Chapter 12 Gravimetric Methods of Analysis Formation of




- Slides: 41

Chapter 12 Gravimetric Methods of Analysis Formation of precipitates and crystals.

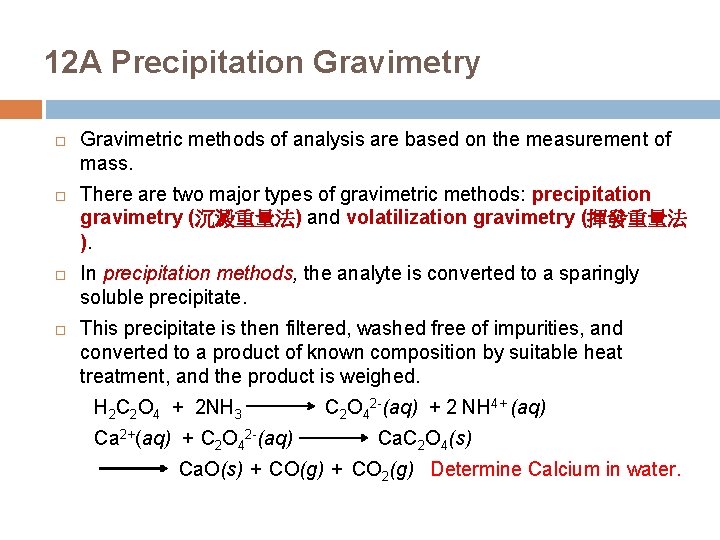
12 A Precipitation Gravimetry Gravimetric methods of analysis are based on the measurement of mass. There are two major types of gravimetric methods: precipitation gravimetry (沉澱重量法) and volatilization gravimetry (揮發重量法 ). In precipitation methods, the analyte is converted to a sparingly soluble precipitate. This precipitate is then filtered, washed free of impurities, and converted to a product of known composition by suitable heat treatment, and the product is weighed. H 2 C 2 O 4 + 2 NH 3 Ca 2+(aq) + C 2 O 42 -(aq) + 2 NH 4+ (aq) Ca. C 2 O 4(s) Ca. O(s) + CO(g) + CO 2(g) Determine Calcium in water.

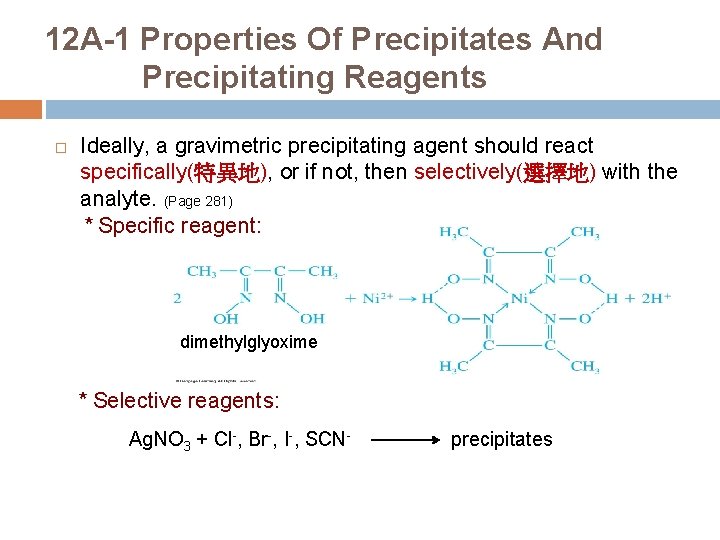
12 A-1 Properties Of Precipitates And Precipitating Reagents Ideally, a gravimetric precipitating agent should react specifically(特異地), or if not, then selectively(選擇地) with the analyte. (Page 281) * Specific reagent: dimethylglyoxime * Selective reagents: Ag. NO 3 + Cl-, Br-, I-, SCN- precipitates

12 A-1 Properties Of Precipitates And Precipitating Reagents The ideal precipitating reagent would react with the analyte to give a product that is 1. Readily filtered and washed free of contaminants 2. Of sufficiently low solubility so that no significant loss of the solid occurs during filtration and washing 3. Unreactive with constituents of the atmosphere 4. Of known composition after it is dried or, if necessary, ignited.

