MEDICAL MANAGEMENT OF CHEMICAL CASUALTIES NERVE AGENTS PRETREAMENT





































































- Slides: 119

MEDICAL MANAGEMENT OF CHEMICAL CASUALTIES NERVE AGENTS & PRETREAMENT • U. S. ARMY MEDICAL RESEARCH INSTITUTE OF CHEMICAL DEFENSE USAMRICD PROTECT, PROJECT, SUSTAIN

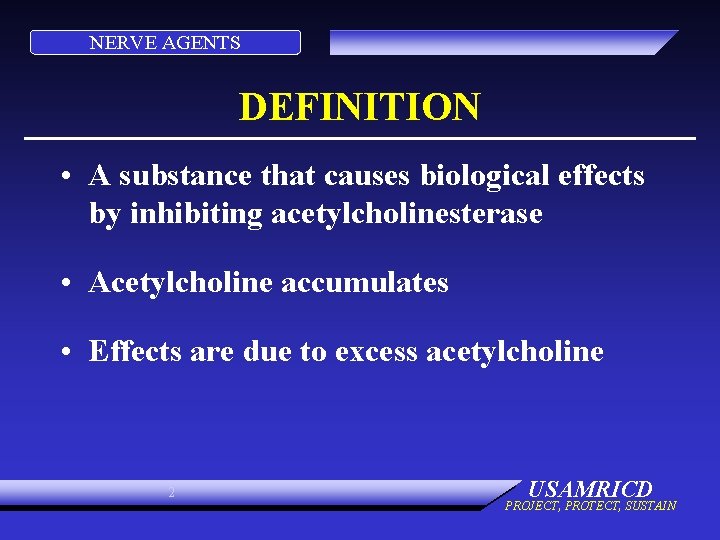
NERVE AGENTS DEFINITION • A substance that causes biological effects by inhibiting acetylcholinesterase • Acetylcholine accumulates • Effects are due to excess acetylcholine 2 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS EXAMPLES • Carbamates – Physostigmine (Antilirium) – Neostigmine (Prostigmine) – Pyridostigmine (Mestinon) – Sevin (insecticide) • Organophosphates – Malathion – Diazinon – “Nerve Agents” 3 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS • GA (Tabun) • GB (Sarin) • GD (Soman) • GF • VX 4 USAMRICD PROJECT, PROTECT, SUSTAIN

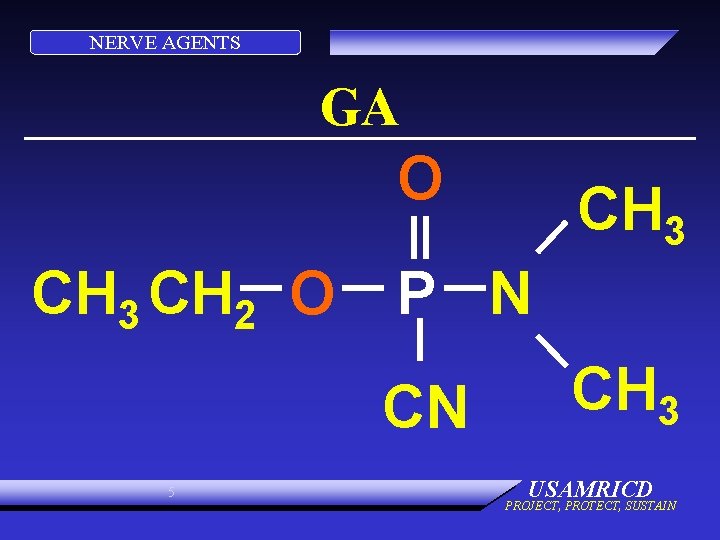
NERVE AGENTS GA O CH 3 CH 2 O P N CN 5 CH 3 USAMRICD PROJECT, PROTECT, SUSTAIN

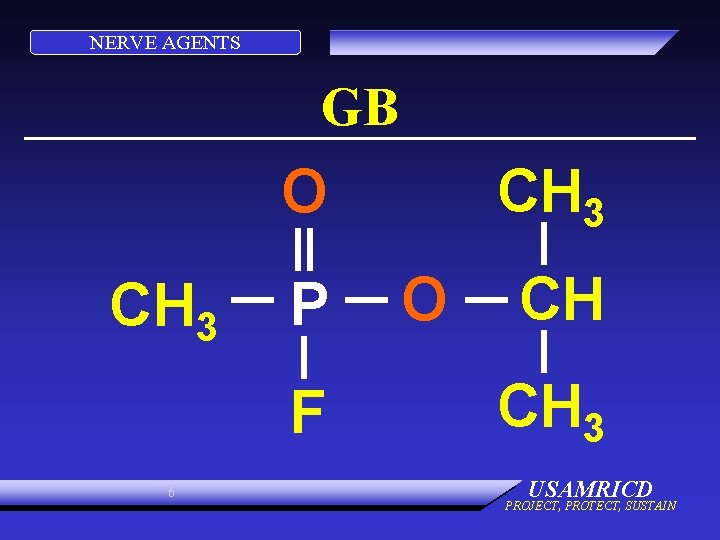
NERVE AGENTS GB O CH 3 P F 6 CH 3 O CH CH 3 USAMRICD PROJECT, PROTECT, SUSTAIN

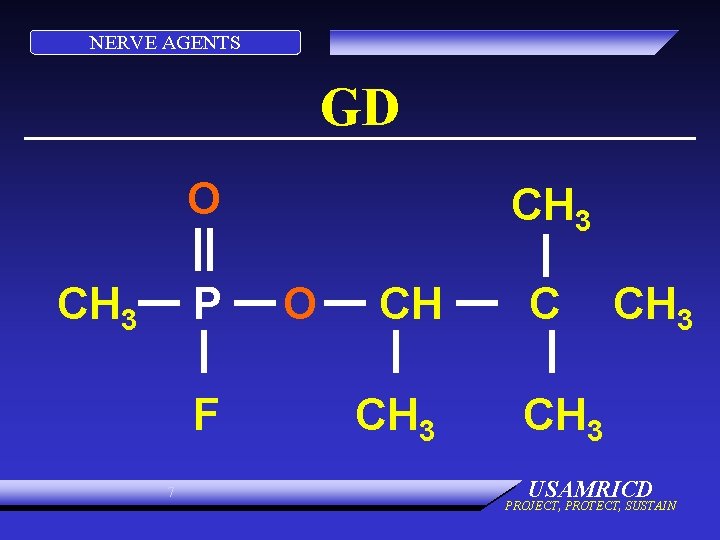
NERVE AGENTS GD O CH 3 P F 7 CH 3 O CH CH 3 USAMRICD PROJECT, PROTECT, SUSTAIN

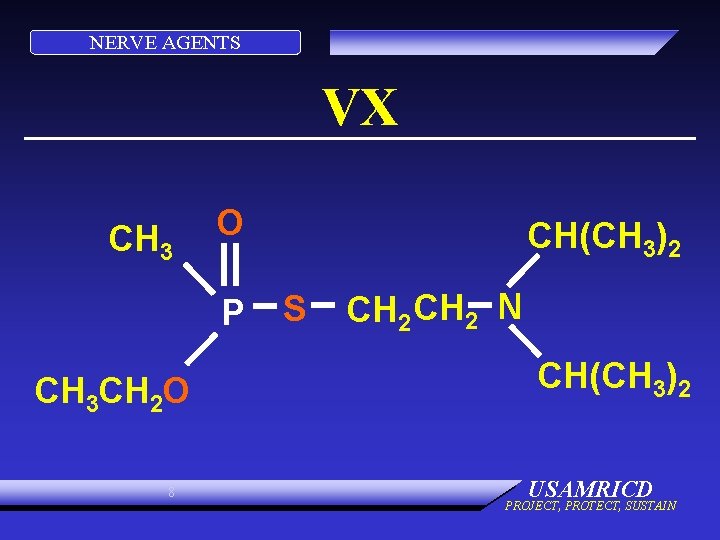
NERVE AGENTS VX CH 3 O P CH 3 CH 2 O 8 CH(CH 3)2 S CH 2 N CH(CH 3)2 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS CONTINUED HISTORY • Germany, WW II, nerve agent munitions • Used by Iraq • In stockpiles 9 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS TERRORIST USE • Matsumoto, 1994 – 7 deaths • Tokyo, 1995 – 12 deaths 10 USAMRICD PROJECT, PROTECT, SUSTAIN

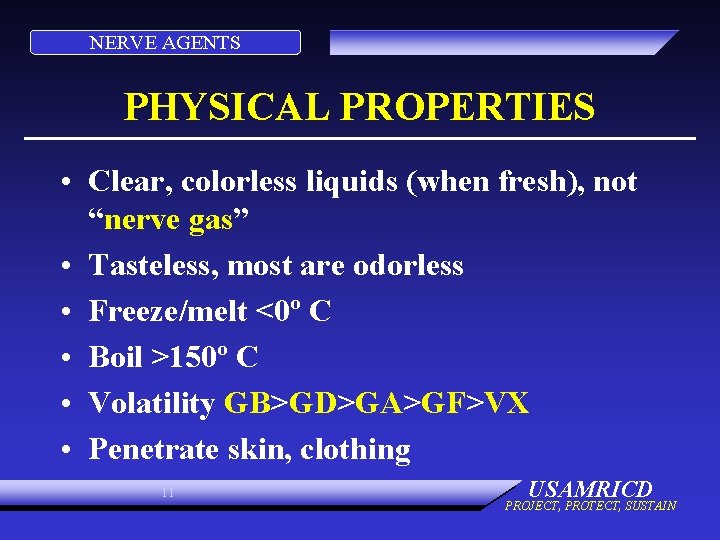
NERVE AGENTS PHYSICAL PROPERTIES • Clear, colorless liquids (when fresh), not “nerve gas” • Tasteless, most are odorless • Freeze/melt <0º C • Boil >150º C • Volatility GB>GD>GA>GF>VX • Penetrate skin, clothing 11 USAMRICD PROJECT, PROTECT, SUSTAIN

