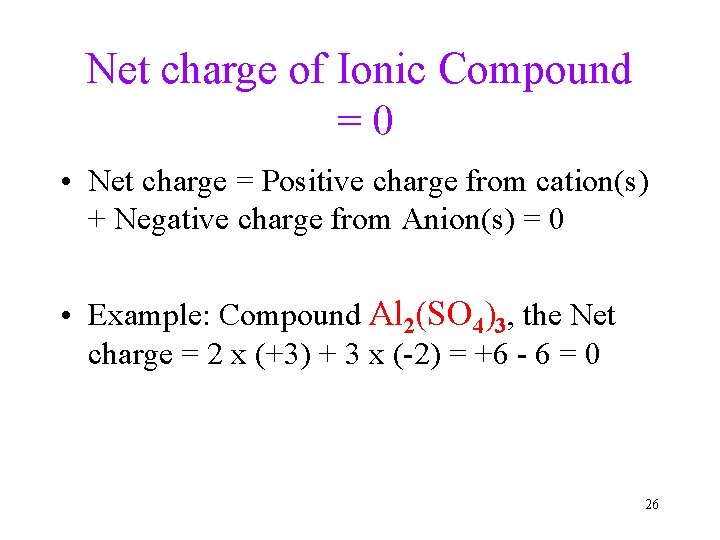






















- Slides: 77

Molecules and Compounds: Nomenclature

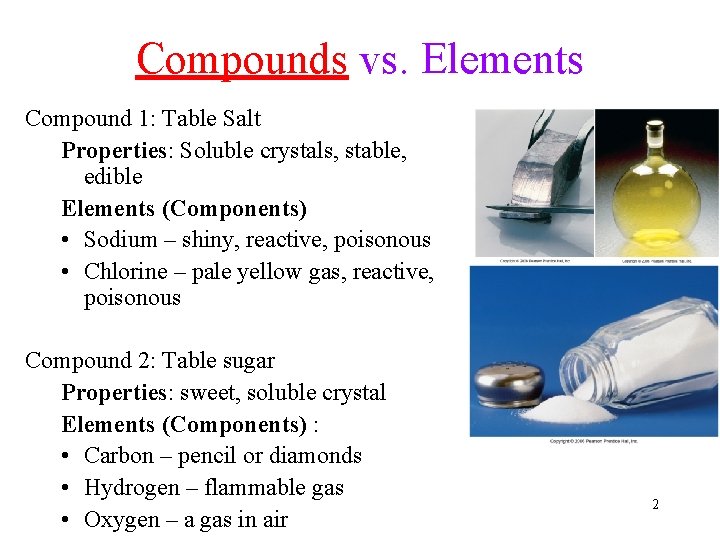
Compounds vs. Elements Compound 1: Table Salt Properties: Soluble crystals, stable, edible Elements (Components) • Sodium – shiny, reactive, poisonous • Chlorine – pale yellow gas, reactive, poisonous Compound 2: Table sugar Properties: sweet, soluble crystal Elements (Components) : • Carbon – pencil or diamonds • Hydrogen – flammable gas • Oxygen – a gas in air 2

Law of Constant Composition Pure substances have constant composition ü all samples of a pure substance contain the same elements in the same percentages (ratios): Water (H: 11%, O: 89%), Table salt (Na: 39%, Cl: 61%), Sugar ü mixtures have variable composition: Air, Seawater, Concrete, Rocky road ice cream, Coke 3

Why do Compounds Show Constant Composition • the smallest piece of a compound is called a molecule: Water molecule, Sugar molecule • every molecule of a compound has the same number and type of atoms. Water molecule: 2 Hydrogen atom + 1 Oxygen atom; Sugar molecule: 12 Carbon atom + 22 Hydrogen atom + 11 Oxygen atom every sample of the compound will have the same ratio of the elements 4

Chemical Formula Chemical formula: describe the compound by describing the number and type of each atom in the simplest unit of the compound ü molecules or ions (Table salt: Cl+, Na-) • Element represented by its letter symbol: H instead of hydrogen; Na instead of Sodium • #Atoms of each element: the right of the element as a subscript, H 2 O (unless if there is only one atom, the 1 subscript is not written) • Polyatomic groups (multiple atoms in group, example: CO 3) are placed in parentheses if more than one 5

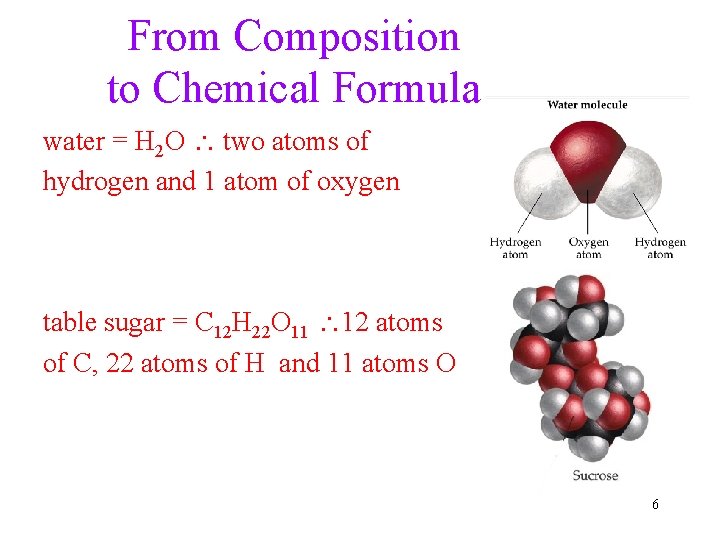
From Composition to Chemical Formula water = H 2 O two atoms of hydrogen and 1 atom of oxygen table sugar = C 12 H 22 O 11 12 atoms of C, 22 atoms of H and 11 atoms O 6

Classifying Pure Substances Element • Atomic: consists of single atoms (Metals, Noble gases) • Molecular: consists of multi-atom molecules (O 2, N 2, Cl 2, etc) Compound • Molecular: consists of molecules made of only nonmetals (CO 2, H 2 O) • Ionic: consists of cations (Na+) and anions (Cl-) 7

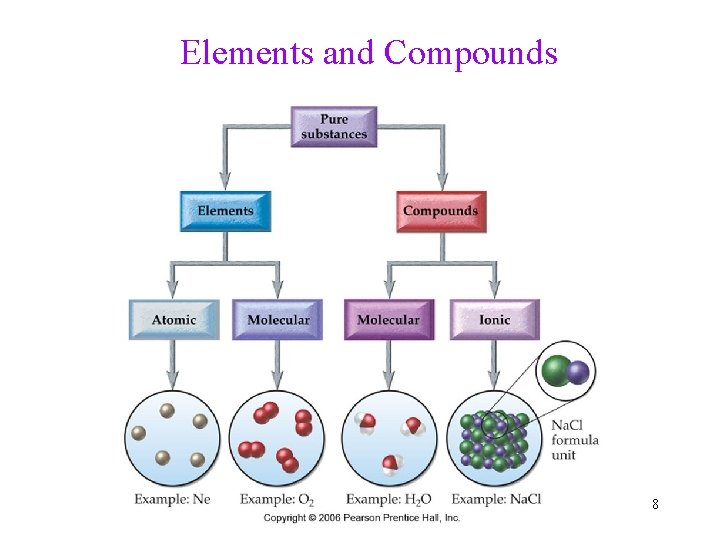
Elements and Compounds 8

Classify each of the following: Element atomic/molecular Compound molecular/ionic • • • aluminum, Al = atomic element aluminum chloride, Al. Cl 3 = ionic compound chlorine, Cl 2 = molecular element acetone, C 3 H 6 O = molecular compound carbon monoxide, CO = molecular compound cobalt, Co = atomic element 9

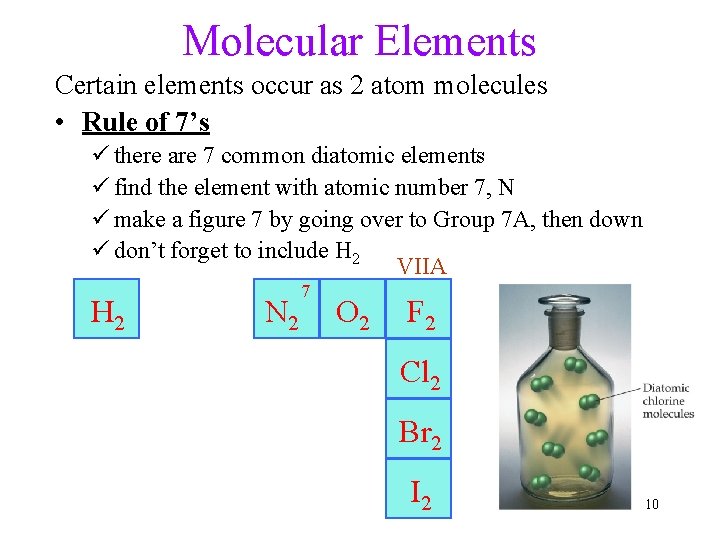
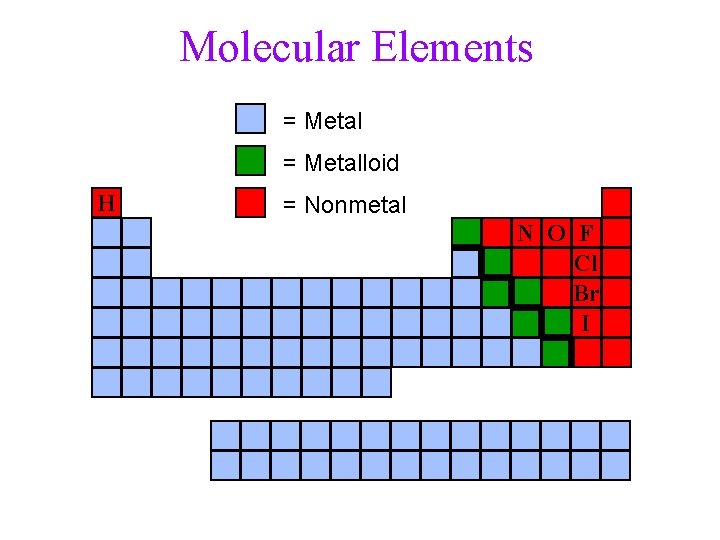
Molecular Elements Certain elements occur as 2 atom molecules • Rule of 7’s ü there are 7 common diatomic elements ü find the element with atomic number 7, N ü make a figure 7 by going over to Group 7 A, then down ü don’t forget to include H 2 VIIA H 2 N 2 7 O 2 F 2 Cl 2 Br 2 I 2 10