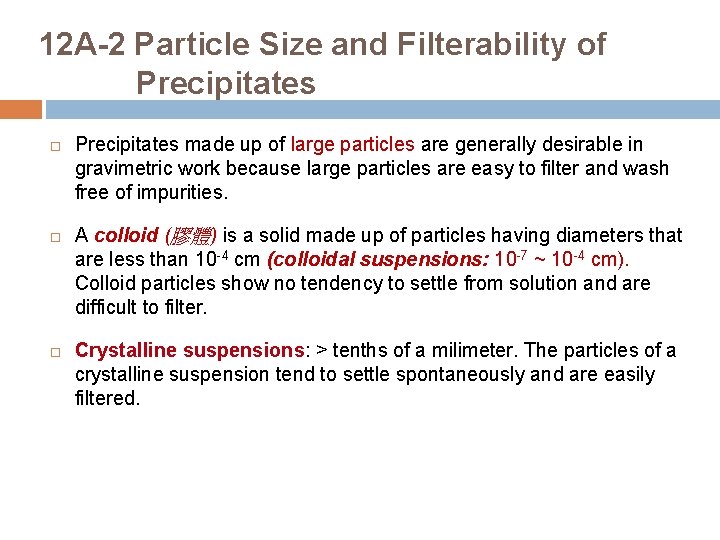
12 A-2 Particle Size and Filterability of Precipitates made up of large particles are generally desirable in gravimetric work because large particles are easy to filter and wash free of impurities. A colloid (膠體) is a solid made up of particles having diameters that are less than 10 -4 cm (colloidal suspensions: 10 -7 ~ 10 -4 cm). Colloid particles show no tendency to settle from solution and are difficult to filter. Crystalline suspensions: > tenths of a milimeter. The particles of a crystalline suspension tend to settle spontaneously and are easily filtered.

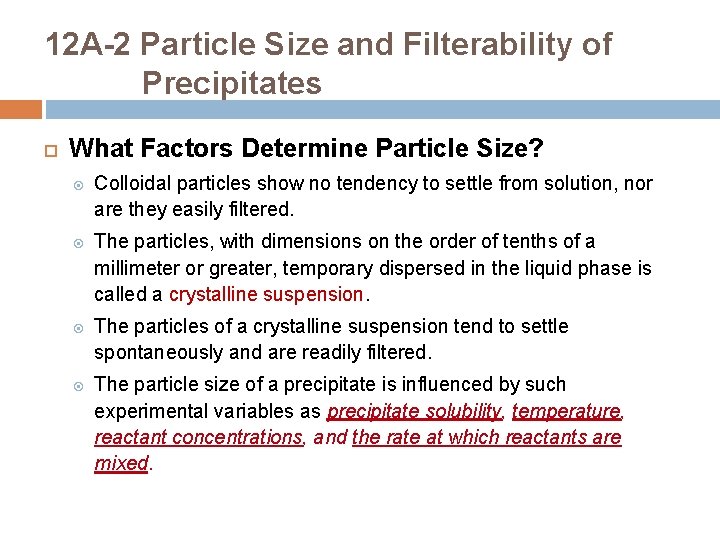
12 A-2 Particle Size and Filterability of Precipitates What Factors Determine Particle Size? Colloidal particles show no tendency to settle from solution, nor are they easily filtered. The particles, with dimensions on the order of tenths of a millimeter or greater, temporary dispersed in the liquid phase is called a crystalline suspension. The particles of a crystalline suspension tend to settle spontaneously and are readily filtered. The particle size of a precipitate is influenced by such experimental variables as precipitate solubility, temperature, reactant concentrations, and the rate at which reactants are mixed.