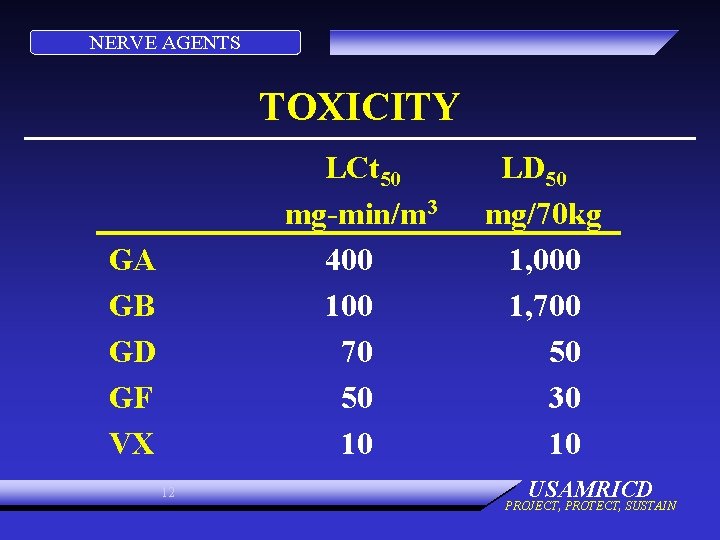
NERVE AGENTS TOXICITY LCt 50 mg-min/m 3 400 100 70 50 10 GA GB GD GF VX 12 LD 50 mg/70 kg 1, 000 1, 700 50 30 10 USAMRICD PROJECT, PROTECT, SUSTAIN

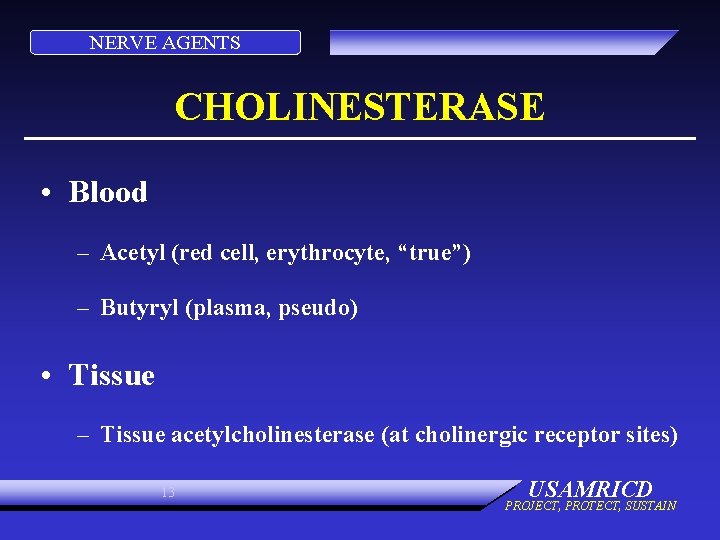
NERVE AGENTS CHOLINESTERASE • Blood – Acetyl (red cell, erythrocyte, “true”) – Butyryl (plasma, pseudo) • Tissue – Tissue acetylcholinesterase (at cholinergic receptor sites) 13 USAMRICD PROJECT, PROTECT, SUSTAIN

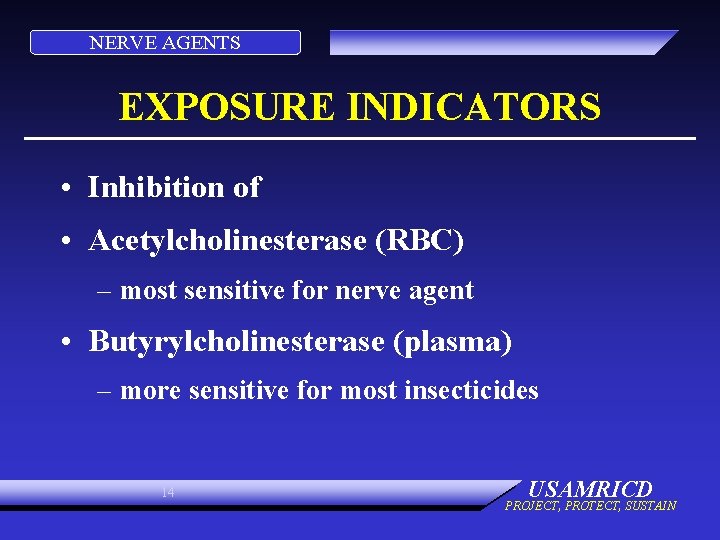
NERVE AGENTS EXPOSURE INDICATORS • Inhibition of • Acetylcholinesterase (RBC) – most sensitive for nerve agent • Butyrylcholinesterase (plasma) – more sensitive for most insecticides 14 USAMRICD PROJECT, PROTECT, SUSTAIN

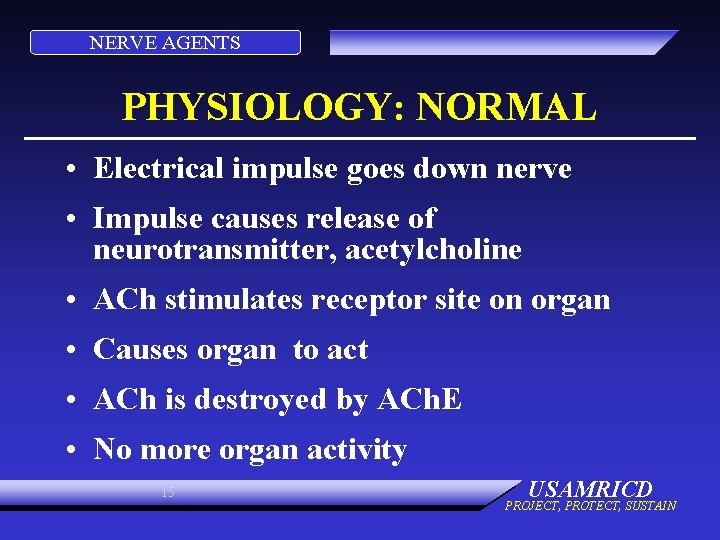
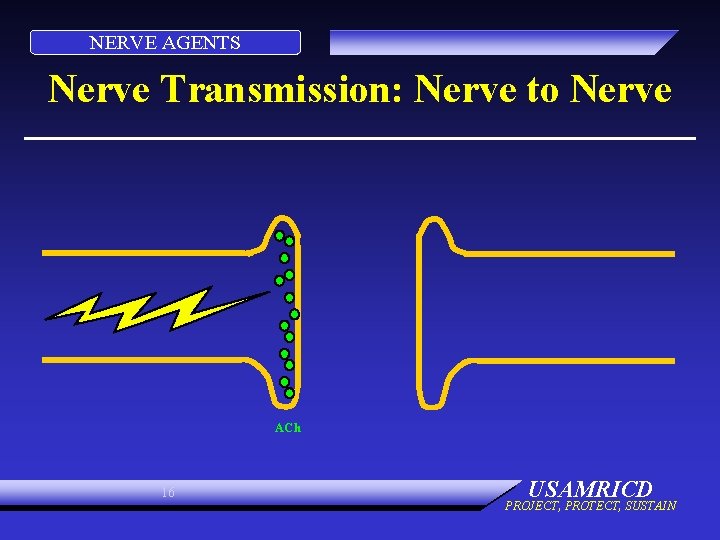
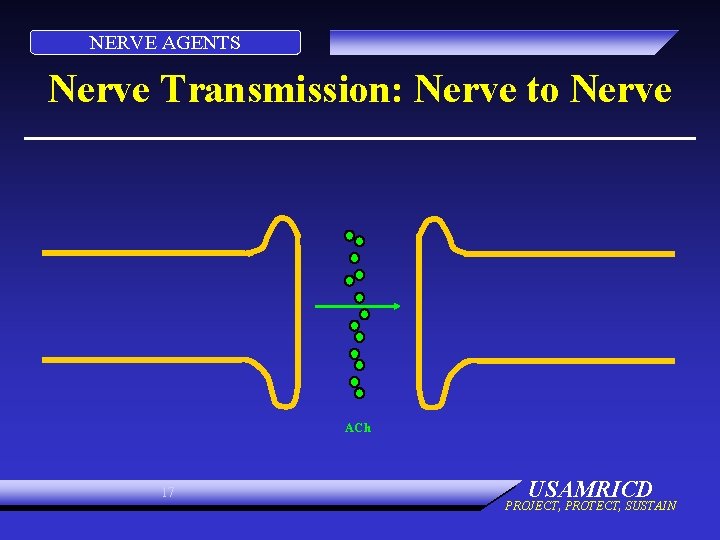
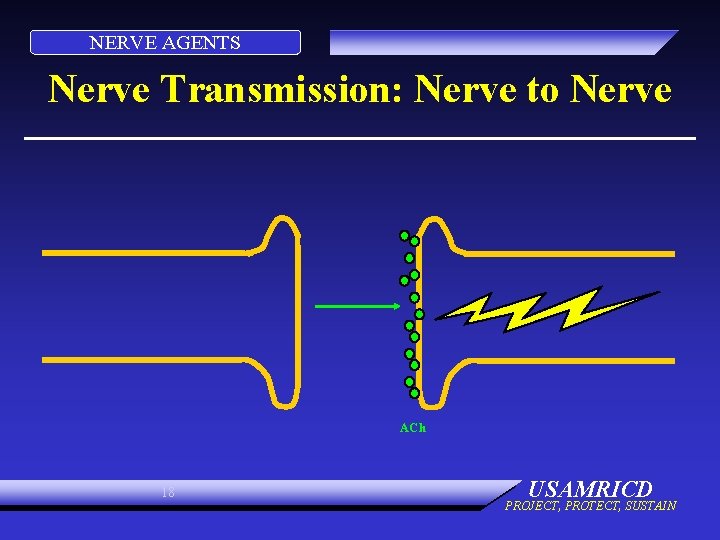
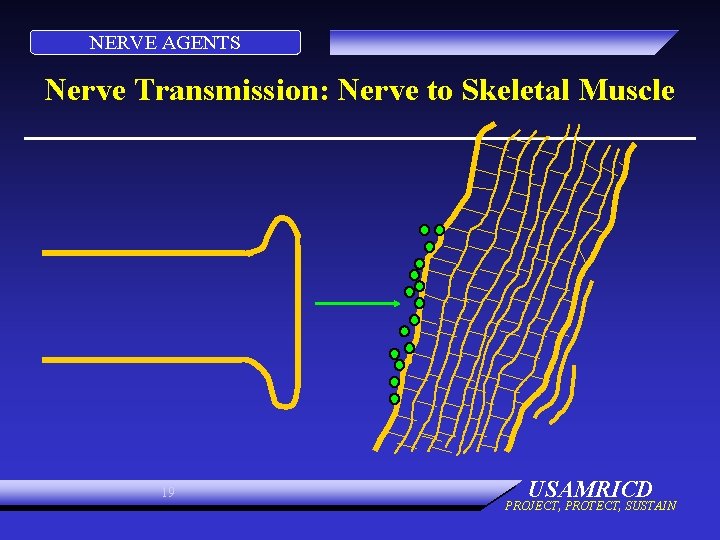
NERVE AGENTS PHYSIOLOGY: NORMAL • Electrical impulse goes down nerve • Impulse causes release of neurotransmitter, acetylcholine • ACh stimulates receptor site on organ • Causes organ to act • ACh is destroyed by ACh. E • No more organ activity 15 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS Nerve Transmission: Nerve to Nerve ACh 16 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS Nerve Transmission: Nerve to Nerve ACh 17 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS Nerve Transmission: Nerve to Nerve ACh 18 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS Nerve Transmission: Nerve to Skeletal Muscle 19 USAMRICD PROJECT, PROTECT, SUSTAIN