Molecular Elements = Metalloid H = Nonmetal N O F Cl Br I

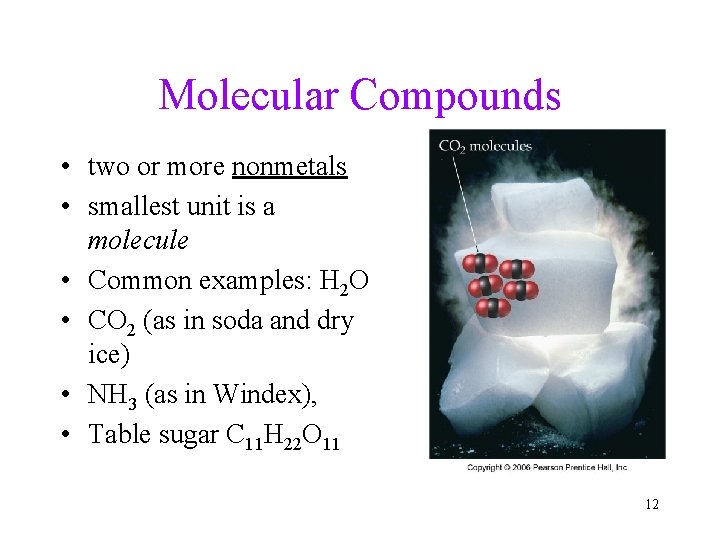
Molecular Compounds • two or more nonmetals • smallest unit is a molecule • Common examples: H 2 O • CO 2 (as in soda and dry ice) • NH 3 (as in Windex), • Table sugar C 11 H 22 O 11 12

Ionic Compounds Ions: Metals (Cation Mx+) and Nonmetals (Anion Ny -) • No individual molecules!! • have a 3 -dimensional array of cations and anions made of formula units: Na. Cl, Mg. O • Na+ Cl- Na+ Cl • Cl- Na+ • Na+ Cl- 13

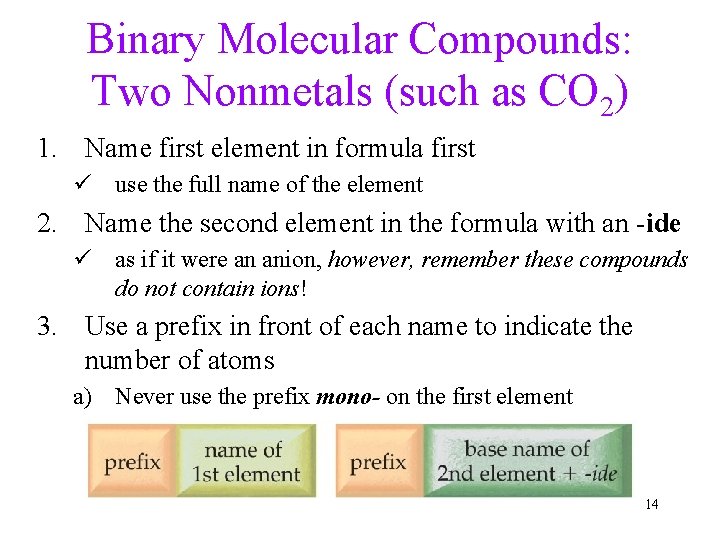
Binary Molecular Compounds: Two Nonmetals (such as CO 2) 1. Name first element in formula first ü use the full name of the element 2. Name the second element in the formula with an -ide ü as if it were an anion, however, remember these compounds do not contain ions! 3. Use a prefix in front of each name to indicate the number of atoms a) Never use the prefix mono- on the first element 14

Subscript - Prefixes • 1 = mono-; ünot used on first nonmetal • 2 = di • 3 = tri • 4 = tetra- • • • 5 = penta 6 = hexa 7 = hepta 8 = octadrop last “a” if name begins with vowel 15

Exceptions when Naming Molecular Compounds of course, water Other common exceptions: • NH 3: ammonia (as in Windex) • H 2 S: hydrogen sulfide • HCl: hydrogen chloride (same for HX, where X = halogen) • CH 4: methane (as in natural gas) • H 2 O 2: hydrogen peroxide 16

Example – Naming Binary Molecular BF 3 1. Is it one of the common exceptions? H 2 O, NH 3, CH 4, C 12 H 22 O 11 = No! 2. Identify Major Class B = is a nonmetal because it is on the right side of the PT F = is a nonmetal because it is on the right side of the PT Molecular 3. Identify the Subclass 2 elements Binary Molecular 17

Example – Naming Binary Molecular BF 3 4. Name the first element boron 5. Name the second element with an –ide fluorine fluoride 6. Add a prefix to each name to indicate the subscript monoboron, trifluoride 7. Write the first element with prefix, then the second element with prefix ü Drop prefix mono from first element boron trifluoride 18

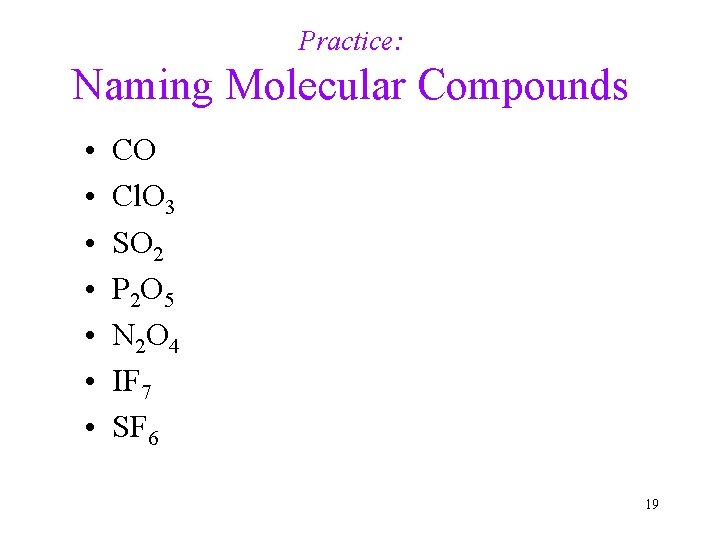
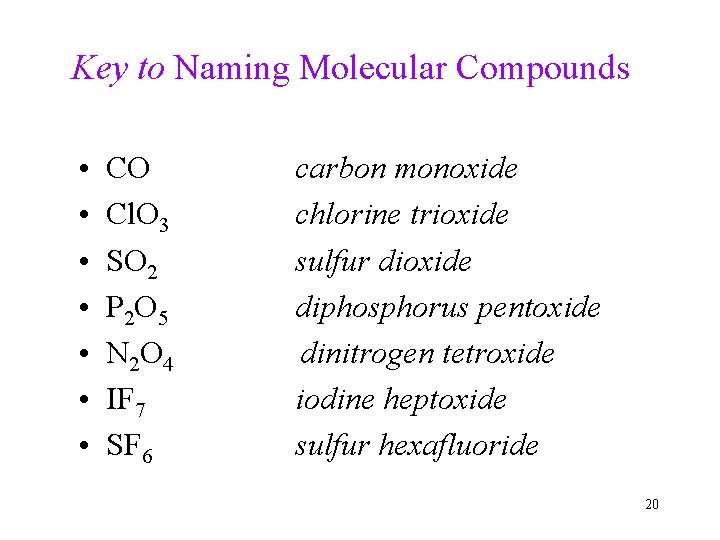
Practice: Naming Molecular Compounds • • CO Cl. O 3 SO 2 P 2 O 5 N 2 O 4 IF 7 SF 6 19

Key to Naming Molecular Compounds • • CO Cl. O 3 SO 2 P 2 O 5 N 2 O 4 IF 7 SF 6 carbon monoxide chlorine trioxide sulfur dioxide diphosphorus pentoxide dinitrogen tetroxide iodine heptoxide sulfur hexafluoride 20

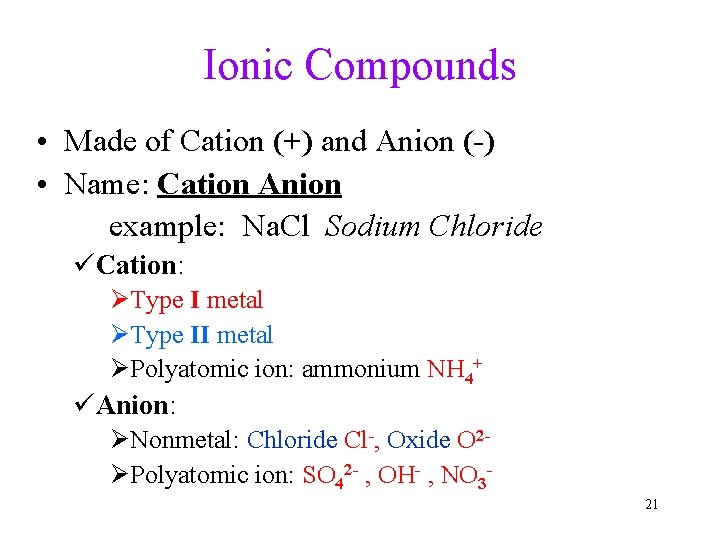
Ionic Compounds • Made of Cation (+) and Anion (-) • Name: Cation Anion example: Na. Cl Sodium Chloride üCation: ØType I metal ØType II metal ØPolyatomic ion: ammonium NH 4+ üAnion: ØNonmetal: Chloride Cl-, Oxide O 2ØPolyatomic ion: SO 42 - , OH- , NO 321

