Gases The Ideal Gas Law General Organic and



- Slides: 13

Gases The Ideal Gas Law General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 1

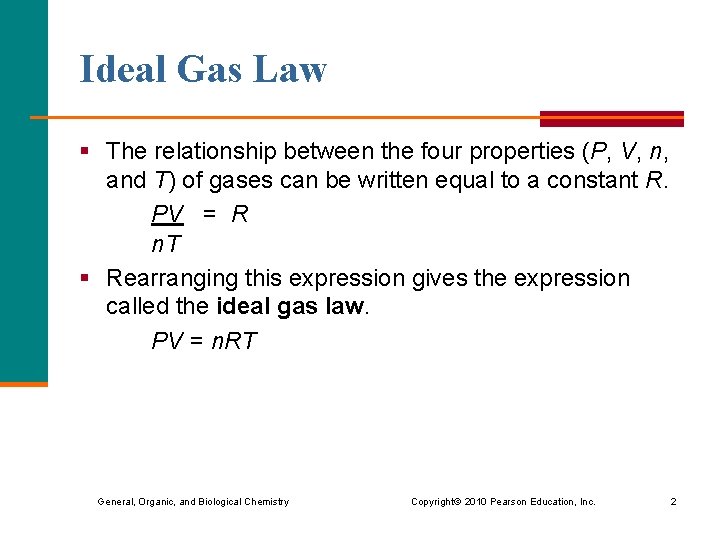
Ideal Gas Law § The relationship between the four properties (P, V, n, and T) of gases can be written equal to a constant R. PV = R n. T § Rearranging this expression gives the expression called the ideal gas law. PV = n. RT General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 2

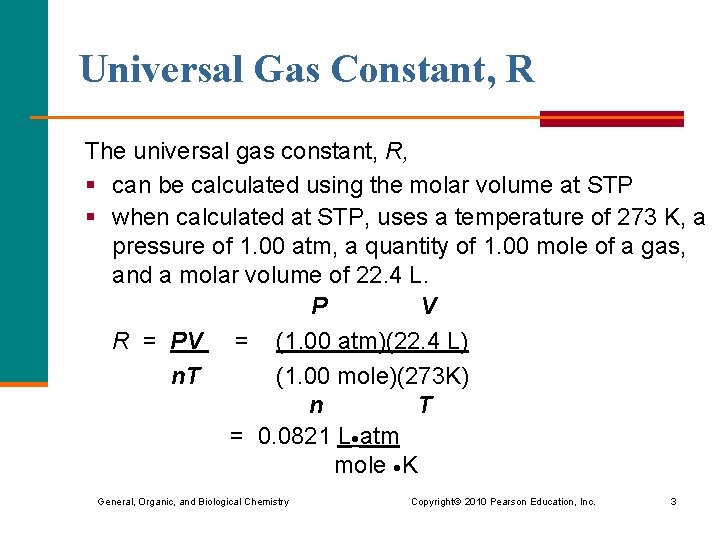
Universal Gas Constant, R The universal gas constant, R, § can be calculated using the molar volume at STP § when calculated at STP, uses a temperature of 273 K, a pressure of 1. 00 atm, a quantity of 1. 00 mole of a gas, and a molar volume of 22. 4 L. P V R = PV = (1. 00 atm)(22. 4 L) n. T (1. 00 mole)(273 K) n T = 0. 0821 L atm mole K General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 3

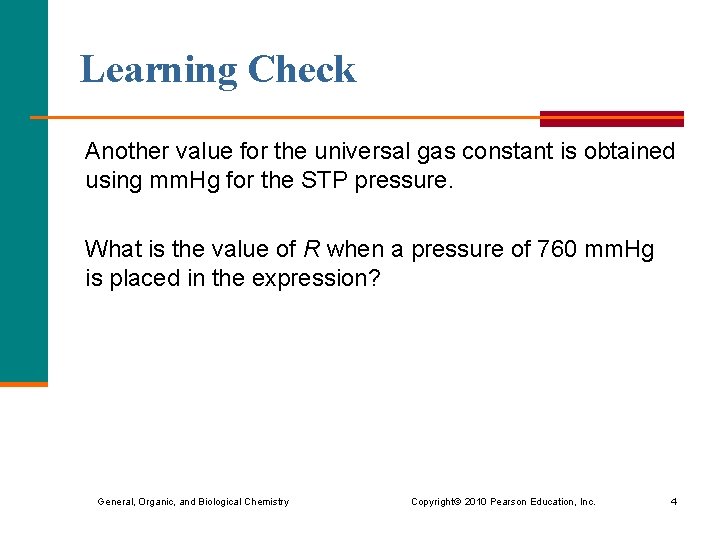
Learning Check Another value for the universal gas constant is obtained using mm. Hg for the STP pressure. What is the value of R when a pressure of 760 mm. Hg is placed in the expression? General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 4