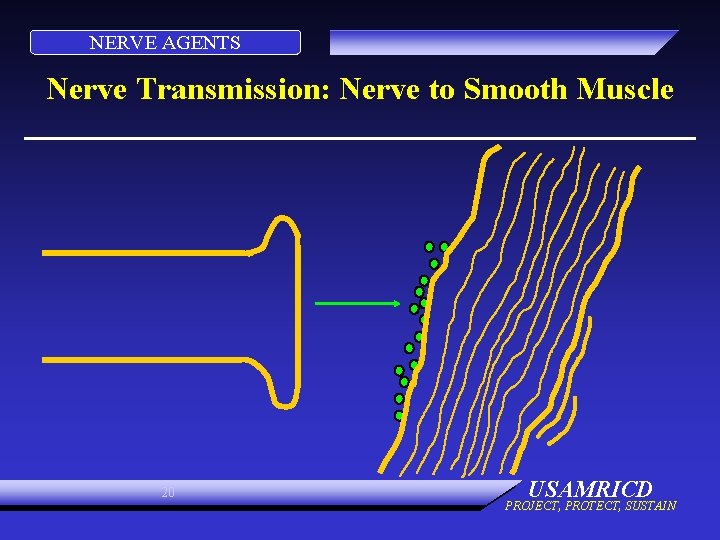
NERVE AGENTS Nerve Transmission: Nerve to Smooth Muscle 20 USAMRICD PROJECT, PROTECT, SUSTAIN

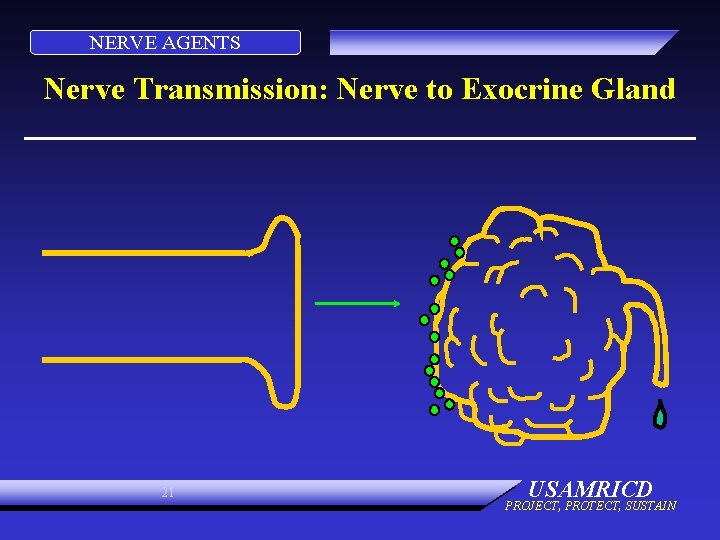
NERVE AGENTS Nerve Transmission: Nerve to Exocrine Gland 21 USAMRICD PROJECT, PROTECT, SUSTAIN

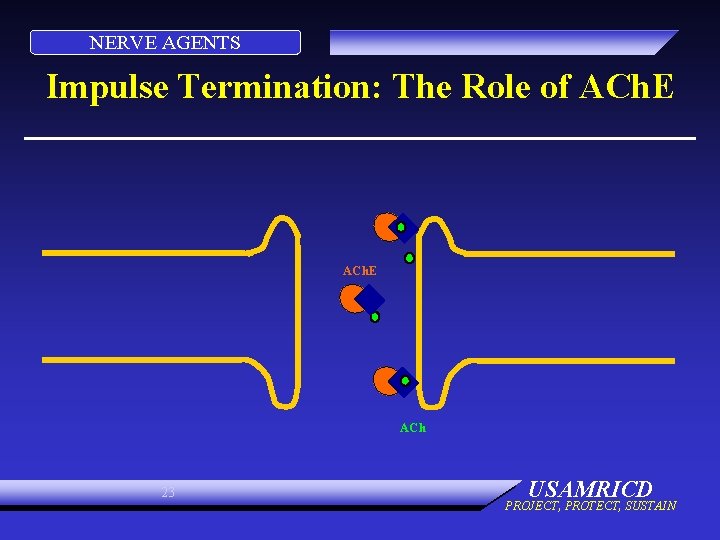
NERVE AGENTS Impulse Termination: The Role of ACh. E ACh 22 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS Impulse Termination: The Role of ACh. E ACh 23 USAMRICD PROJECT, PROTECT, SUSTAIN

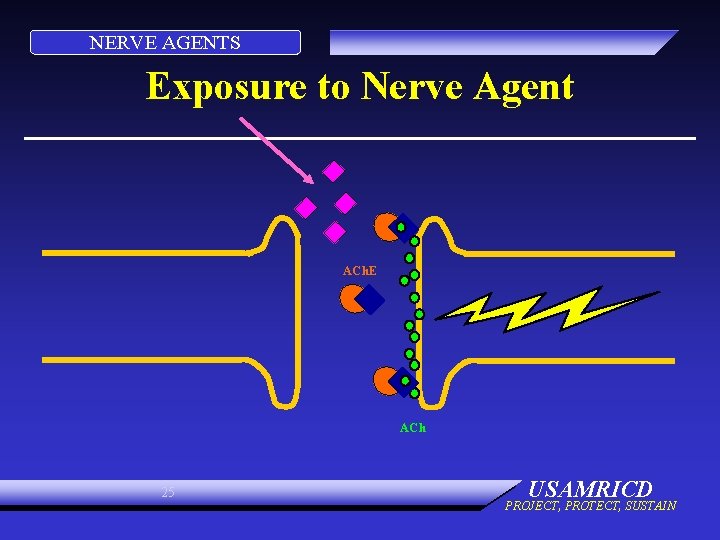
NERVE AGENTS PHYSIOLOGY: NERVE AGENT • Enzyme (ACh. E) is inhibited • Does not destroy ACh • Excess ACh continues to stimulate organ • Organ overstimulation 24 USAMRICD PROJECT, PROTECT, SUSTAIN

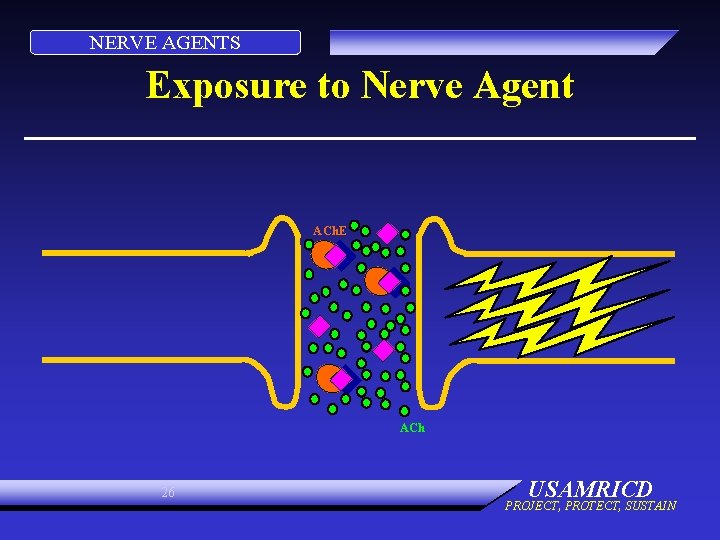
NERVE AGENTS Exposure to Nerve Agent ACh. E ACh 25 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS Exposure to Nerve Agent ACh. E ACh 26 USAMRICD PROJECT, PROTECT, SUSTAIN

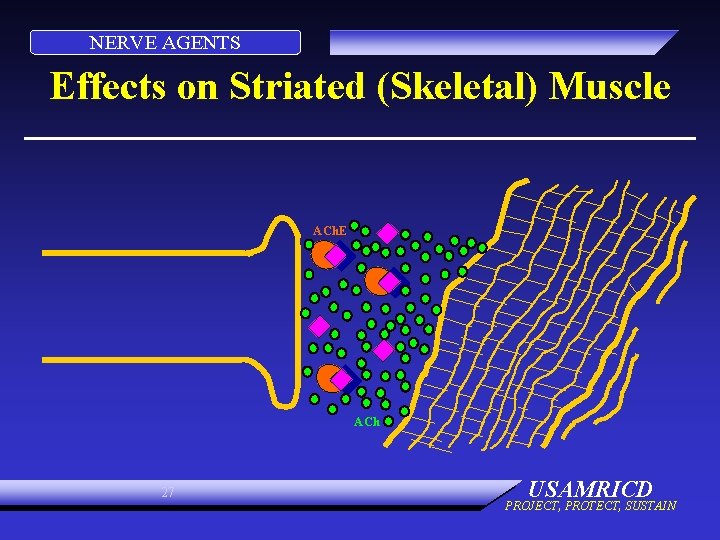
NERVE AGENTS Effects on Striated (Skeletal) Muscle ACh. E ACh 27 USAMRICD PROJECT, PROTECT, SUSTAIN