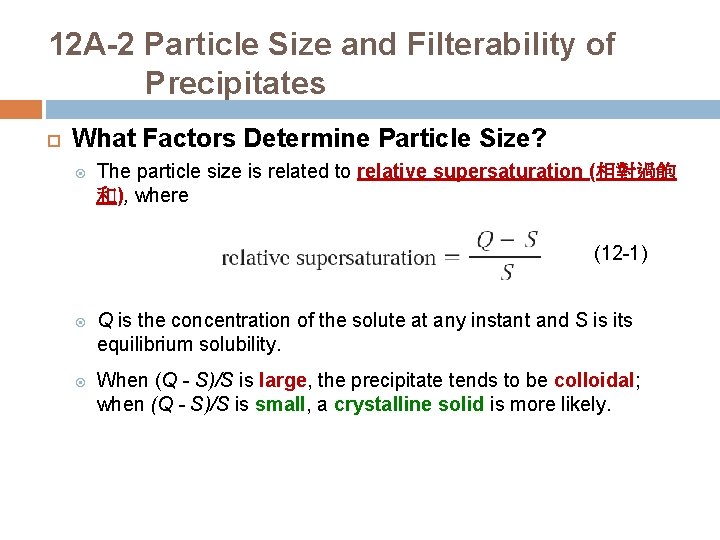
12 A-2 Particle Size and Filterability of Precipitates What Factors Determine Particle Size? The particle size is related to relative supersaturation (相對過飽 和), where (12 -1) Q is the concentration of the solute at any instant and S is its equilibrium solubility. When (Q - S)/S is large, the precipitate tends to be colloidal; when (Q - S)/S is small, a crystalline solid is more likely.

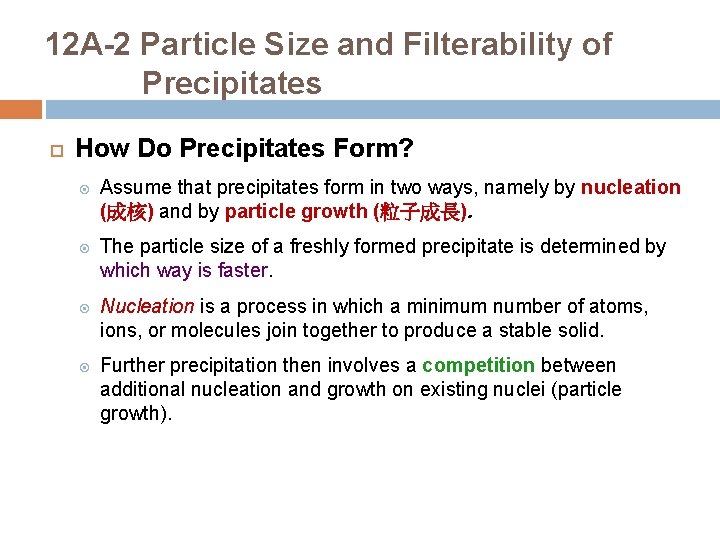
12 A-2 Particle Size and Filterability of Precipitates How Do Precipitates Form? Assume that precipitates form in two ways, namely by nucleation (成核) and by particle growth (粒子成長). The particle size of a freshly formed precipitate is determined by which way is faster. Nucleation is a process in which a minimum number of atoms, ions, or molecules join together to produce a stable solid. Further precipitation then involves a competition between additional nucleation and growth on existing nuclei (particle growth).

12 A-2 Particle Size and Filterability of Precipitates How Do Precipitates Form? If nucleation predominates, a precipitate containing a large number of small particles results. If particle growth predominates, a smaller number of larger particles is produced. The rate of nucleation is believed to increase enormously with increasing relative supersaturation [(Q-S)/S]. In contrast, the rate of particle growth is only moderately enhanced by high relative supersaturations.

12 A-2 Particle Size and Filterability of Precipitates Controlling Particle Size Experimental variables lead to crystalline precipitates include elevated temperatures to increase the solubility of the precipitate (increase S), dilute solutions (to minimize Q), and slow addition of the precipitating agent with good stirring (to minimize Q). Larger particles can also be obtained by p. H control, provided the solubility of the precipitate depends on p. H. (ex) Ca 2+(aq) + C 2 O 42 -(aq) H 2 C 2 O 4 + 2 NH 3 Ca. C 2 O 4(s) in mild acidic environment. C 2 O 42 -(aq) + 2 NH 4+(aq) NH 3 is added slowly.

12 A-3 Colloidal Precipitates Coagulate, or agglomerate, the individual particles of most colloids to give a filterable, amorphous mass that will settle out of solution. Coagulation (凝結,凝集) of Colloids Coagulation can be hastened by heating, stirring, and adding an electrolyte to the medium. Why colloidal suspensions are stable and do not coagulate spontaneously?