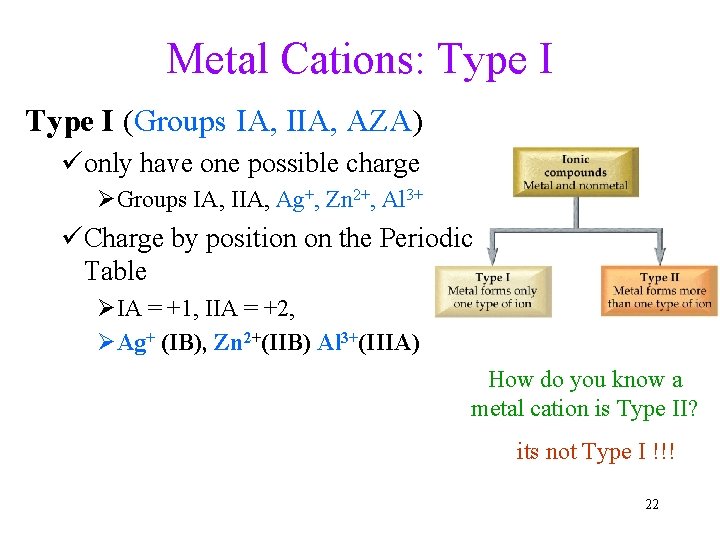
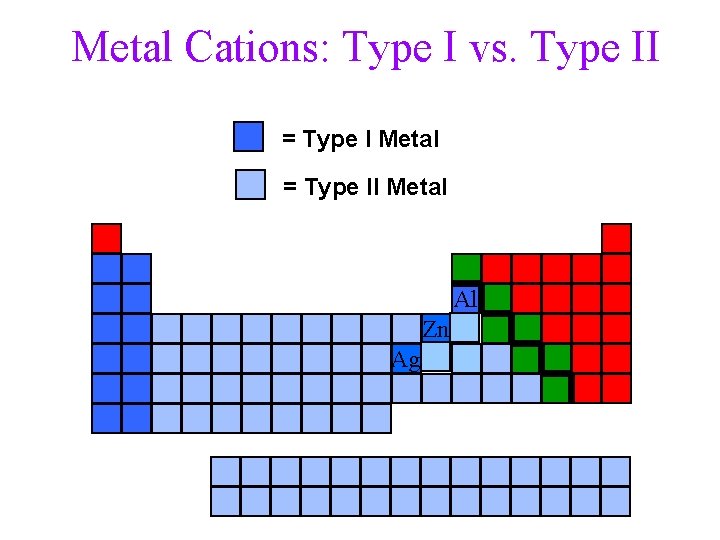
Metal Cations: Type I (Groups IA, IIA, AZA) üonly have one possible charge ØGroups IA, IIA, Ag+, Zn 2+, Al 3+ üCharge by position on the Periodic Table ØIA = +1, IIA = +2, ØAg+ (IB), Zn 2+(IIB) Al 3+(IIIA) How do you know a metal cation is Type II? its not Type I !!! 22

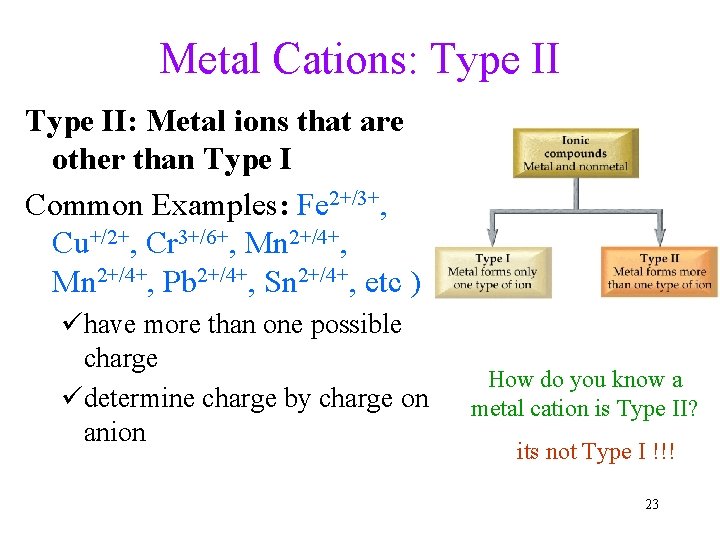
Metal Cations: Type II: Metal ions that are other than Type I Common Examples: Fe 2+/3+, Cu+/2+, Cr 3+/6+, Mn 2+/4+, Pb 2+/4+, Sn 2+/4+, etc ) ühave more than one possible charge üdetermine charge by charge on anion How do you know a metal cation is Type II? its not Type I !!! 23

Metal Cations: Type I vs. Type II = Type I Metal = Type II Metal Al Zn Ag

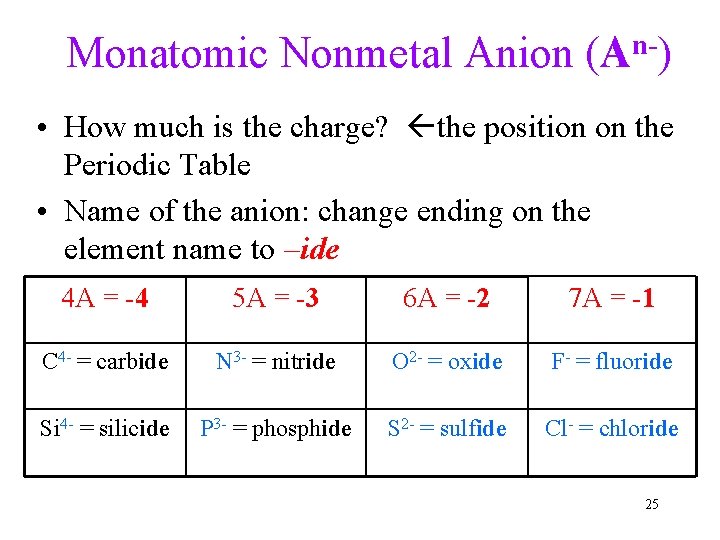
Monatomic Nonmetal Anion (An-) • How much is the charge? the position on the Periodic Table • Name of the anion: change ending on the element name to –ide 4 A = -4 5 A = -3 6 A = -2 7 A = -1 C 4 - = carbide N 3 - = nitride O 2 - = oxide F- = fluoride Si 4 - = silicide P 3 - = phosphide S 2 - = sulfide Cl- = chloride 25
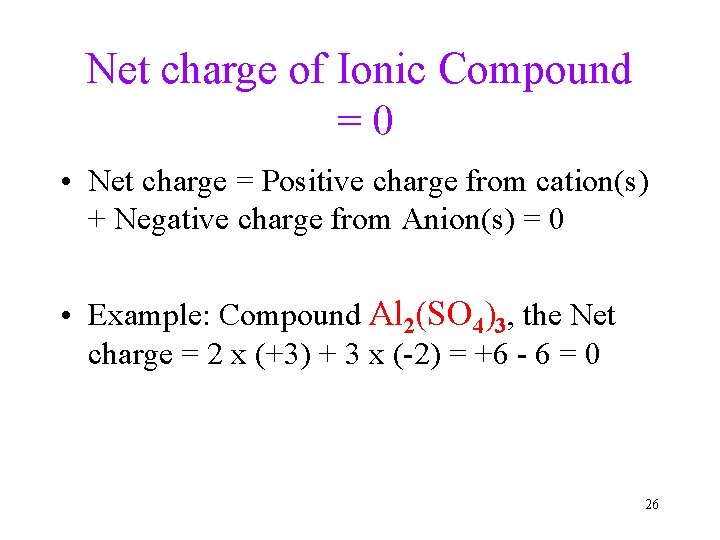
Net charge of Ionic Compound =0 • Net charge = Positive charge from cation(s) + Negative charge from Anion(s) = 0 • Example: Compound Al 2(SO 4)3, the Net charge = 2 x (+3) + 3 x (-2) = +6 - 6 = 0 26

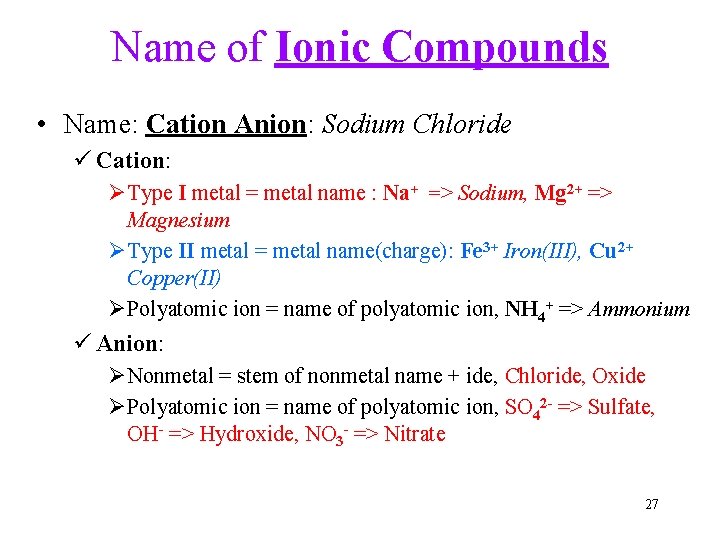
Name of Ionic Compounds • Name: Cation Anion: Sodium Chloride ü Cation: ØType I metal = metal name : Na+ => Sodium, Mg 2+ => Magnesium ØType II metal = metal name(charge): Fe 3+ Iron(III), Cu 2+ Copper(II) ØPolyatomic ion = name of polyatomic ion, NH 4+ => Ammonium ü Anion: ØNonmetal = stem of nonmetal name + ide, Chloride, Oxide ØPolyatomic ion = name of polyatomic ion, SO 42 - => Sulfate, OH- => Hydroxide, NO 3 - => Nitrate 27

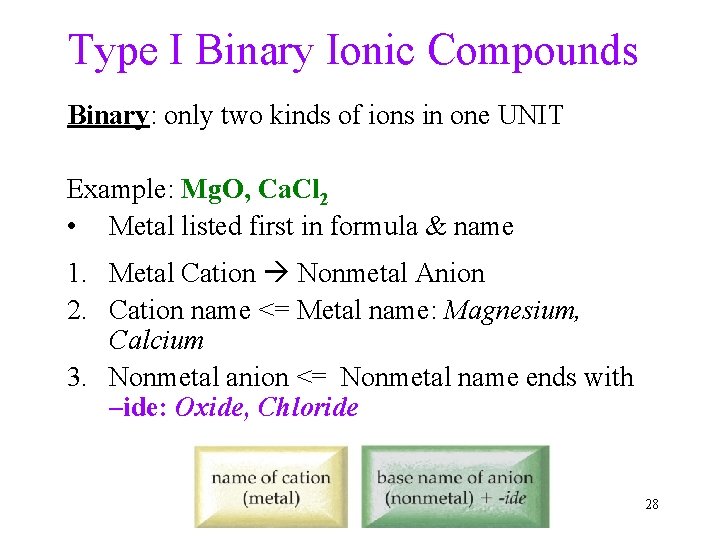
Type I Binary Ionic Compounds Binary: only two kinds of ions in one UNIT Example: Mg. O, Ca. Cl 2 • Metal listed first in formula & name 1. Metal Cation Nonmetal Anion 2. Cation name <= Metal name: Magnesium, Calcium 3. Nonmetal anion <= Nonmetal name ends with –ide: Oxide, Chloride 28