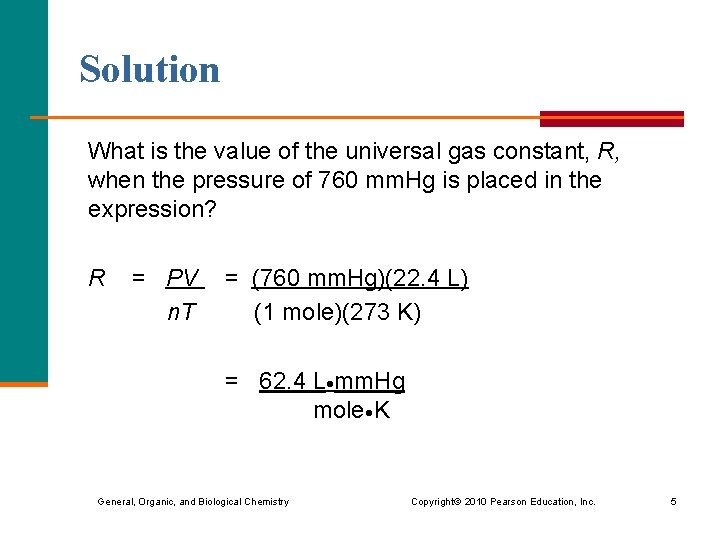
Solution What is the value of the universal gas constant, R, when the pressure of 760 mm. Hg is placed in the expression? R = PV = (760 mm. Hg)(22. 4 L) n. T (1 mole)(273 K) = 62. 4 L mm. Hg mole K General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 5

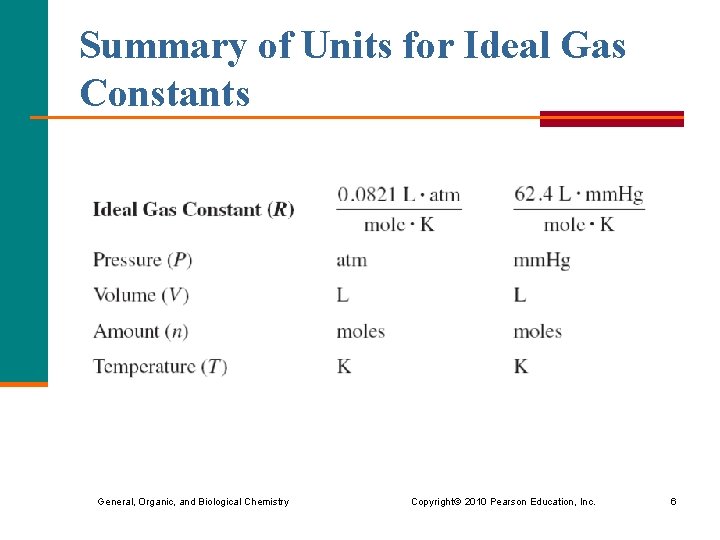
Summary of Units for Ideal Gas Constants General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 6

Guide to Using the Ideal Gas Law General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 7

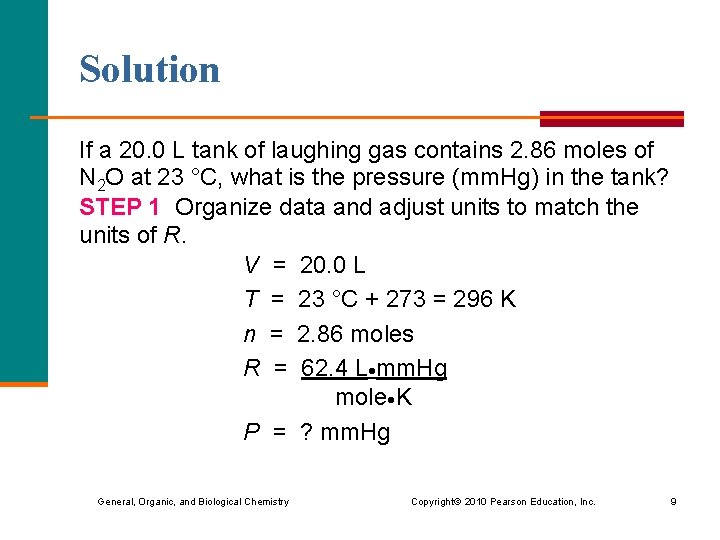
Learning Check Dinitrogen oxide (N 2 O), laughing gas, is used by dentists as an anesthetic. If a 20. 0 L tank of laughing gas contains 2. 86 moles of N 2 O at 23 °C, what is the pressure (mm. Hg) in the tank? General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 8

Solution If a 20. 0 L tank of laughing gas contains 2. 86 moles of N 2 O at 23 °C, what is the pressure (mm. Hg) in the tank? STEP 1 Organize data and adjust units to match the units of R. V = 20. 0 L T = 23 °C + 273 = 296 K n = 2. 86 moles R = 62. 4 L mm. Hg mole K P = ? mm. Hg General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 9