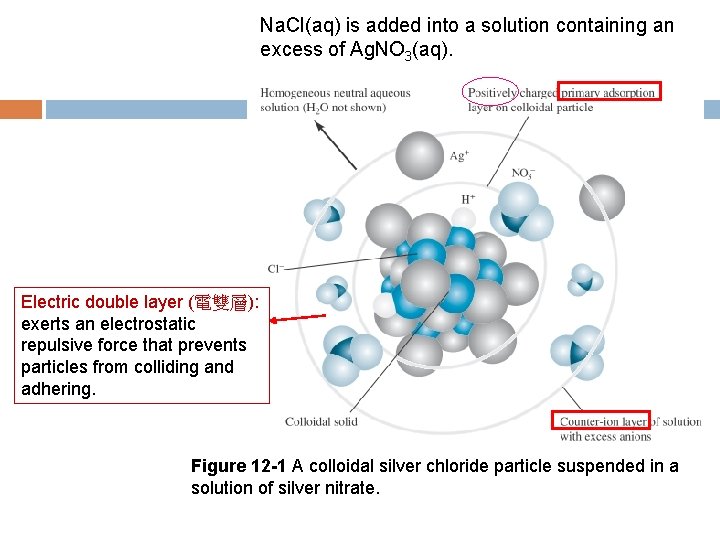
12 A-3 Colloidal Precipitates Coagulation of Colloids Colloidal suspensions are stable because the particles are either positively charged or negatively charged and thus repel one another. Cations or anions are adsorbed to the particle surface. Adsorption (吸附) is a process in which a substance (gas, liquid, or solid) is held on the surface of a solid. In contrast, absorption (吸收) involves retention of a substance within the pores of a solid. The charge on a colloidal particle formed in a gravimetric analysis is determined by the charge of the lattice ion (晶格離子 ) that is in excess when the precipitation is complete.

Na. Cl(aq) is added into a solution containing an excess of Ag. NO 3(aq). Electric double layer (電雙層): exerts an electrostatic repulsive force that prevents particles from colliding and adhering. Figure 12 -1 A colloidal silver chloride particle suspended in a solution of silver nitrate.

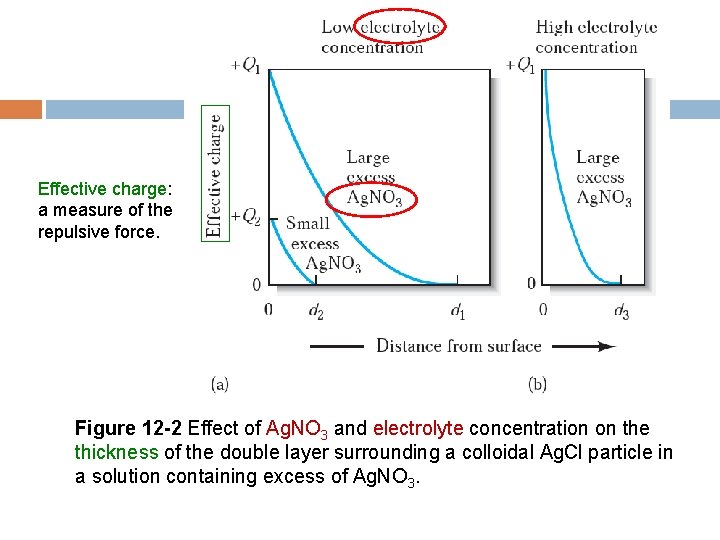
Effective charge: a measure of the repulsive force. Figure 12 -2 Effect of Ag. NO 3 and electrolyte concentration on the thickness of the double layer surrounding a colloidal Ag. Cl particle in a solution containing excess of Ag. NO 3.