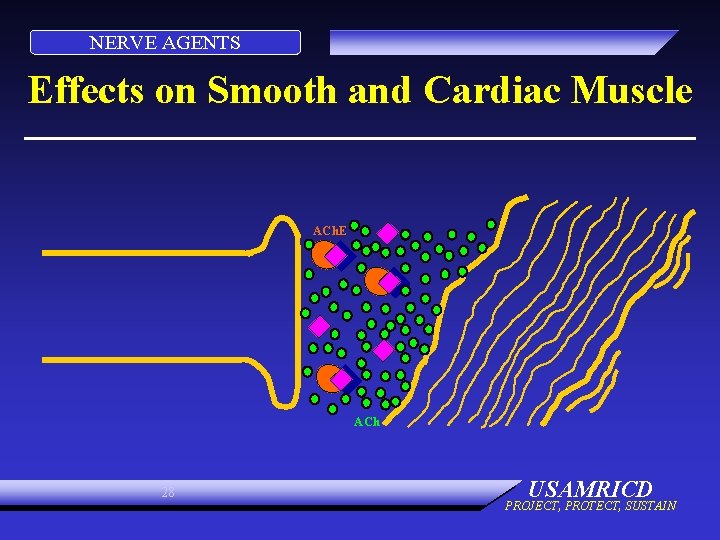
NERVE AGENTS Effects on Smooth and Cardiac Muscle ACh. E ACh 28 USAMRICD PROJECT, PROTECT, SUSTAIN

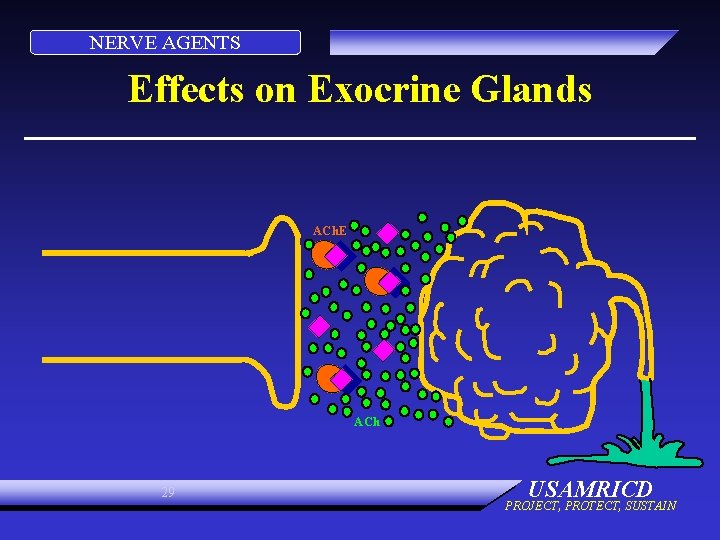
NERVE AGENTS Effects on Exocrine Glands ACh. E ACh 29 USAMRICD PROJECT, PROTECT, SUSTAIN

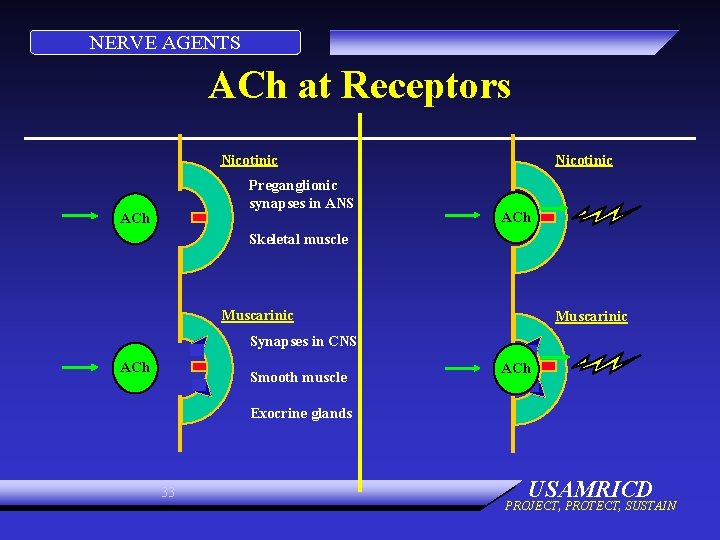
NERVE AGENTS ORGANS • Muscarinic – Smooth muscles – Exocrine glands – Cranial nerves (vagus) • Nicotinic – Skeletal muscles – Pre-ganglionic nerves • Both – CNS 30 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS EFFECTS • Muscarinic – Smooth muscles • Airways - constrict • GI tract - constrict • Pupils - constrict – Glands • Eyes, nose, mouth, sweat, airways, GI – Heart, bradycardia (vagal) 31 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS NICOTINIC • Skeletal muscles – Fasciculations, twitching, fatigue, flaccid paralysis • Pre-ganglionic – Tachycardia, hypertension 32 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS ACh at Receptors Nicotinic Preganglionic synapses in ANS ACh Nicotinic ACh Skeletal muscle Muscarinic Synapses in CNS ACh Smooth muscle ACh Exocrine glands 33 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS HEART RATE • Muscarinic (vagal) decreases • Nicotinic (ganglionic) increases • May be high, low, normal 34 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS CNS • Acutely, large exposure – Loss of consciousness – Seizures – Apnea – Death 35 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS CONTINUED CNS • Acutely, small exposure – Minor CNS effects • Slowness in thinking and decision making • Sleep disturbances • Poor concentration • Emotional problems • Other minor problems 36 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS CONTINUED CNS • Minor CNS effects – May last for 3 to 6 weeks – May follow any exposure – Not always present – Very slight, subtle 37 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS VAPOR • Small exposure – Eyes: Miosis; injection; dim, blurred vision; pain; maybe nausea, vomiting – Nose: Rhinorrhea – Mouth: Salivation – Airways: Shortness of breath 38 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS VAPOR - NOSE and MOUTH • Runny nose – Worse than cold or hay fever – Leaking faucet • Mouth – Excessive saliva – May run out corners 39 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS VAPOR - RESPIRATORY TRACT • Small exposure – Tight chest • Moderate exposure – Severe breathing difficulty – Gasping, irregular breathing – Compounded by excessive secretions 40 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS VAPOR - GASTROINTESTINAL • Exposure to a large but not lethal concentration may cause: – Nausea, vomiting – Pain in abdomen – Diarrhea, involuntary defecation or urination 41 USAMRICD PROJECT, PROTECT, SUSTAIN

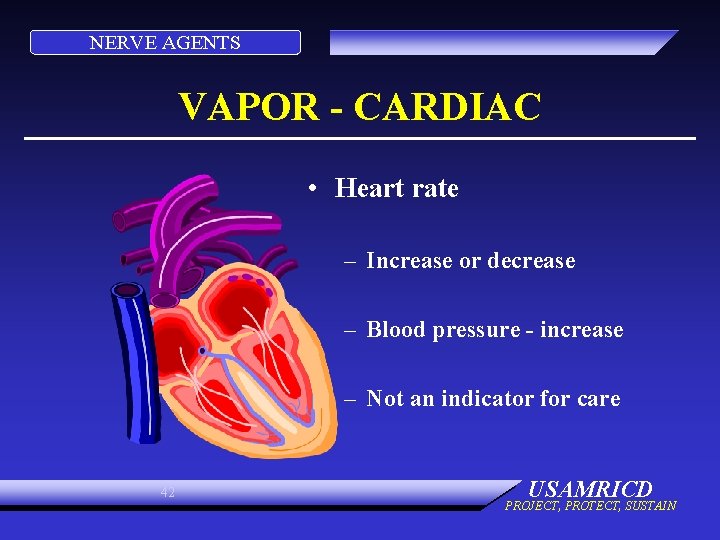
NERVE AGENTS VAPOR - CARDIAC • Heart rate – Increase or decrease – Blood pressure - increase – Not an indicator for care 42 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS CONTINUED VAPOR • Onset of effects: seconds to minutes • After removal from vapor – Effects do not worsen – May improve • No late-onset effects 43 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS CONTINUED VAPOR • Large exposure • Previously listed effects plus. . . – Loss of consciousness – Seizures – Apnea – Flaccid paralysis – Death 44 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS LIQUID ON SKIN • Small droplet: local effects – Sweating, fasciculations • Medium droplet: systemic effects – GI • Large droplet: CNS – Loss of consciousness, seizures, apnea, flaccid paralysis, death 45 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS CONTINUED LIQUID ON SKIN • Onset of effects – Small, medium drop • As long as 18 hours – Large, lethal drop • Usually <30 minutes 46 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS CONTINUED LIQUID ON SKIN • Onset time, penetration – Skin site – Temperature – Moisture 47 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS CONTINUED LIQUID ON SKIN • Effects may occur despite initial decontamination • Effects may worsen 48 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS MIOSIS • Almost always after vapor • After liquid on skin: – Small: no – Moderate: maybe – Severe: yes 49 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS MANAGEMENT • ABCs • Drugs • Decontamination • Supportive • Not necessarily in that order 50 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS CONTINUED MANAGEMENT • MOST IMPORTANT • Protect self – Protective gear – Decontaminate casualty • Protect medical facility – Decontaminate casualty 51 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS DETECTION • M 256 A 1 • Chemical Agent Monitor • M 8 and M 9 paper • M 8 A 1 Automatic Chemical Agent Alarm 52 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS PROTECTIVE POSTURE MOPP 4!!!!!! 53 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS SKIN DECONTAMINATION • Early is best, within 1 to 2 minutes – Little benefit after 30 minutes • Physical removal is best – Forceful flush with water – Stick, dirt, cloth, M 291 • Solutions (hypochlorite, etc. ) – Detoxify after many minutes 54 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS VENTILATION • Possibly less need after pyridostigmine • None forward of Battalion Aid Station • Very high airway resistance until atropine is given 55 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS ANTIDOTES • Too much acetylcholine – Block excess acetylcholine • Enzyme inhibited – Reactivate enzyme 56 USAMRICD PROJECT, PROTECT, SUSTAIN