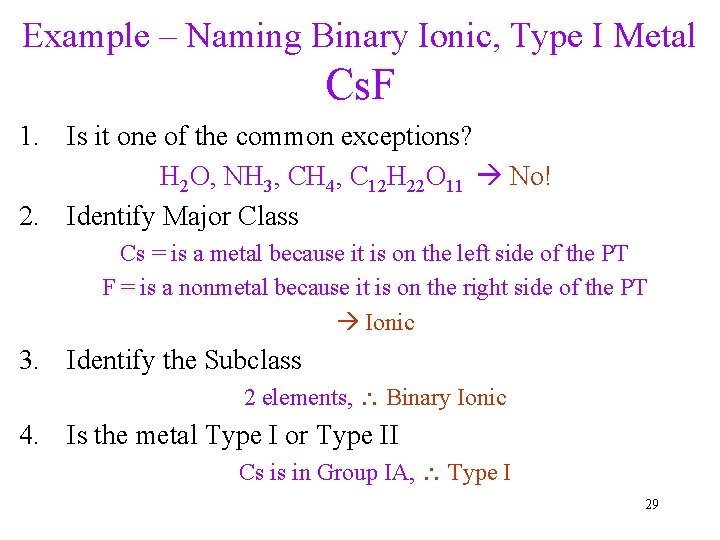
Example – Naming Binary Ionic, Type I Metal Cs. F 1. Is it one of the common exceptions? H 2 O, NH 3, CH 4, C 12 H 22 O 11 No! 2. Identify Major Class Cs = is a metal because it is on the left side of the PT F = is a nonmetal because it is on the right side of the PT Ionic 3. Identify the Subclass 2 elements, Binary Ionic 4. Is the metal Type I or Type II Cs is in Group IA, Type I 29

Example – Naming Binary Ionic, Type I Metal Cs. F 5. Identify cation and anion Cs = Cs+ because it is Group 1 F = F- because it is Group 7 6. Name the cation Cs+ = cesium 7. Name the anion F- = fluoride 8. Full name: Cation name first, Anion name last cesium fluoride 30

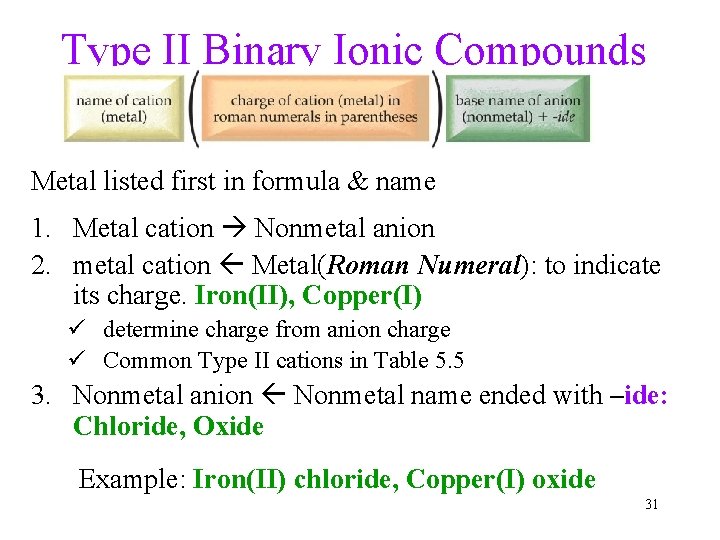
Type II Binary Ionic Compounds Metal listed first in formula & name 1. Metal cation Nonmetal anion 2. metal cation Metal(Roman Numeral): to indicate its charge. Iron(II), Copper(I) ü determine charge from anion charge ü Common Type II cations in Table 5. 5 3. Nonmetal anion Nonmetal name ended with –ide: Chloride, Oxide Example: Iron(II) chloride, Copper(I) oxide 31

How to find the charge on Type II metal ions? • Example: Name Compound Fe 2(SO 4)3 Since the sum of all charges equals zero, the charge on iron ions are unknown and sulfate each has – 2 charge, then we have 2 x Fe + 3 x (-2) = 0 Fe = +3, each iron ion has a charge of +3 Name: iron(III) sulfate Key: knowing the charge on ANIONs! 32

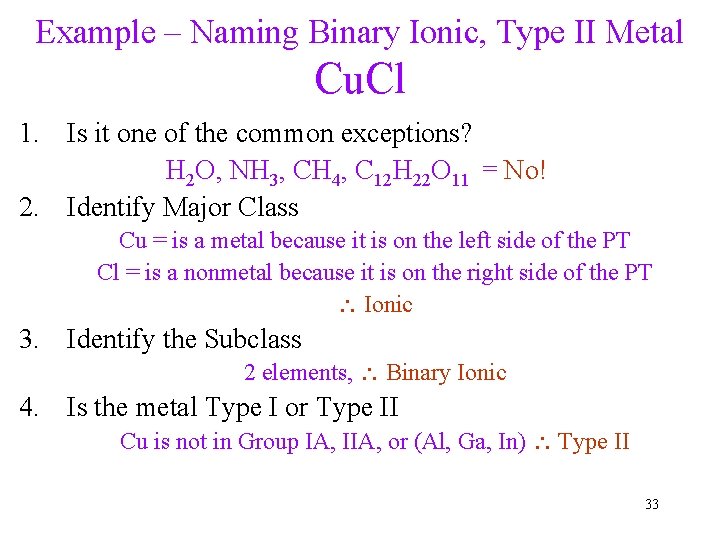
Example – Naming Binary Ionic, Type II Metal Cu. Cl 1. Is it one of the common exceptions? H 2 O, NH 3, CH 4, C 12 H 22 O 11 = No! 2. Identify Major Class Cu = is a metal because it is on the left side of the PT Cl = is a nonmetal because it is on the right side of the PT Ionic 3. Identify the Subclass 2 elements, Binary Ionic 4. Is the metal Type I or Type II Cu is not in Group IA, IIA, or (Al, Ga, In) Type II 33

Example – Naming Binary Ionic, Type II Metal Cu. Cl 5. Identify cation and anion Cl = Cl- because it is Group 7 Cu = Cu+ to balance the charge 6. Name the cation Cu+ = copper(I) 7. Name the anion Cl- = chloride 8. Write the cation name first, then the anion name copper(I) chloride 34

Practice: Naming Ionic compounds • • Hg. F 2 Cu. I 2 Ca. Cl 2 Fe 2 O 3 Sn. Cl 4 Mg 3 N 2 Ag 2 S 35

Naming Ionic compounds Hints: find type II ion charge from anion • • Hg. F 2 : Two F- = -2 Hg = +2 Cu. I 2 : Two I- = -2 Cu = +2 Ca. Cl 2 : both fixed charges Fe 2 O 3 : Three O 2 - = -6 Fe = +3 Sn. Br 4 : Four Br- = -4 Sn = +4 Mg 3 N 2 : both fixed charges Ag 2 S : both fixed charges 36

Answer key: names of ionic compounds • • Hg. F 2 = Mercury(II) fluoride Cu. I 2 = copper(II) iodide Ca. Cl 2 = calcium chloride Fe 2 O 3 = Iron(III) oxide Sn. Br 4 = tin(IV) bromide Mg 3 N 2 = magnesium nitride Ag 2 S = silver sulfide 37

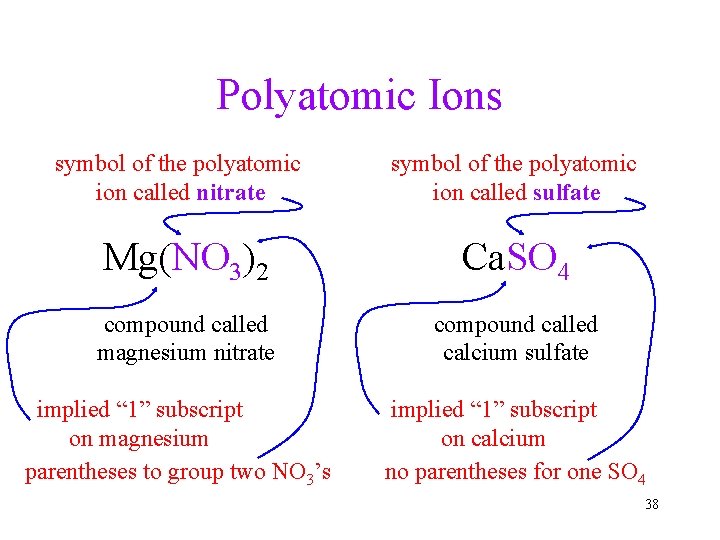
Polyatomic Ions symbol of the polyatomic ion called nitrate symbol of the polyatomic ion called sulfate Mg(NO 3)2 Ca. SO 4 compound called magnesium nitrate compound called calcium sulfate implied “ 1” subscript on magnesium parentheses to group two NO 3’s implied “ 1” subscript on calcium no parentheses for one SO 4 38

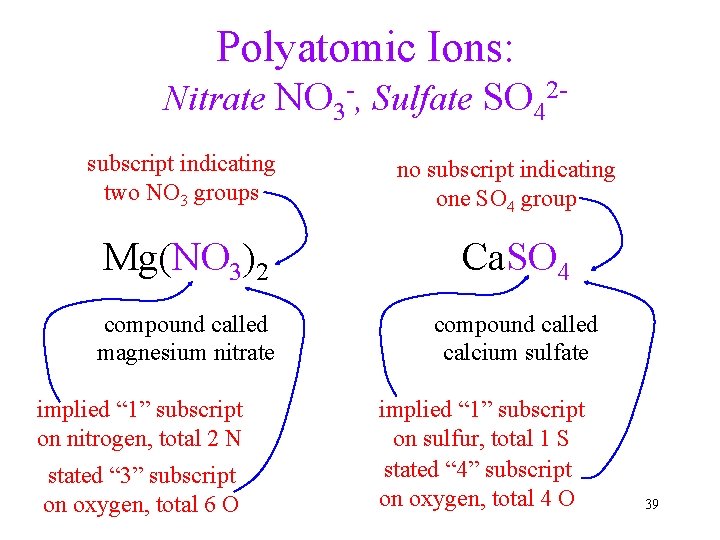
Polyatomic Ions: Nitrate NO 3 -, Sulfate SO 42 subscript indicating two NO 3 groups no subscript indicating one SO 4 group Mg(NO 3)2 Ca. SO 4 compound called magnesium nitrate compound called calcium sulfate implied “ 1” subscript on nitrogen, total 2 N stated “ 3” subscript on oxygen, total 6 O implied “ 1” subscript on sulfur, total 1 S stated “ 4” subscript on oxygen, total 4 O 39