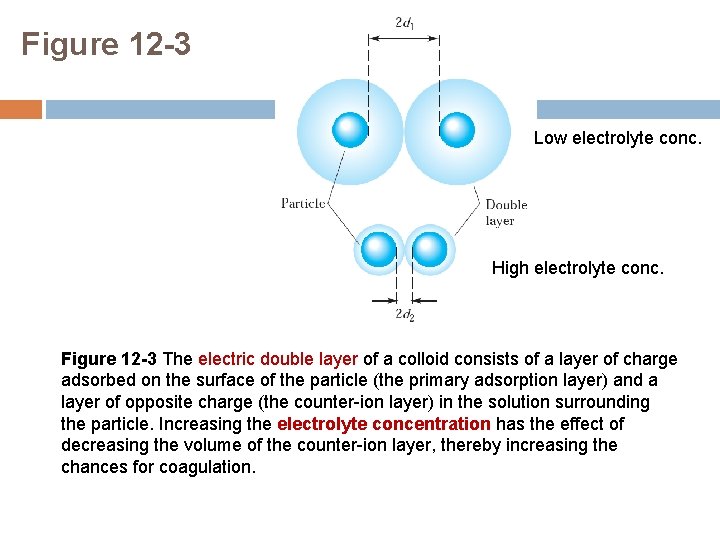
Figure 12 -3 Low electrolyte conc. High electrolyte conc. Figure 12 -3 The electric double layer of a colloid consists of a layer of charge adsorbed on the surface of the particle (the primary adsorption layer) and a layer of opposite charge (the counter-ion layer) in the solution surrounding the particle. Increasing the electrolyte concentration has the effect of decreasing the volume of the counter-ion layer, thereby increasing the chances for coagulation.

12 A-3 Colloidal Precipitates Coagulation of Colloids Coagulation of a colloidal suspension can often be brought about by a short period of heating (decrease di), particularly if accompanied by stirring. An even more effective way to coagulate a colloid is to increase the electrolyte concentration of the solution (decrease di). (Fig 12 -3) Peptization of Colloids Peptization (膠溶,解膠) is a process by which a coagulated colloid returns to its dispersed state (electrolyte has been leached by washing), which can be solved by washing with a volatile electrolyte such as nitric acid. Treatment of Colloidal Precipitates Colloids are best precipitated from hot, stirred solutions containing sufficient electrolyte to ensure coagulation.

12 A-4 Crystalline Precipitates Improving Particle Size and Filterability The particle size of crystalline solids can often be improved significantly by minimizing Q, maximizing S, or both. Digestion (浸煮) of crystalline precipitates (without stirring) for some time after formation frequently yields a purer, more filterable product. Digestion is a process in which a precipitate is heated an hour or more in the solution from which it was formed (mother liquor). Water is expelled from the solid.

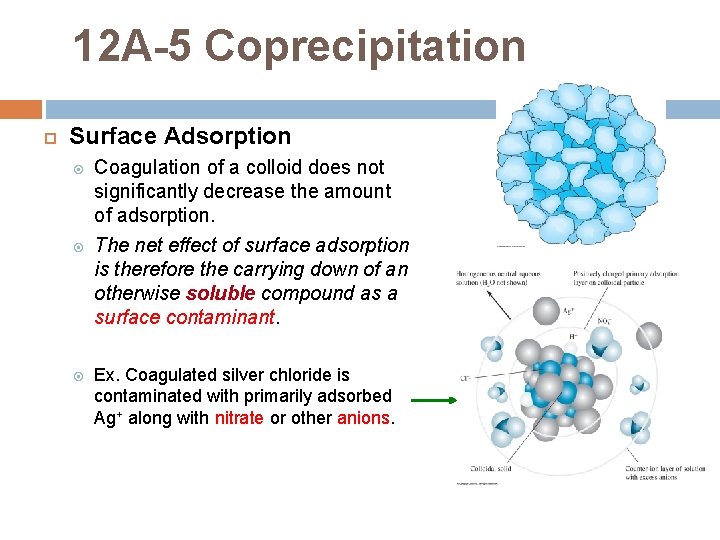
12 A-5 Coprecipitation (共沉澱) is a process in which normally soluble compounds are carried out of solution by a precipitate. There are four types of coprecipitation: (1) surface adsorption, (2) mixed-crystal formation, (3) occlusion, and (4) mechanical entrapment. (1) & (2) are equilibrium processes, while (3) & (4) arise from kinetics of crystal growth. Surface Adsorption (表面吸附) Adsorption is a common source of coprecipitation that is likely to cause significant contamination of precipitates with large specific surface areas (coagulated colloids).

12 A-5 Coprecipitation Surface Adsorption Coagulation of a colloid does not significantly decrease the amount of adsorption. The net effect of surface adsorption is therefore the carrying down of an otherwise soluble compound as a surface contaminant. Ex. Coagulated silver chloride is contaminated with primarily adsorbed Ag+ along with nitrate or other anions.