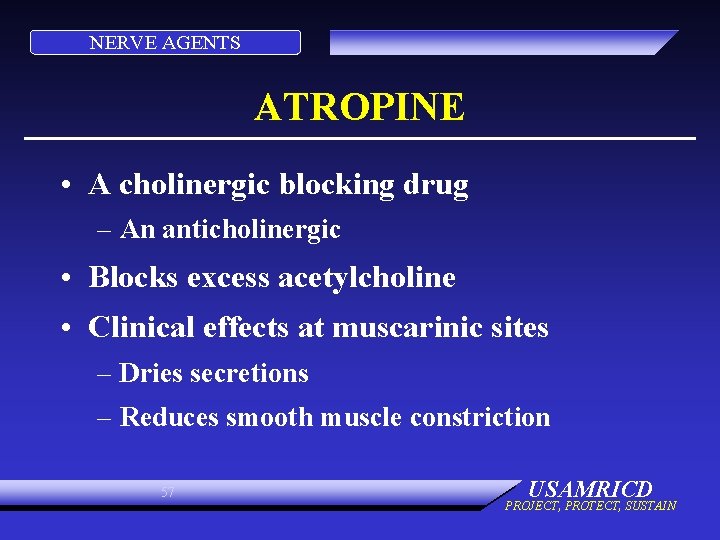
NERVE AGENTS ATROPINE • A cholinergic blocking drug – An anticholinergic • Blocks excess acetylcholine • Clinical effects at muscarinic sites – Dries secretions – Reduces smooth muscle constriction 57 USAMRICD PROJECT, PROTECT, SUSTAIN

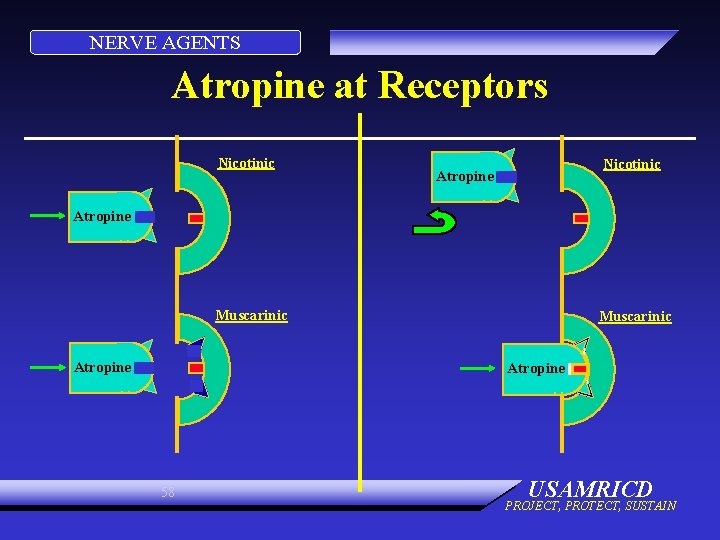
NERVE AGENTS Atropine at Receptors Nicotinic Atropine Muscarinic Atropine 58 USAMRICD PROJECT, PROTECT, SUSTAIN

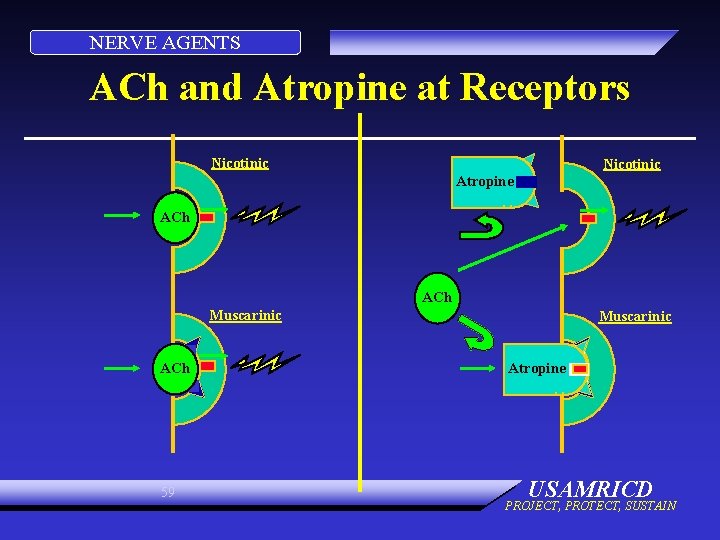
NERVE AGENTS ACh and Atropine at Receptors Nicotinic Atropine ACh Muscarinic ACh 59 Muscarinic Atropine USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS CONTINUED ATROPINE • • Side effects in unexposed Starting dose 2 mg or 6 mg More, 2 mg every 5 to 10 minutes Until – Secretions drying – Ventilation improved • Usual dose: (severe casualty) 15 to 20 mg – 1000 s of mgs in insecticide 60 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS CONTINUED ATROPINE • Not for – Skeletal muscle effects – Miosis, unless used topically • Use will cause blurred vision for 24 hours 61 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS Action of Atropine on Smooth Muscle Atr Atr ACh. E Atr Atr ACh Atr Atr Atr 62 USAMRICD PROJECT, PROTECT, SUSTAIN

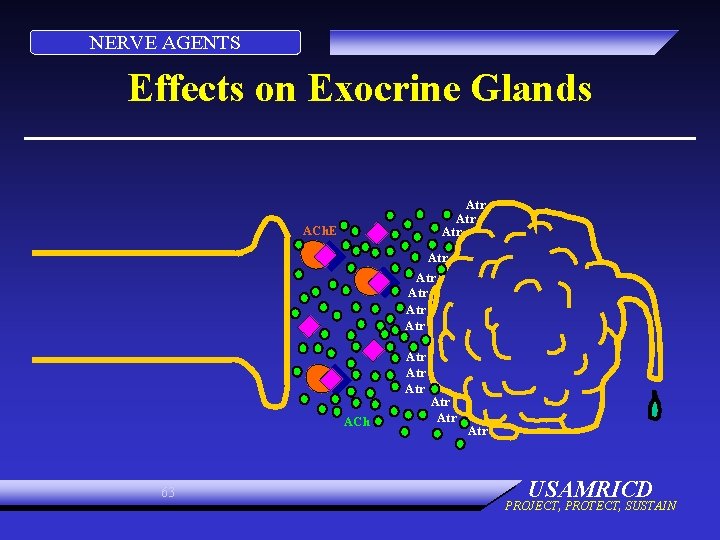
NERVE AGENTS Effects on Exocrine Glands Atr Atr ACh. E Atr Atr ACh 63 Atr Atr USAMRICD PROJECT, PROTECT, SUSTAIN

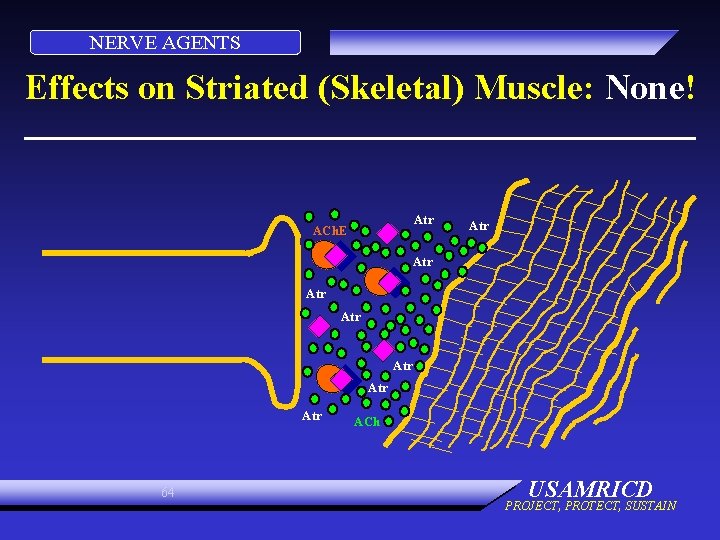
NERVE AGENTS Effects on Striated (Skeletal) Muscle: None! Atr ACh. E Atr Atr 64 ACh USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS OXIMES • Effects at nicotinic sites – Increase skeletal muscle strength • No clinical effects at muscarinic sites 65 USAMRICD PROJECT, PROTECT, SUSTAIN

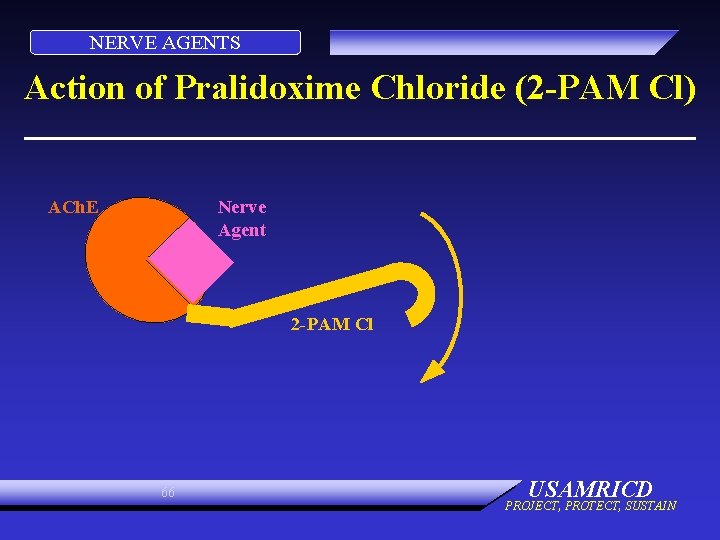
NERVE AGENTS Action of Pralidoxime Chloride (2 -PAM Cl) Nerve Agent ACh. E 2 -PAM Cl 66 USAMRICD PROJECT, PROTECT, SUSTAIN