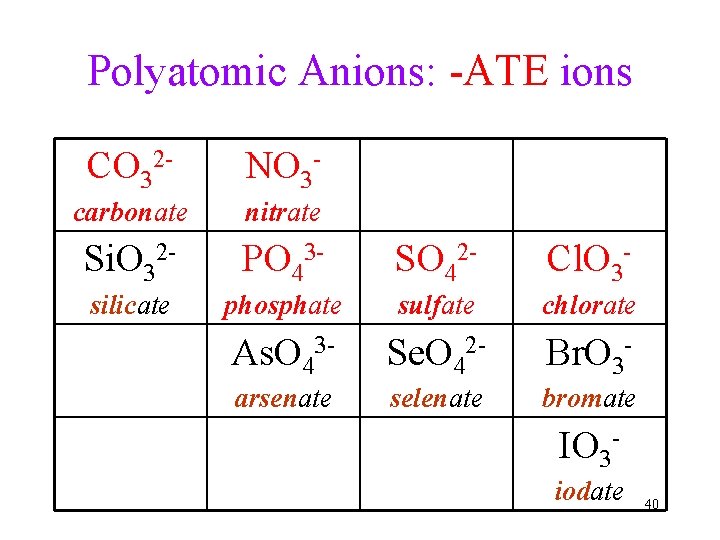
Polyatomic Anions: -ATE ions CO 32 - NO 3 - carbonate nitrate Si. O 32 - PO 43 - SO 42 - Cl. O 3 - silicate phosphate sulfate chlorate As. O 43 - Se. O 42 - Br. O 3 - arsenate selenate bromate IO 3 iodate 40

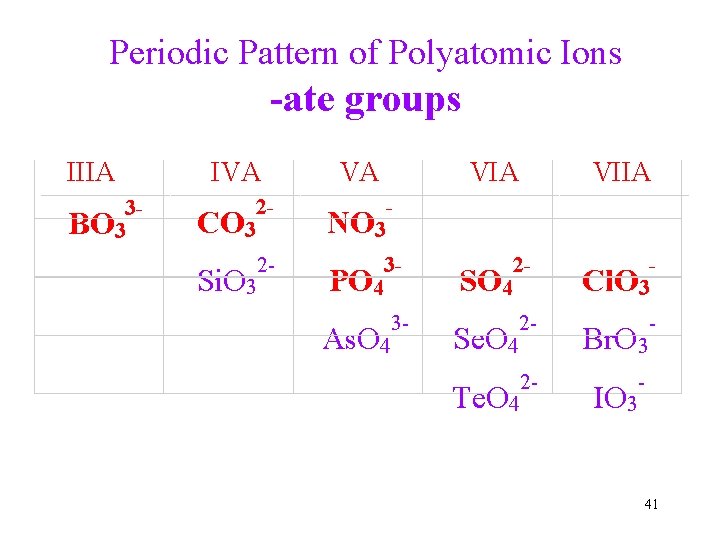
Periodic Pattern of Polyatomic Ions -ate groups IIIA 3 BO 3 IVA VA VIIA 2 CO 3 NO 3 2 Si. O 3 3 PO 4 2 SO 4 Cl. O 3 3 As. O 4 2 Se. O 4 Br. O 3 2 Te. O 4 IO 3 41

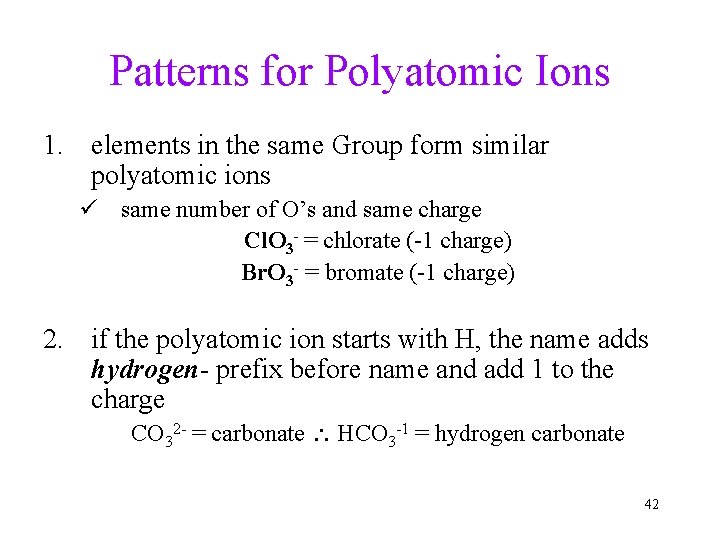
Patterns for Polyatomic Ions 1. elements in the same Group form similar polyatomic ions ü same number of O’s and same charge Cl. O 3 - = chlorate (-1 charge) Br. O 3 - = bromate (-1 charge) 2. if the polyatomic ion starts with H, the name adds hydrogen- prefix before name and add 1 to the charge CO 32 - = carbonate HCO 3 -1 = hydrogen carbonate 42

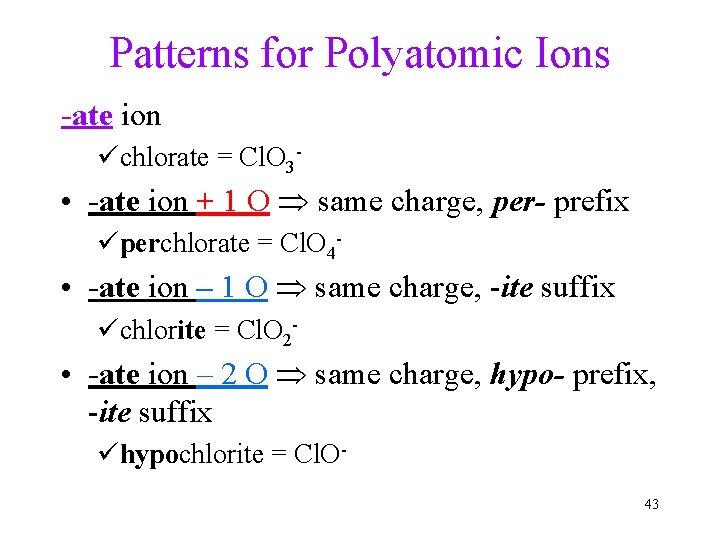
Patterns for Polyatomic Ions -ate ion üchlorate = Cl. O 3 - • -ate ion + 1 O same charge, per- prefix üperchlorate = Cl. O 4 - • -ate ion – 1 O same charge, -ite suffix üchlorite = Cl. O 2 - • -ate ion – 2 O same charge, hypo- prefix, -ite suffix ühypochlorite = Cl. O 43

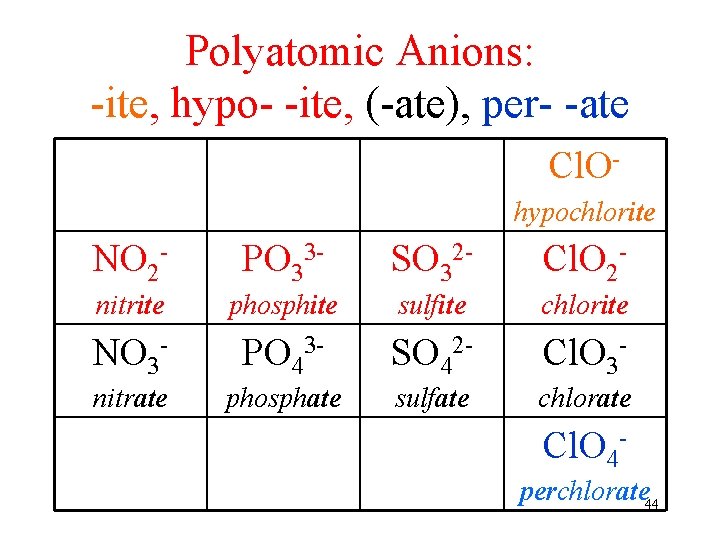
Polyatomic Anions: -ite, hypo- -ite, (-ate), per- -ate Cl. Ohypochlorite NO 2 - PO 33 - SO 32 - Cl. O 2 - nitrite phosphite sulfite chlorite NO 3 - PO 43 - SO 42 - Cl. O 3 - nitrate phosphate sulfate chlorate Cl. O 4 perchlorate 44

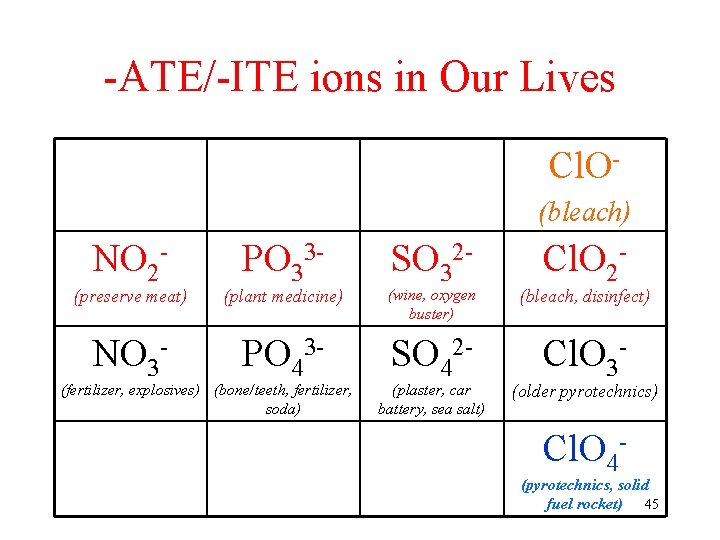
-ATE/-ITE ions in Our Lives Cl. O(bleach) NO 2 - PO 33 - SO 32 - Cl. O 2 - (preserve meat) (plant medicine) (wine, oxygen buster) (bleach, disinfect) NO 3 - PO 43 - SO 42 - Cl. O 3 - (fertilizer, explosives) (bone/teeth, fertilizer, soda) (plaster, car battery, sea salt) (older pyrotechnics) Cl. O 4 - (pyrotechnics, solid fuel rocket) 45