12 A-5 Coprecipitation Surface Adsorption Minimizing Adsorbed Impurities on Colloids The purity of many coagulated colloids is improved by digestion ( 浸煮) or washing with a solution containing a volatile electrolyte. Regardless of the method of treatment, a coagulated colloid is always contaminated to some degree, even after extensive washing. Reprecipitation. A drastic but effective way to minimize the effects of adsorption is reprecipitation (再沉澱), or double precipitation. (Because the first precipitate carries down only a fraction of the contaminant present in the original solvent)

12 A-5 Coprecipitation Mixed-Crystal Formation (混合晶體形成) In mixed-crystal formation, one of the ions in the crystal lattice of a solid is replaced by an ion of another element. (ex) The extent of mixed-crystal contamination is governed by the law of mass action and increases as the ratio of contaminant to analyte concentration increases. Occlusion (包留) and Mechanical Entrapment (力學誘陷) When a crystal is growing rapidly during precipitate formation, foreign ions in the counter-ion layer may become trapped, or occluded, within the growing crystal. Occlusion is a type of coprecipitation in which a compound is trapped within a pocket formed during rapid crystal growth.

12 A-5 Coprecipitation Occlusion and Mechanical Entrapment Mixed-crystal formation may occur in both colloidal and crystalline precipitates, whereas occlusion and mechanical entrapment are confined to crystalline precipitates. Mechanical entrapment occurs when crystals lie close together during growth. Occlusion and Mechanical Entrapment Both occlusion and mechanical entrapment are at a minimum when the rate of precipitate formation is low, that is, under conditions of low supersaturation. In addition, digestion (浸煮) is often remarkably helpful in reducing these types of coprecipitation.

12 A-5 Coprecipitation Errors Coprecipitation cause either negative or positive errors. The contaminant is not a compound of the ion being determined: positive error. (ex. Ag. Cl adsorbs Ag. NO 3) The contaminant does contain the ion being determined: positive or negative error. Ba. SO 4 occlusion of Ba(NO 3)2: positive error Ba. SO 4 occlusion of Ba. Cl: negative error

12 A-6 Precipitation from Homogeneous Solution Homogeneous precipitation is a process in which a precipitate is formed by slow generation of a precipitating reagent homogeneously throughout a solution. [relative supersaturation (QS)/S is kept low] In general, homogeneously formed precipitates, both colloidal and crystalline, are better suited for analysis than a solid formed by direct addition of a precipitating reagent. Ex. Urea is often used for homogeneous generation of hydroxide ion. (<100 o. C, 1~2 hour) (NH 2)2 CO + 3 H 2 O CO 2 + 2 NH 4+ + 2 OH- Al(OH)3

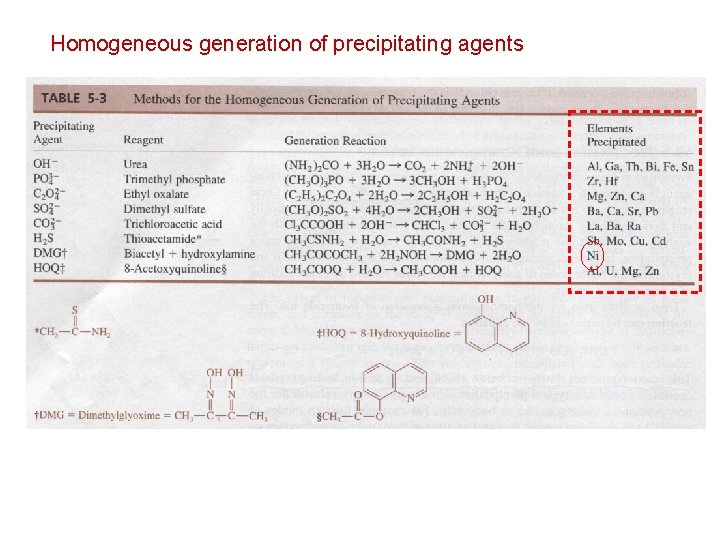
Homogeneous generation of precipitating agents

12 A-7 Drying And Ignition Of Precipitates After filtration, a gravimetric precipitate is heated until its mass becomes constant. Some precipitates are also ignited to decompose the solid and form a compound of known composition. This new compound is often called the weighing form (稱量形式). Recording thermal decomposition curves is often called thermogravimetry or thermogravimetric analysis (TGA: 熱重分析), and the mass versus temperature curves are called thermograms ( 熱圖).