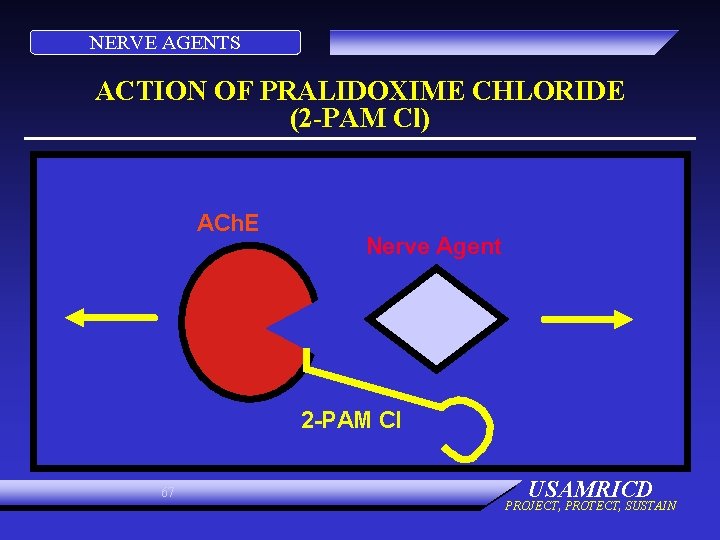
NERVE AGENTS ACTION OF PRALIDOXIME CHLORIDE (2 -PAM Cl) ACh. E Nerve Agent 2 -PAM Cl 67 USAMRICD PROJECT, PROTECT, SUSTAIN

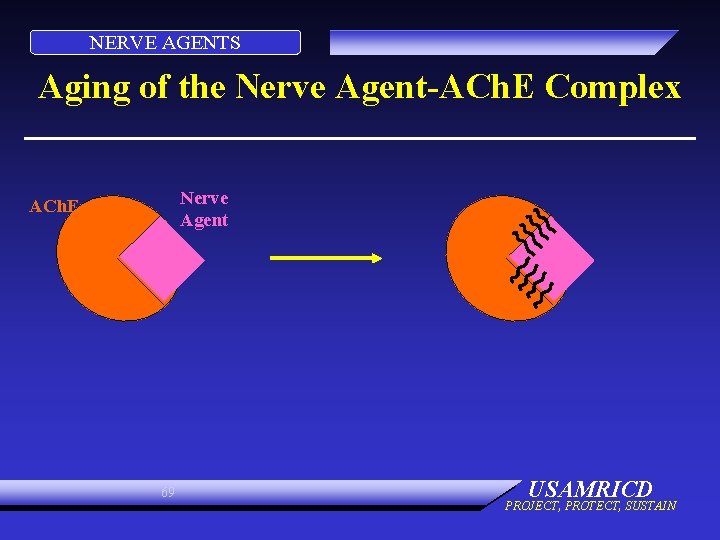
NERVE AGENTS CONTINUED OXIMES • Remove agent from enzyme, unless aging has occurred • Aging: agent-enzyme complex changes • Oximes cannot reactivate enzyme • Aging times: GD 2 min GB 3 to 4 hours Others longer 68 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS Aging of the Nerve Agent-ACh. E Complex Nerve Agent ACh. E 69 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS Introduction of 2 -PAM Cl after Aging 70 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS CONTINUED OXIMES • Other countries have different ones – England: P 2 S – Some European countries: obidoxime – Israel: TMB 4 – Japan: 2 -PAMI 71 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS 2 -PAMCL DOSE • NAAK (MARK I): contains 600 mg – One or three Combopens; repeat in one hour • IV: One gram slowly (20 to 30 min) – Repeat in one hour 72 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS SEIZURES • Without pyridostigmine – Not prolonged – Anticonvulsant seldom necessary • Prolonged after pyridostigmine – Possible brain damage from prolonged seizures – Anticonvulsant needed (diazepam) • Give diazepam to any severe casualty 73 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS ARRHYTHMIAS • Initial, transient from agent, atropine • Terminal after hypoxia • V-fib if atropine given IV with hypoxia 74 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS RECOVERY • Severe casualty: – Without complications, conscious, breathing, in 2 to 3 hours 75 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS RETURN TO DUTY • Dose-dependent, need dependent • Could be hours with minor exposure, great need • Many days after severe exposure • Consider: – Vision – Minor, subtle mental effects 76 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS LONG TERM • EEG changes not detected in individuals – Minor changes detected in an averaged group – Significance unknown 77 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS MARK I • Spring powered injectors – Atropine, 2 mg/0. 7 ml – 2 -PAMCl, 600 mg/2 ml 78 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS MARK I AUTO-INJECTOR 79 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS MILD VAPOR EXPOSURE • Miosis, rhinorrhea • Rx: Probably none unless rhinorrhea is severe • Atropine IM will not help miosis 80 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS MODERATE VAPOR EXPOSURE • Miosis, rhinorrhea, moderate or severe dyspnea • Walking and talking • Rx: 1 MARK I (if dyspnea is quite severe: 2 MARK Is) 81 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS SEVERE VAPOR EXPOSURE • Conscious or unconscious • Seizing or post-ictal • Breathing or not • Or effects in two or more systems (airway, GI, muscular, CNS) 82 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS SEVERE VAPOR EXPOSURE • Rx: 3 MARK Is and diazepam ASAP • Ventilation • Rx even after cardiac arrest 83 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS MILD LIQUID EXPOSURE • Localized twitching, sweating • Rx: 1 MARK I (agent has been absorbed) 84 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS MODERATE SKIN EXPOSURE • GI effects: vomiting, diarrhea, cramps • Rx: 1 MARK I • Watch carefully for 18 hours 85 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS SEVERE SKIN EXPOSURE • Conscious or unconscious • Seizing or post-ictal • Breathing or not • Or effects in two or more systems (airway, GI, muscular, CNS) 86 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS SEVERE SKIN EXPOSURE • Rx: 3 MARK Is and diazepam • Ventilation • Rx after cardiac arrest 87 USAMRICD PROJECT, PROTECT, SUSTAIN

MEDICAL MANAGEMENT OF CHEMICAL CASUALTIES NERVE AGENTS A Case Study From the Tokyo Subway Incident • U. S. ARMY MEDICAL RESEARCH INSTITUTE OF CHEMICAL DEFENSE USAMRICD PROTECT, PROJECT, SUSTAIN

Tokyo Subway Victim NERVE AGENTS • 35 -year-old man • Exposed to sarin during the Tokyo subway attack 20 MAR 95 • For approximately 7 minutes after exposure: – Had tonic-clonic convulsions – Episodes of dyspnea, during which he needed artificial respiration • In the hospital emergency room he was comatose and mildly cyanosed • Both pupils were constricted to 1. 5 mm • Had increased oral and nasal secretions and USAMRICD profuse 89 sweating and vomiting PROJECT, PROTECT, SUSTAIN

Tokyo Subway Victim NERVE AGENTS • Atropine sulphate and pralidoxime iodide were given intravenously • The patient began to regain consciousness 8 hours after exposure • Regained full mobility after 54 hours • He was, however, disoriented and had an impaired short-term memory • His electroencephalogram showed mild slowing of alpha activity, intermittent theta bursts, and the development of delta busts during hyperventilation – disappeared 3 months after exposure • CT and MRI imaging showed no focal lesions • Plasma cholinesterase activity, which was markedly low at 6% of normal levels after exposure, was normal within 3 weeks • RBC cholinesterase activity was normal after 3 months 90 USAMRICD PROJECT, PROTECT, SUSTAIN

Tokyo Subway Victim NERVE AGENTS • Neuropsychological tests 6 months after exposure showed no global intellectual impairment or defects in immediate recall • All his errors on the Mini Mental State were related to recall and temporal orientation • Performance was particularly impaired on the Logical Memory and Associate Learning scales from the Wechsler Memory Scale-Revised • Ability to copy the Rey-Osterrieth complex figure was normal (36/36) • However, when he was asked to reproduce the drawing 3 and 30 minutes later, his performance was worse (18/36 and 3/36, respectively) • These results suggest a defect in his ability to consolidate new learning and memory • Furthermore, without confabulation, he showed retrograde amnesia that extended to 70 days before exposure to sarin USAMRICD • Personality 91 changes characterized by passivity and shallow affect were PROJECT, PROTECT, SUSTAIN also evident

Tokyo Subway Victim NERVE AGENTS • The extent and consequences of brain injury and incapacity due to nerve gas poisoning in human beings are not understood • Patient had amnesia similar to that caused by severe acute hypoxia • Hypoxia may have been a factor in our patient during the first 7 minutes after exposure • Defects such as retrograde amnesia and character changes might be associated with the direct effects of excess choline 92 l USAMRICD Hatta K et al. , Lancet 347: 1343, 1996 PROJECT, PROTECT, SUSTAIN

PRETREATMENT FOR NERVE AGENT POISONING U. S. ARMY MEDICAL RESEARCH INSTITUTE OF CHEMICAL DEFENSE USAMRICD PROTECT, PROJECT, SUSTAIN

NERVE AGENTS TERMINAL OBJECTIVE • Apply principles of pyridostigmine use in enhancing drug therapy for nerve agent intoxication 94 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS WHY? • Major threat agent: Soman • Therapy for soman: relatively ineffective 95 USAMRICD PROJECT, PROTECT, SUSTAIN

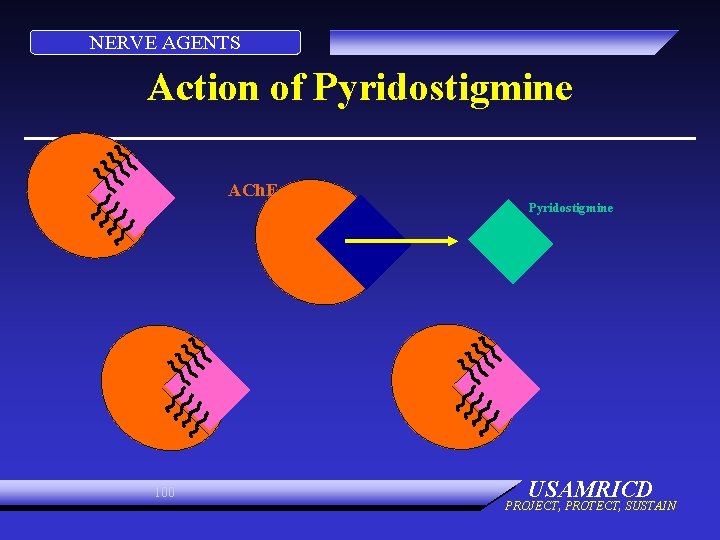
NERVE AGENTS CARBAMATES • Transient carbamylation of ACh. E • Protects site from OP (nerve agent) • Carbamylation of only small amount of ACh. E needed 96 USAMRICD PROJECT, PROTECT, SUSTAIN