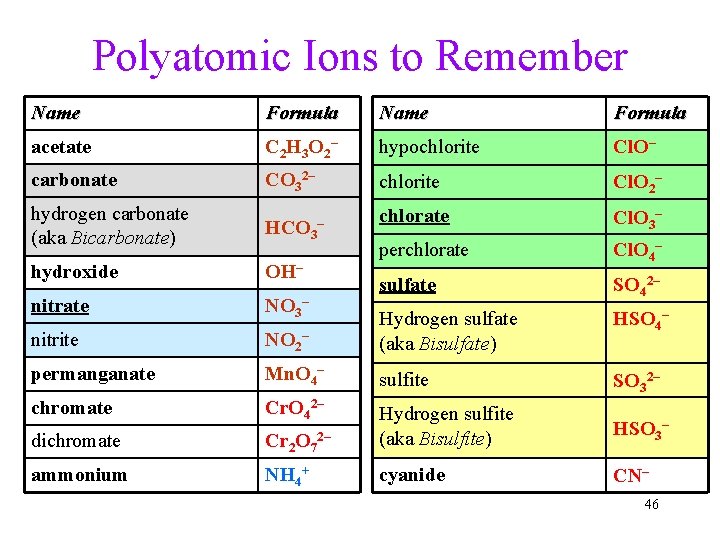
Polyatomic Ions to Remember Name Formula acetate C 2 H 3 O 2 – hypochlorite Cl. O– carbonate CO 32– chlorite Cl. O 2– hydrogen carbonate (aka Bicarbonate) HCO 3 chlorate Cl. O 3– hydroxide OH– perchlorate Cl. O 4– nitrate NO 3– sulfate SO 42– nitrite NO 2– Hydrogen sulfate (aka Bisulfate) HSO 4– permanganate Mn. O 4– sulfite SO 32– chromate Cr. O 42– dichromate Cr 2 O 7 Hydrogen sulfite (aka Bisulfite) HSO 3– ammonium NH 4+ cyanide CN– – 2– 46

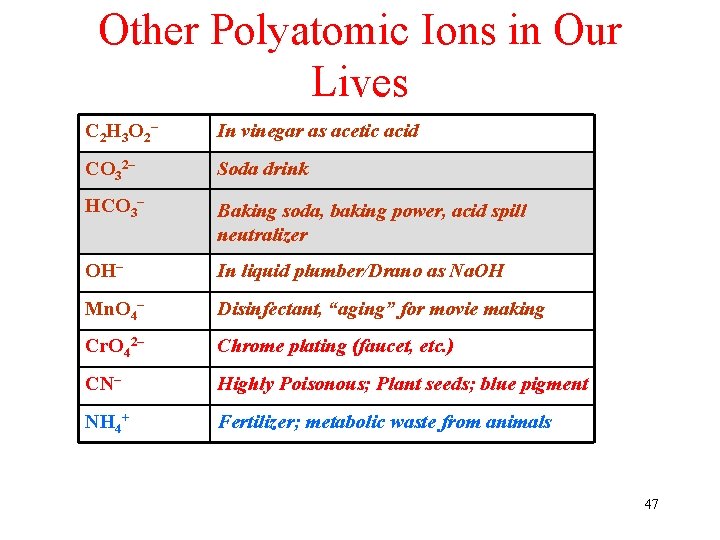
Other Polyatomic Ions in Our Lives C 2 H 3 O 2 – In vinegar as acetic acid CO 32– Soda drink HCO 3– Baking soda, baking power, acid spill neutralizer OH– In liquid plumber/Drano as Na. OH Mn. O 4– Disinfectant, “aging” for movie making Cr. O 42– Chrome plating (faucet, etc. ) CN– Highly Poisonous; Plant seeds; blue pigment NH 4+ Fertilizer; metabolic waste from animals 47

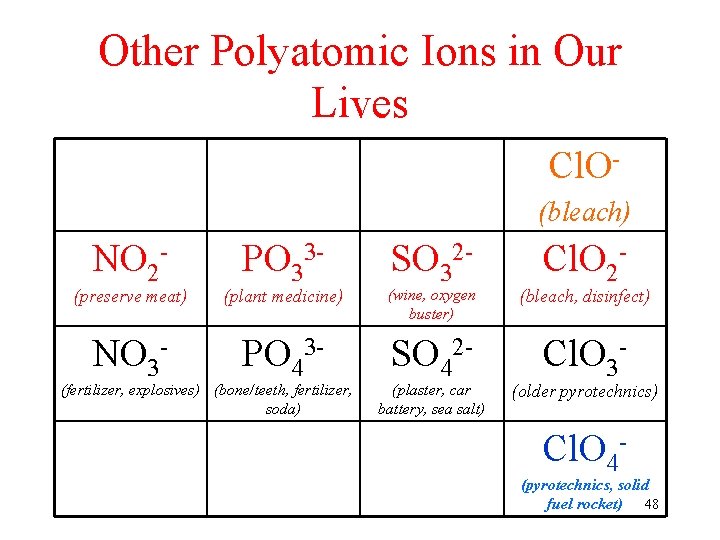
Other Polyatomic Ions in Our Lives Cl. O(bleach) NO 2 - PO 33 - SO 32 - Cl. O 2 - (preserve meat) (plant medicine) (wine, oxygen buster) (bleach, disinfect) NO 3 - PO 43 - SO 42 - Cl. O 3 - (fertilizer, explosives) (bone/teeth, fertilizer, soda) (plaster, car battery, sea salt) (older pyrotechnics) Cl. O 4 - (pyrotechnics, solid fuel rocket) 48

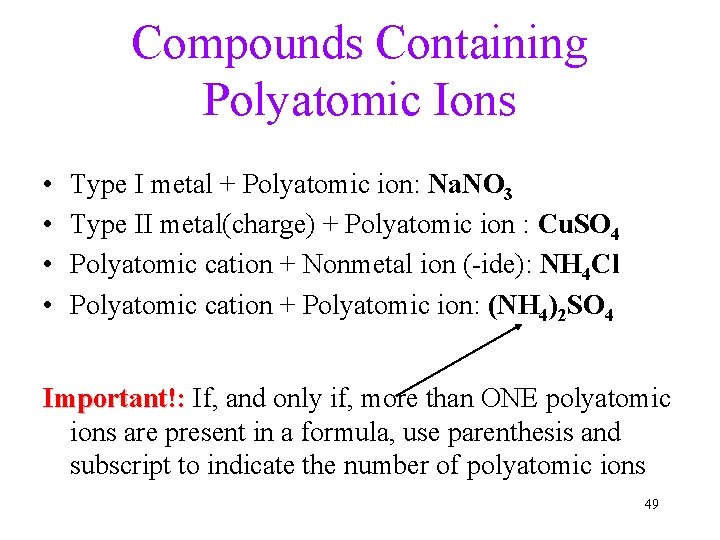
Compounds Containing Polyatomic Ions • • Type I metal + Polyatomic ion: Na. NO 3 Type II metal(charge) + Polyatomic ion : Cu. SO 4 Polyatomic cation + Nonmetal ion (-ide): NH 4 Cl Polyatomic cation + Polyatomic ion: (NH 4)2 SO 4 Important!: If, and only if, more than ONE polyatomic ions are present in a formula, use parenthesis and subscript to indicate the number of polyatomic ions 49

Example – Naming Ionic with Polyatomic Ion Na 2 SO 4 1. Is it one of the common exceptions? H 2 O, NH 3, CH 4, C 12 H 22 O 11 = No! 2. Identify Major Class Na = is a metal because it is on the left side of the PT SO 4 = is a polyatomic ion Ionic 3. Identify the Subclass compound has 3 elements Ionic with Polyatomic Ion 4. Is the metal Type I or Type II Na is in Group IA, Type I 50

Example – Naming Ionic with Polyatomic Ion Na 2 SO 4 5. Identify the ions Na = Na+ because in Group 1 SO 4 = SO 42 - a polyatomic ion 6. Name the cation Na+ = sodium (Type I) 7. Name the anion SO 42 - = sulfate 8. Write the name of the cation followed by the name of the anion sodium sulfate 51

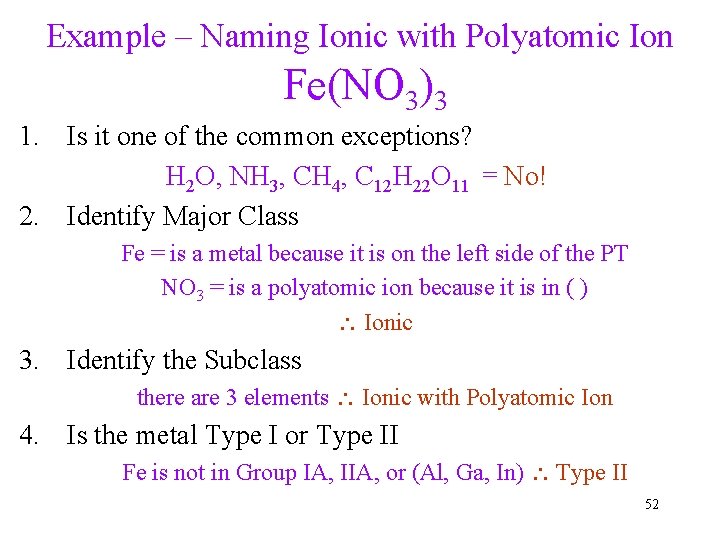
Example – Naming Ionic with Polyatomic Ion Fe(NO 3)3 1. Is it one of the common exceptions? H 2 O, NH 3, CH 4, C 12 H 22 O 11 = No! 2. Identify Major Class Fe = is a metal because it is on the left side of the PT NO 3 = is a polyatomic ion because it is in ( ) Ionic 3. Identify the Subclass there are 3 elements Ionic with Polyatomic Ion 4. Is the metal Type I or Type II Fe is not in Group IA, IIA, or (Al, Ga, In) Type II 52