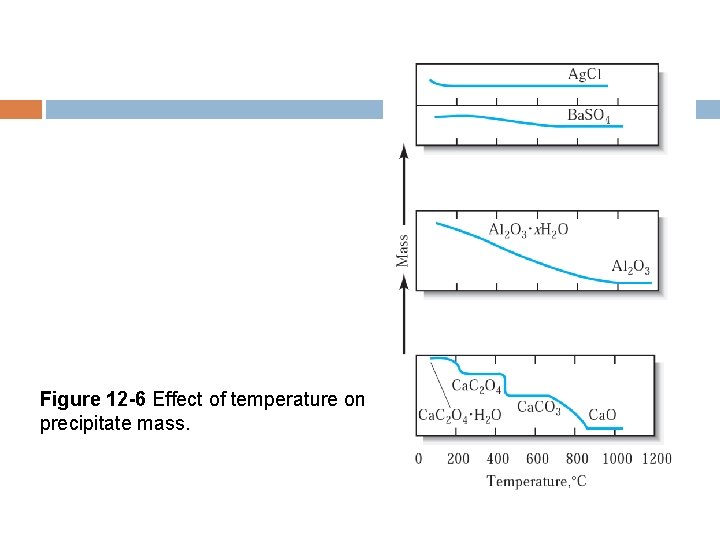
Figure 12 -6 Effect of temperature on precipitate mass.

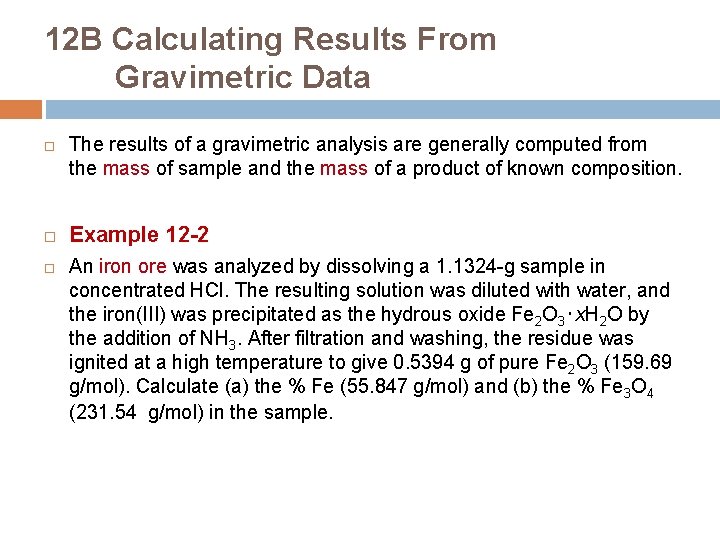
12 B Calculating Results From Gravimetric Data The results of a gravimetric analysis are generally computed from the mass of sample and the mass of a product of known composition. Example 12 -2 An iron ore was analyzed by dissolving a 1. 1324 -g sample in concentrated HCl. The resulting solution was diluted with water, and the iron(III) was precipitated as the hydrous oxide Fe 2 O 3‧x. H 2 O by the addition of NH 3. After filtration and washing, the residue was ignited at a high temperature to give 0. 5394 g of pure Fe 2 O 3 (159. 69 g/mol). Calculate (a) the % Fe (55. 847 g/mol) and (b) the % Fe 3 O 4 (231. 54 g/mol) in the sample.