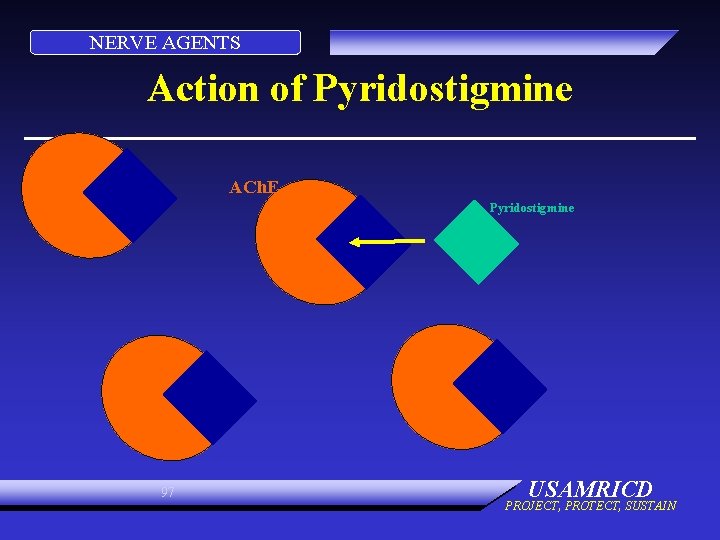
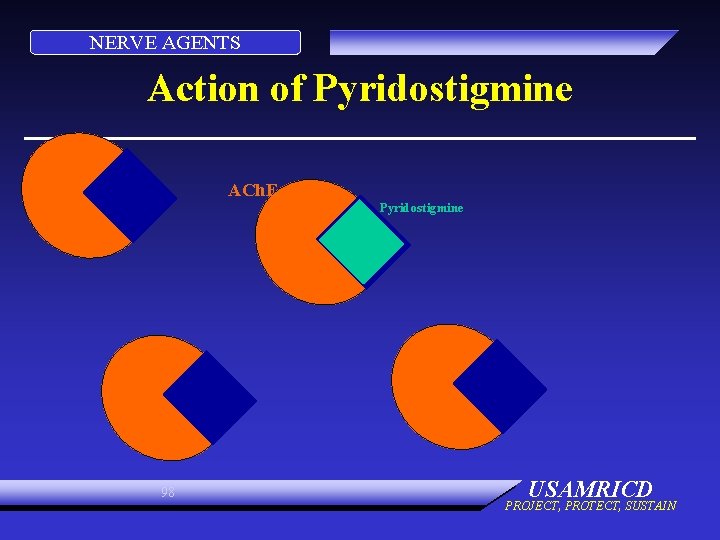
NERVE AGENTS Action of Pyridostigmine ACh. E Pyridostigmine 97 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS Action of Pyridostigmine ACh. E Pyridostigmine 98 USAMRICD PROJECT, PROTECT, SUSTAIN

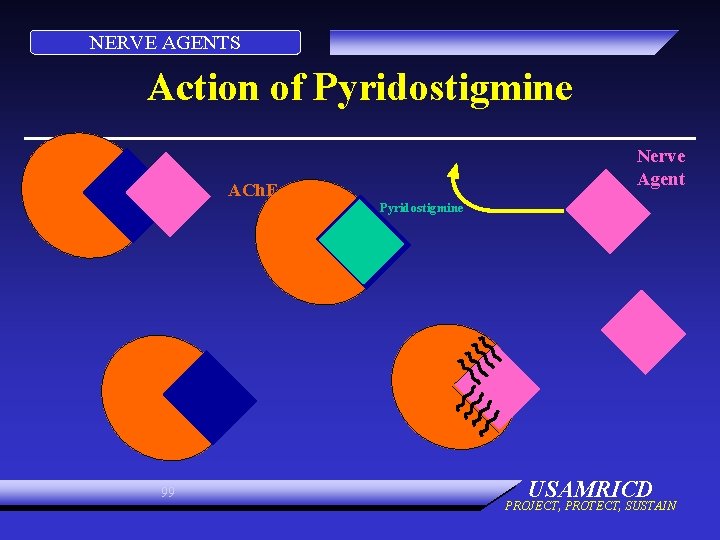
NERVE AGENTS Action of Pyridostigmine Nerve Agent ACh. E Pyridostigmine 99 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS Action of Pyridostigmine ACh. E Pyridostigmine 100 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS Pretreatment • Pretreatment alone, without therapy provides no benefit • Pretreatment followed by antidotes after nerve agent: beneficial 101 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS Protective Ratio • PR = LD 50 (treated) LD 50 (untreated) • PR of 1. 0: No effect • PR of 5. 0 desirable for the battlefield • PR of antidotes against GD: 1. 6 102 USAMRICD PROJECT, PROTECT, SUSTAIN

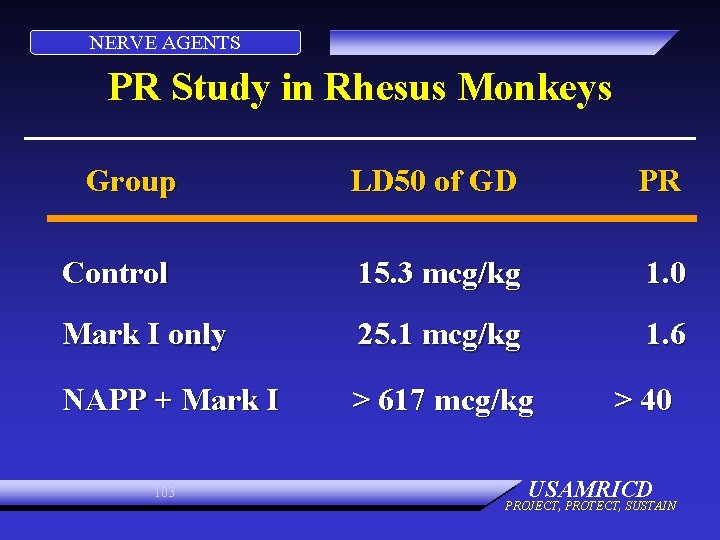
NERVE AGENTS PR Study in Rhesus Monkeys Group LD 50 of GD PR Control 15. 3 mcg/kg 1. 0 Mark I only 25. 1 mcg/kg 1. 6 NAPP + Mark I > 617 mcg/kg 103 > 40 USAMRICD PROJECT, PROTECT, SUSTAIN

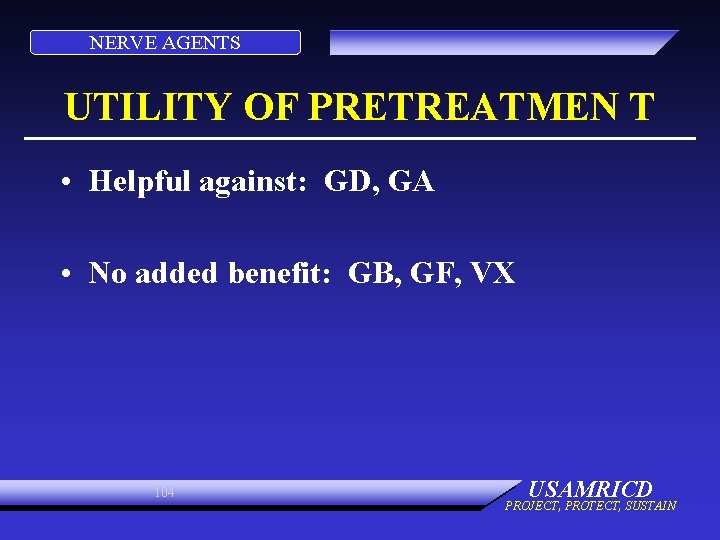
NERVE AGENTS UTILITY OF PRETREATMEN T • Helpful against: GD, GA • No added benefit: GB, GF, VX 104 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS BEFORE USE • Efficacy • Safety – Short-term • Side Effects • Performance – Long-term 105 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS SHORT TERM • Side effects: <5% of those taking it • Performance: No decrements in military tasks (including shooting, flying, driving, physical tasks) 106 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS LONG TERM • Animal studies • Myasthenia gravis patients – Starting dose usually 60 mg q 8 h, can go much higher – Usual course of treatment is years, not weeks 107 USAMRICD PROJECT, PROTECT, SUSTAIN

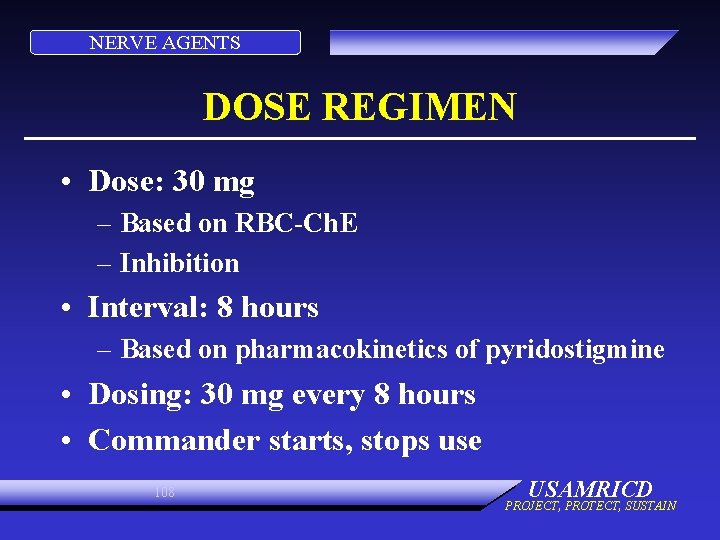
NERVE AGENTS DOSE REGIMEN • Dose: 30 mg – Based on RBC-Ch. E – Inhibition • Interval: 8 hours – Based on pharmacokinetics of pyridostigmine • Dosing: 30 mg every 8 hours • Commander starts, stops use 108 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS WHAT DO YOU SAY IF YOUR COMMANDER ASKS: • How long after I order pyridostigmine do I have to wait until my troops are protected? • How soon after I order them to stop taking it can I consider them at risk? 109 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS PYRIDOSTIGMINE: USE • Mestinon : five decades for myasthenia gravis • Regonal : anesthesia 110 USAMRICD PROJECT, PROTECT, SUSTAIN