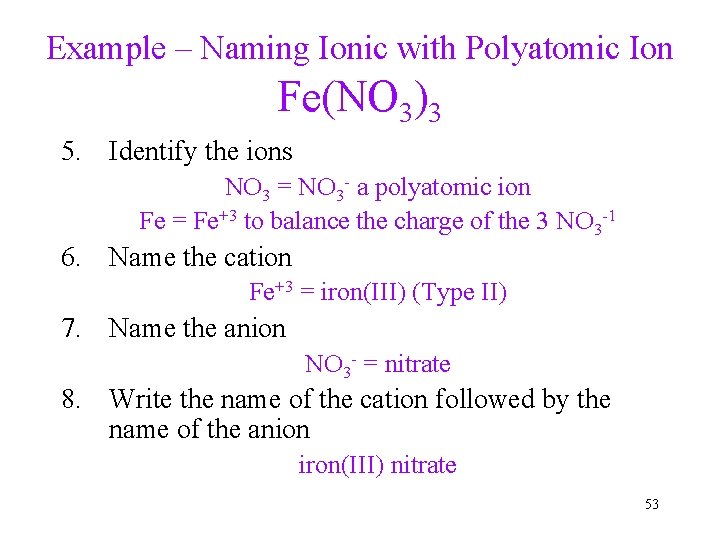
Example – Naming Ionic with Polyatomic Ion Fe(NO 3)3 5. Identify the ions NO 3 = NO 3 - a polyatomic ion Fe = Fe+3 to balance the charge of the 3 NO 3 -1 6. Name the cation Fe+3 = iron(III) (Type II) 7. Name the anion NO 3 - = nitrate 8. Write the name of the cation followed by the name of the anion iron(III) nitrate 53

Practice: Naming Ionic compounds • • Hg 2 SO 4 Cu. Cl. O 3 Zn(NO 3)2 Fe. CO 3 Sn(SO 3)2 Co. PO 4 Al(Cl. O 4)3 54

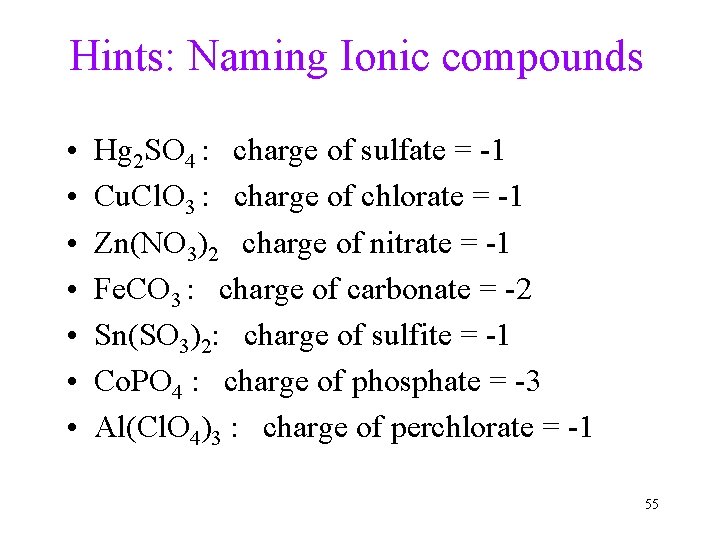
Hints: Naming Ionic compounds • • Hg 2 SO 4 : charge of sulfate = -1 Cu. Cl. O 3 : charge of chlorate = -1 Zn(NO 3)2 charge of nitrate = -1 Fe. CO 3 : charge of carbonate = -2 Sn(SO 3)2: charge of sulfite = -1 Co. PO 4 : charge of phosphate = -3 Al(Cl. O 4)3 : charge of perchlorate = -1 55

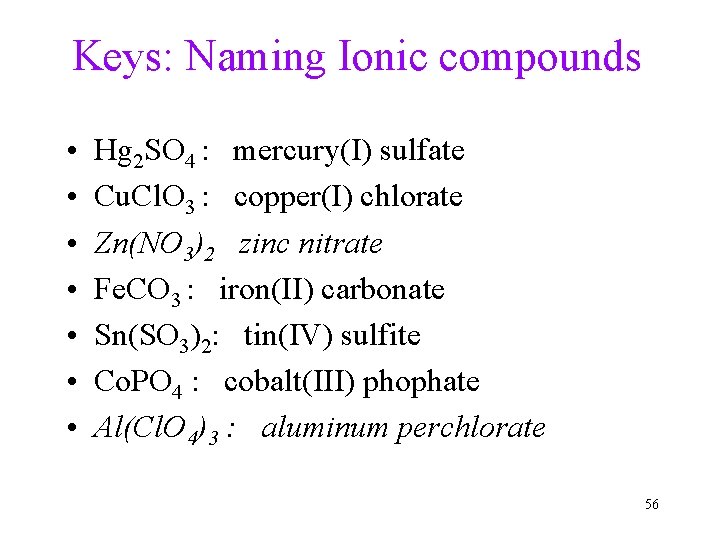
Keys: Naming Ionic compounds • • Hg 2 SO 4 : mercury(I) sulfate Cu. Cl. O 3 : copper(I) chlorate Zn(NO 3)2 zinc nitrate Fe. CO 3 : iron(II) carbonate Sn(SO 3)2: tin(IV) sulfite Co. PO 4 : cobalt(III) phophate Al(Cl. O 4)3 : aluminum perchlorate 56

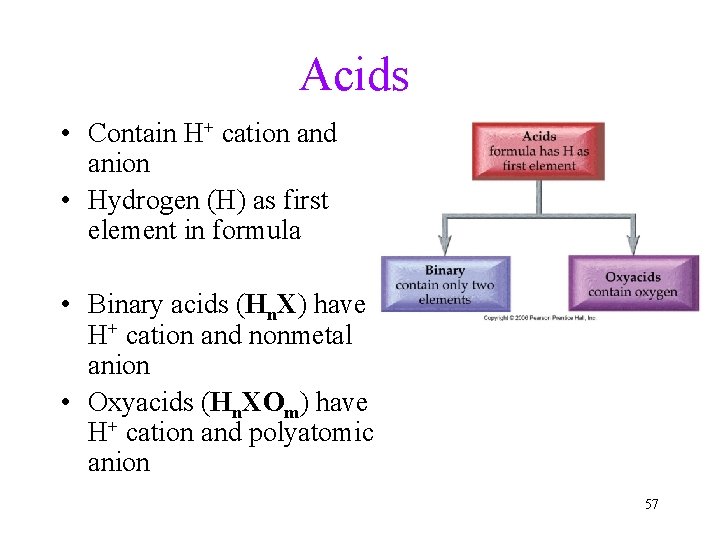
Acids • Contain H+ cation and anion • Hydrogen (H) as first element in formula • Binary acids (Hn. X) have H+ cation and nonmetal anion • Oxyacids (Hn. XOm) have H+ cation and polyatomic anion 57

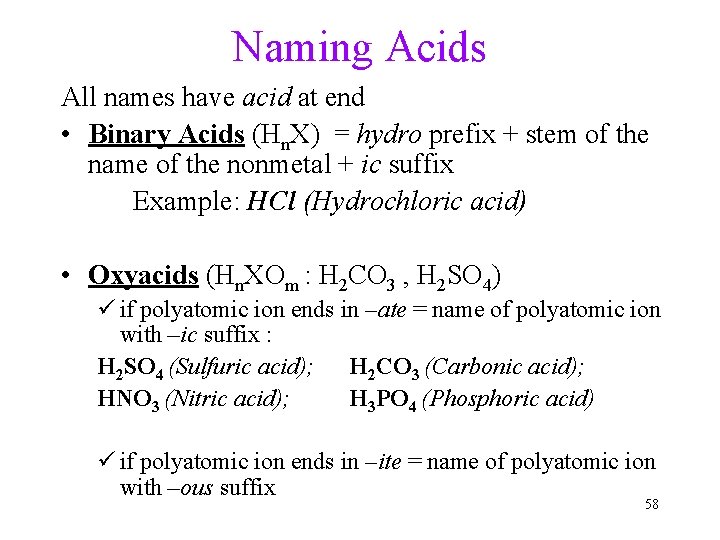
Naming Acids All names have acid at end • Binary Acids (Hn. X) = hydro prefix + stem of the name of the nonmetal + ic suffix Example: HCl (Hydrochloric acid) • Oxyacids (Hn. XOm : H 2 CO 3 , H 2 SO 4) ü if polyatomic ion ends in –ate = name of polyatomic ion with –ic suffix : H 2 SO 4 (Sulfuric acid); H 2 CO 3 (Carbonic acid); HNO 3 (Nitric acid); H 3 PO 4 (Phosphoric acid) ü if polyatomic ion ends in –ite = name of polyatomic ion with –ous suffix 58

Naming Binary Acids – HF 1. First of all, it is binary acid HX 2. Identify the anion F F-, fluoride because Group 7 A 2. Name the anion with an –ic suffix F- = fluoride fluoric 3. Add a hydro- prefix to the anion name hydrofluoric 4. Add the word acid to the end hydrofluoric acid 59

Naming Oxyacids: H 2 SO 4 1. Identify the anion SO 4 = SO 42 - = sulfate 2. If the anion has –ate suffix, change it to –ic. If the anion has –ite suffix, change it to -ous SO 42 - = sulfate sulfuric 3. Write the name of the anion followed by the word acid sulfuric acid (kind of an exception, to make it sound nicer!) 60

Practice: Naming Acids first: what is the anion? • • HNO 3 HCl. O 3 HBr H 2 CO 3 H 2 SO 3 H 3 PO 4 HCl. O 4 nitrate chlorate bromide carbonate sulfite phosphate perchlorate 61

Practice: Naming Acids • • HNO 3 nitrate nitric acid HCl. O 3 chlorate chloric acid HBr bromide hydrobromic acid H 2 CO 3 carbonate carbonic acid H 2 SO 3 sulfite sulfurous acid H 3 PO 4 phosphate phosphoric acid HCl. O 4 perchlorate perchloric acid 62