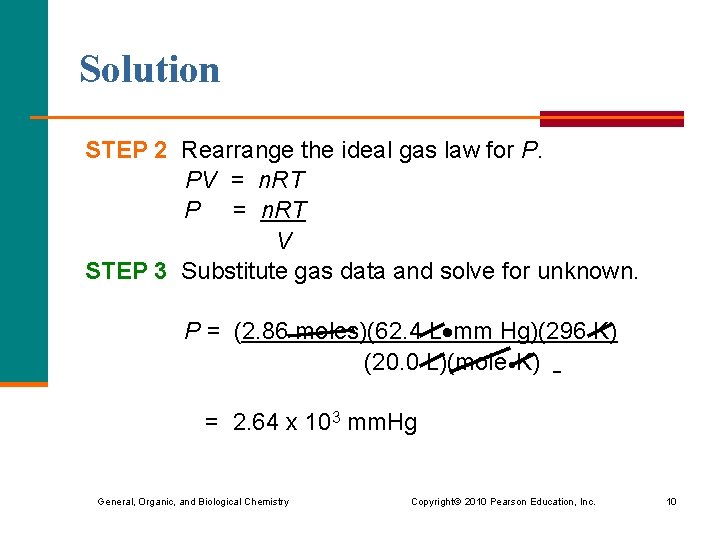
Solution STEP 2 Rearrange the ideal gas law for P. PV = n. RT P = n. RT V STEP 3 Substitute gas data and solve for unknown. P = (2. 86 moles)(62. 4 L mm Hg)(296 K) (20. 0 L)(mole K) = 2. 64 x 103 mm. Hg General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 10

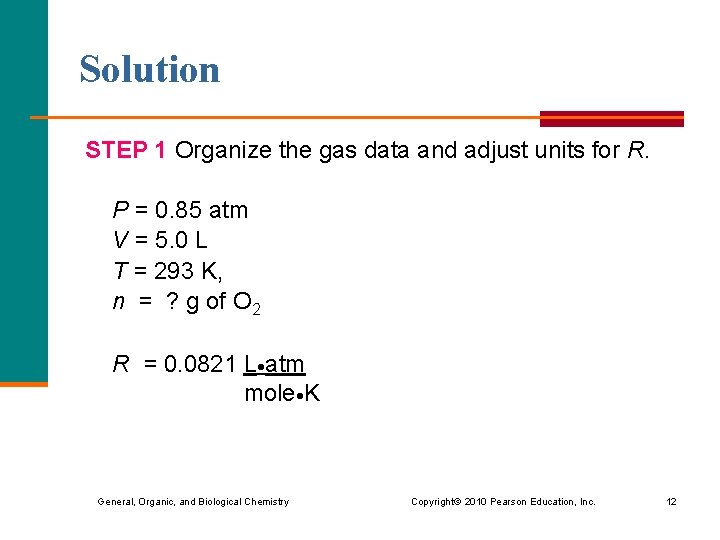
Learning Check A cylinder contains 5. 0 L of O 2 at 20. 0 °C and 0. 85 atm. How many grams of oxygen are in the cylinder? General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 11

Solution STEP 1 Organize the gas data and adjust units for R. P = 0. 85 atm V = 5. 0 L T = 293 K, n = ? g of O 2 R = 0. 0821 L atm mole K General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 12

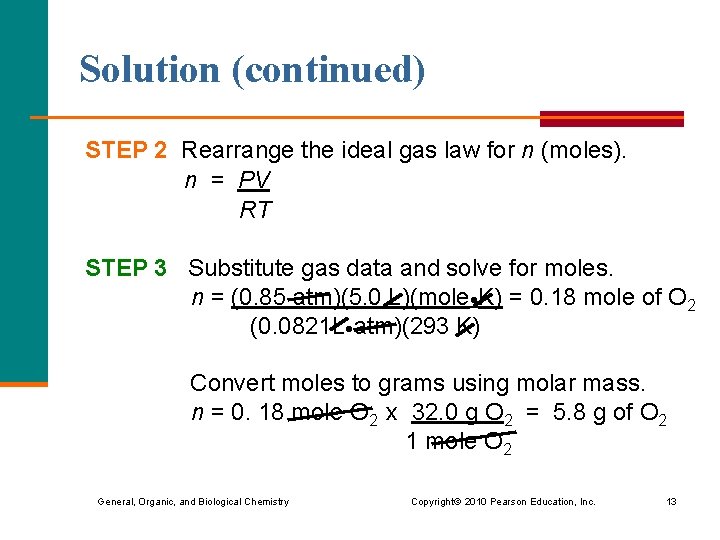
Solution (continued) STEP 2 Rearrange the ideal gas law for n (moles). n = PV RT STEP 3 Substitute gas data and solve for moles. n = (0. 85 atm)(5. 0 L)(mole K) = 0. 18 mole of O 2 (0. 0821 L atm)(293 K) Convert moles to grams using molar mass. n = 0. 18 mole O 2 x 32. 0 g O 2 = 5. 8 g of O 2 1 mole O 2 General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 13