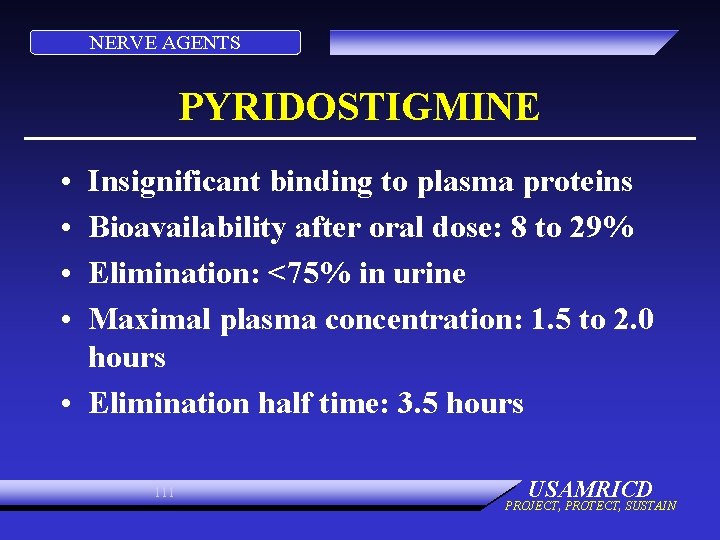
NERVE AGENTS PYRIDOSTIGMINE • • Insignificant binding to plasma proteins Bioavailability after oral dose: 8 to 29% Elimination: <75% in urine Maximal plasma concentration: 1. 5 to 2. 0 hours • Elimination half time: 3. 5 hours 111 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS PYRIDOSTIGMINE: USE IN GULF WAR • Compliance unknown • High incidence (>50%) of side effects • Most related to pharmacology of drug – GI >50% – GU 5 to 30% • Medical assistance 1% • Discontinuance drug <0. 1% 112 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS Israeli Study • Effects of Pyridostigmine on troops in field conditions – Done under FTX conditions at basic training on 80 troops – Half of them given pyridostigmine 30 mg q 8 h or placebo – Studied before and after 8 -day period on drug or placebo – Study design is double blinded but not crossover 113 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS Results of the Israeli study • Pyridostigmine-treated soldiers had “mild” GI symptoms in most cases • Pyridostigmine-treated soldiers had changes on order of 10% in their scores on vertical addition and four-choice (perceptual speed) tasks. – Other neuropsychiatric parameters were unaffected. • The two groups had no difference in their endocrine or stress tests including cortisol 114 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS Conclusions: • Soldiers did as well functionally with as without pyridostigmine • Functional significance of neuropsychiatric changes is unclear • Commanders and their troops had no complaints and those with mild changes were functionally unaware of them. Limitation: No systematic long-term follow-up –MAJ Givoni Israeli Defence Force 115 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS PYRIDOSTIGMINE • After pretreatment, nerve agent, antidotes: breathing and seizures continue • Potential brain damage • Anticonvulsant (diazepam) needed (10 mg via auto-injector) 116 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS Effects of Long-term Administration • In vitro and in vivo evidence of myopathy • Complaints of weakness, fatigue, etc. • U. K. 60 -day study • U. S. doctrine does not advocate long-term use 117 USAMRICD PROJECT, PROTECT, SUSTAIN

NERVE AGENTS PYRIDOSTIGMINE: SUMMARY • Pretreatment, not a substitute for treatment • “Hides” or protects a fraction of ACh. E (creates a “reserve force” • Increases the amount of nerve agent a person can be exposed to and survive • Causes predictable side effect profile • Does not interfere with military function 118 USAMRICD PROJECT, PROTECT, SUSTAIN

MEDICAL MANAGEMENT OF CHEMICAL CASUALTIES SUMMARY ANY QUESTIONS? U. S. ARMY MEDICAL RESEARCH INSTITUTE OF CHEMICAL DEFENSE USAMRICD PROTECT, PROJECT, SUSTAIN
 Casualties
Casualties Casualties
Casualties Korban jiwa perang dunia 2
Korban jiwa perang dunia 2 Trigeminal nerve which cranial nerve
Trigeminal nerve which cranial nerve Types of weathering process
Types of weathering process What is mechanical weathering
What is mechanical weathering Different types of raising agents
Different types of raising agents Weathering process
Weathering process Agents of physical weathering
Agents of physical weathering Hp insight management agent
Hp insight management agent Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Empirical formula pogil
Empirical formula pogil Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 1 chemical changes
Section 1 chemical changes Chemical formulas and chemical compounds chapter 7
Chemical formulas and chemical compounds chapter 7 Are kc and kp equal
Are kc and kp equal Ptal california medical board
Ptal california medical board Greater baltimore medical center medical records
Greater baltimore medical center medical records Difference between medical report and medical certificate
Difference between medical report and medical certificate Torrance memorial hospital medical records
Torrance memorial hospital medical records Cartersville medical center medical records
Cartersville medical center medical records Scientific management
Scientific management Management pyramid
Management pyramid Top management middle management first line management
Top management middle management first line management 5 agents of erosion
5 agents of erosion Bitter taste masking agents
Bitter taste masking agents Networked insurance agents
Networked insurance agents Agents of erosion
Agents of erosion Identify three agents of mechanical weathering
Identify three agents of mechanical weathering Agents of weathering
Agents of weathering Protective agent definition
Protective agent definition Streptokinase دواء
Streptokinase دواء Thrombolytic drugs mechanism of action
Thrombolytic drugs mechanism of action Superficial and deep heating modalities
Superficial and deep heating modalities Formal agents of social control
Formal agents of social control What is suspension in pharmacy
What is suspension in pharmacy Structure activity relationship of sympathomimetic drugs
Structure activity relationship of sympathomimetic drugs Stocks and sauces
Stocks and sauces Classification of thickening agents
Classification of thickening agents Agent of socialization definition
Agent of socialization definition Secondary agents of socialization
Secondary agents of socialization Agents of socialization
Agents of socialization Types of socialization
Types of socialization Internal and external social control
Internal and external social control Mother sauces and derivatives
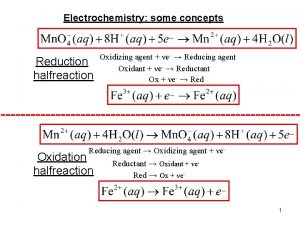
Mother sauces and derivatives How to identify the oxidizing agent
How to identify the oxidizing agent Formal agents of social control
Formal agents of social control What leavening agents are used in quick breads
What leavening agents are used in quick breads Surface active agents
Surface active agents Negative inotropic drugs
Negative inotropic drugs Strong oxidizing agent
Strong oxidizing agent Dulalax
Dulalax Weathering and erosion agents
Weathering and erosion agents 5 agents of evolutionary change
5 agents of evolutionary change Signalling economics example
Signalling economics example Leavening agent
Leavening agent If replicated what will be the complementary strand
If replicated what will be the complementary strand Types of travel agents
Types of travel agents Structure of intelligent agents
Structure of intelligent agents Peas description for house cleaning robot
Peas description for house cleaning robot Inotropic agents
Inotropic agents Mac isoflurane
Mac isoflurane What are examples of non-quick breads?
What are examples of non-quick breads? Give the 8 baking ingredients
Give the 8 baking ingredients Agents of microevolution
Agents of microevolution Vrg
Vrg Classification of inhalational agents
Classification of inhalational agents Classification of inhalational agents
Classification of inhalational agents General anesthesia drugs chart
General anesthesia drugs chart Prokinetic agents
Prokinetic agents Linda wozniewski
Linda wozniewski Deposition agents
Deposition agents Classification of emulsifying agent
Classification of emulsifying agent Theory of emulsification
Theory of emulsification Wet gum method of emulsion
Wet gum method of emulsion Cell potential table
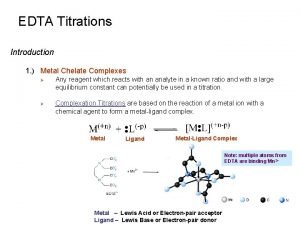
Cell potential table What is chelate
What is chelate 3 agents of erosion
3 agents of erosion Cholinergic agents
Cholinergic agents Dialogflow natural language processing
Dialogflow natural language processing Formal agents of social control
Formal agents of social control Knowledge-based agents
Knowledge-based agents N-cholinomimetics
N-cholinomimetics Cholinergic receptors
Cholinergic receptors 6 agents of socialization
6 agents of socialization Known malicious user-agents
Known malicious user-agents Parasympathomimetic drugs
Parasympathomimetic drugs Structure of intelligent agents
Structure of intelligent agents Antimycobacterial agents
Antimycobacterial agents Antimycobacterial agents
Antimycobacterial agents Mechloroethamine
Mechloroethamine Differentiate between oxidizing and reducing agents
Differentiate between oxidizing and reducing agents Role of economic agents
Role of economic agents Define primary agents of socialization
Define primary agents of socialization Fluent7
Fluent7 Sympathomimetic drugs
Sympathomimetic drugs Mother sauces and derivatives
Mother sauces and derivatives 4 agents of socialization
4 agents of socialization Language artinya
Language artinya Examples of pour batter
Examples of pour batter Food improvement agents
Food improvement agents Pictures of unsafe dented cans
Pictures of unsafe dented cans Difference between wet gum method and dry gum method
Difference between wet gum method and dry gum method Four cleaning agents
Four cleaning agents Problem solving agents
Problem solving agents Known vs unknown environment
Known vs unknown environment Occlusion and mixed-crystal formation
Occlusion and mixed-crystal formation Mechanical entrapment coprecipitation
Mechanical entrapment coprecipitation O.w.c.a. agents
O.w.c.a. agents Define topical agents
Define topical agents Topical agents definition
Topical agents definition Antibacterial agents
Antibacterial agents 5 agents in developing personality
5 agents in developing personality Relative strengths of oxidizing and reducing agents
Relative strengths of oxidizing and reducing agents Logical agents
Logical agents Wumpus world prolog
Wumpus world prolog Primary adsorption layer
Primary adsorption layer Les agents de la géodynamique externe
Les agents de la géodynamique externe Glacial till
Glacial till Goanywhere agent
Goanywhere agent