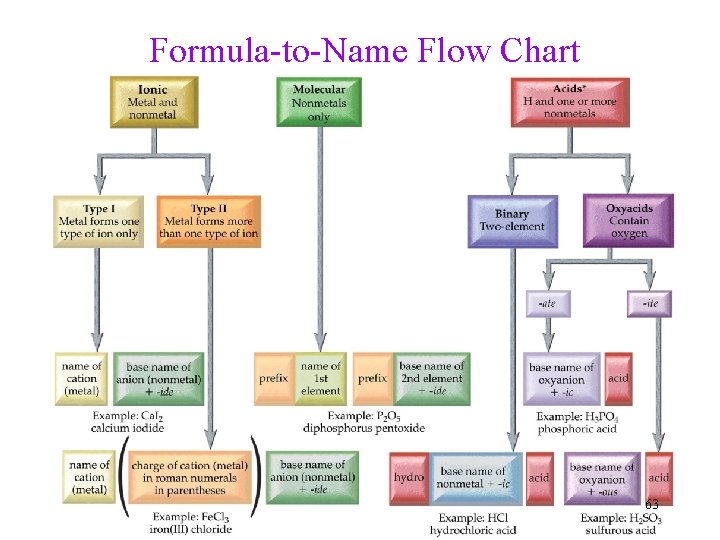
Formula-to-Name Flow Chart 63

Review: Naming Compounds • • Cu. SO 3 Ag. Cl. O N 2 O 5 H 2 S Fe. I 2 Sn(NO 3)4 Ba 3(PO 4)2 (NH 4)2 S 1. Common exceptions? H 2 O, NH 3, CH 4, C 12 H 22 O 11 2. Identify as Molecular or Ionic? 3. Identify • • Binary molecular Type I or II metal ion 64

Review: Naming Compounds • • Cu. SO 3 Ag. Cl. O N 2 O 5 H 2 S Fe. I 2 Sn(NO 3)4 Ba 3(PO 4)2 (NH 4)2 S copper(II) sulfite silver hypochlorite dinitrogen pentoxide hydrosulfuric acid iron(II) iodide tin(IV) nitrate barium phosphate ammonium sulfide 65

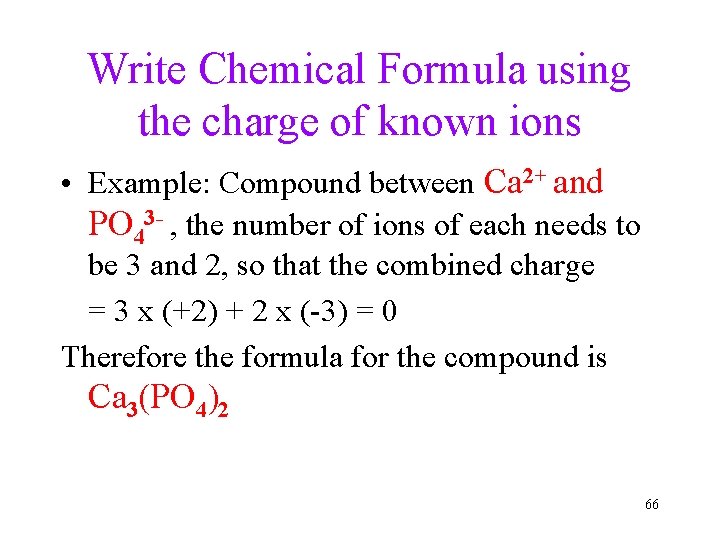
Write Chemical Formula using the charge of known ions • Example: Compound between Ca 2+ and PO 43 - , the number of ions of each needs to be 3 and 2, so that the combined charge = 3 x (+2) + 2 x (-3) = 0 Therefore the formula for the compound is Ca 3(PO 4)2 66

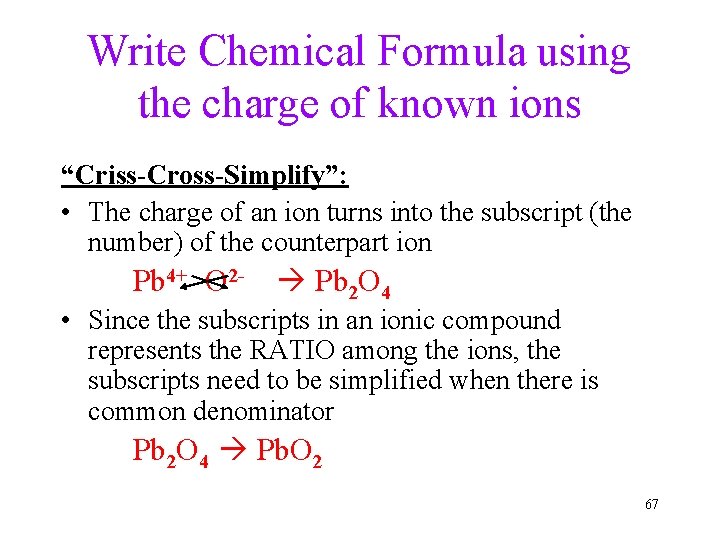
Write Chemical Formula using the charge of known ions “Criss-Cross-Simplify”: • The charge of an ion turns into the subscript (the number) of the counterpart ion Pb 4+ O 2 - Pb 2 O 4 • Since the subscripts in an ionic compound represents the RATIO among the ions, the subscripts need to be simplified when there is common denominator Pb 2 O 4 Pb. O 2 67

Practice: Writing formulas (I) • • copper(II) chloride aluminum oxide magnesium phosphide iron(II) bromide lead(II) sulfide zinc iodide sodium nitride 68

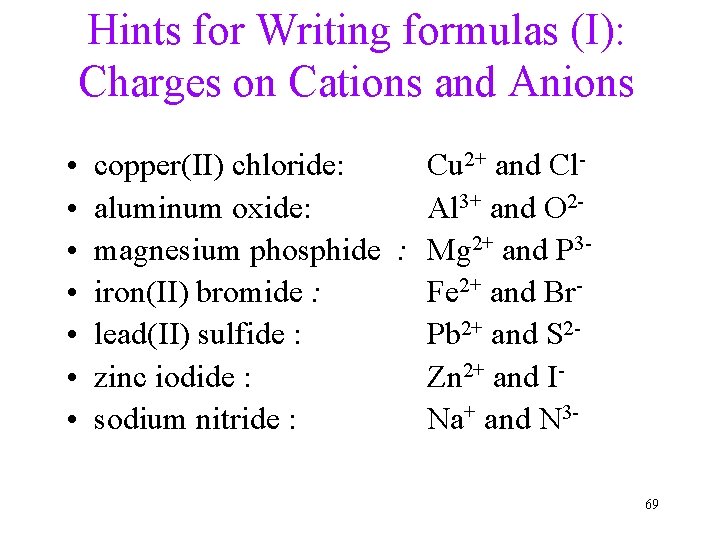
Hints for Writing formulas (I): Charges on Cations and Anions • • copper(II) chloride: Cu 2+ and Claluminum oxide: Al 3+ and O 2 magnesium phosphide : Mg 2+ and P 3 iron(II) bromide : Fe 2+ and Brlead(II) sulfide : Pb 2+ and S 2 zinc iodide : Zn 2+ and Isodium nitride : Na+ and N 369

Key for Writing formulas (I): use criss-cross-reduce • • copper(II) chloride aluminum oxide magnesium phosphide iron(II) bromide lead(II) sulfide zinc iodide sodium nitride Cu. Cl 2 Al 2 O 3 Mg 3 P 2 Fe. Br 2 Fe. S Zn. I 2 Na 3 N 70

Practice: Write Chemical Formulae • • Chromium(II) Chloride Cesium phosphate Lead(II) oxide Zinc nitrate Iron(III) sulfite Strontium nitride Ammonium carbonate 71

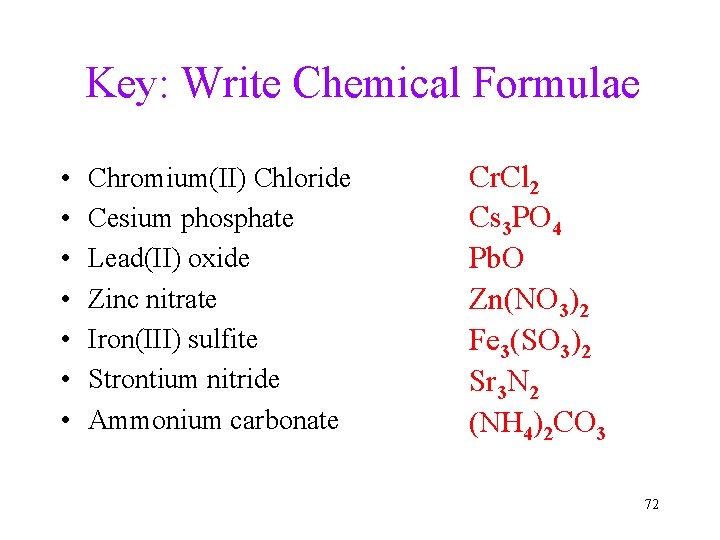
Key: Write Chemical Formulae • • Chromium(II) Chloride Cesium phosphate Lead(II) oxide Zinc nitrate Iron(III) sulfite Strontium nitride Ammonium carbonate Cr. Cl 2 Cs 3 PO 4 Pb. O Zn(NO 3)2 Fe 3(SO 3)2 Sr 3 N 2 (NH 4)2 CO 3 72

Practice: Writing formulas (I) • • copper(I) sulfate aluminum chlorate magnesium phosphate iron(II) carbonate lead(II) acetate zinc sulfite sodium nitrite Nitrogen gas 73

Hints for Writing formulas (I): Charges on Anions • • copper(I) sulfate: -2 for sulfate aluminum chlorate: -1 for chlorate magnesium phosphate: -3 for phosphate iron(II) carbonate : -2 for carbonate lead(II) acetate: -1 for acetate zinc sulfite : -2 for sulfite sodium nitrite: -1 for nitrite Nitrogen gas: atomic or molecular element? 74

Key: Writing formulas (I) • • copper(I) sulfate: Cu 2 SO 4 aluminum chlorate: Al(Cl. O 3)3 magnesium phosphate: Mg 3(PO 4)2 iron(II) carbonate : Fe. CO 3 lead(II) acetate: Pb(C 2 H 3 O 2)2 zinc sulfite : Zn. SO 3 sodium nitrite: Na. NO 2 Nitrogen gas: N 2 75

More on Writing formulae • • copper(II) sulfate aluminum perchlorate hydroiodic acid iron(III) bromide Diphosphorus pentoxide lead(IV) nitride zinc carbonate helium gas 76

Key: Writing formulae • • • copper(II) sulfate Cu. SO 4 aluminum perchlorate Al(Cl. O 4)3 hydroiodic acid HI iron(III) bromide Fe. Br 3 Diphosphorus pentoxide P 2 O 5 lead(IV) nitride Pb 3 N 4 zinc carbonate Zn. CO 3 ammonium nitrite NH 4 NO 2 helium gas He 77