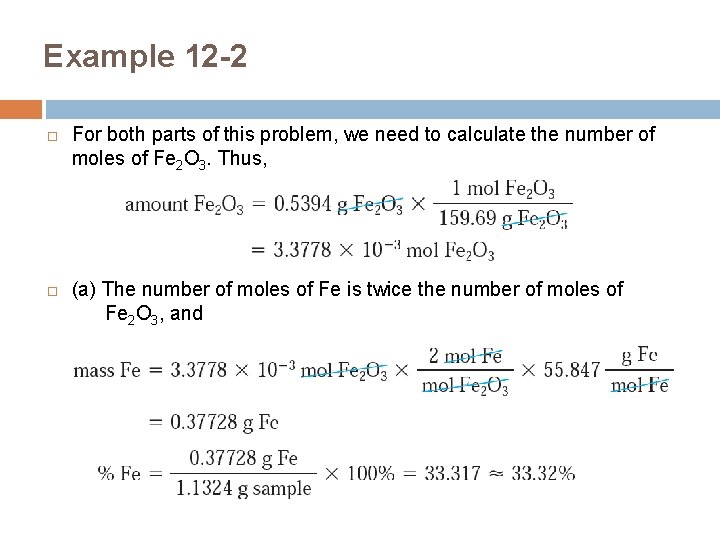
Example 12 -2 For both parts of this problem, we need to calculate the number of moles of Fe 2 O 3. Thus, (a) The number of moles of Fe is twice the number of moles of Fe 2 O 3, and

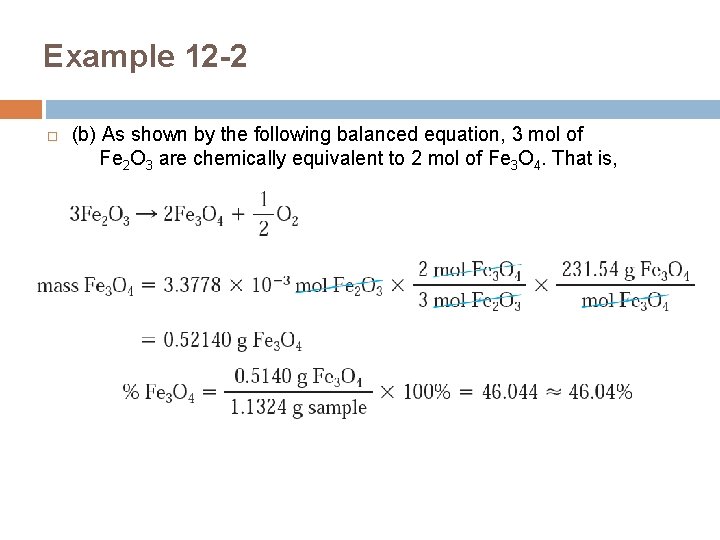
Example 12 -2 (b) As shown by the following balanced equation, 3 mol of Fe 2 O 3 are chemically equivalent to 2 mol of Fe 3 O 4. That is,

12 C Applications Of Gravimetric Methods Gravimetric methods have been developed for most inorganic anions and cations as well as for such neutral species. A variety of organic substances can also be readily determined gravimetrically. Gravimetric methods do not require a calibration or standardization step because the results are calculated directly from the experimental data and molar masses.

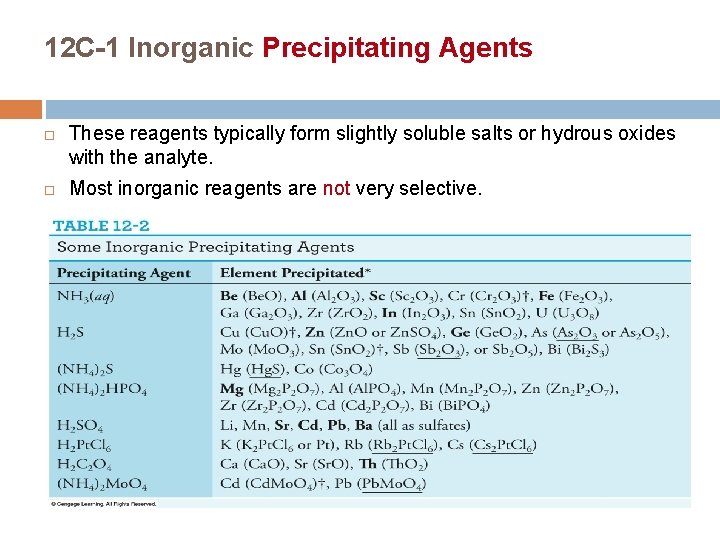
12 C-1 Inorganic Precipitating Agents These reagents typically form slightly soluble salts or hydrous oxides with the analyte. Most inorganic reagents are not very selective.

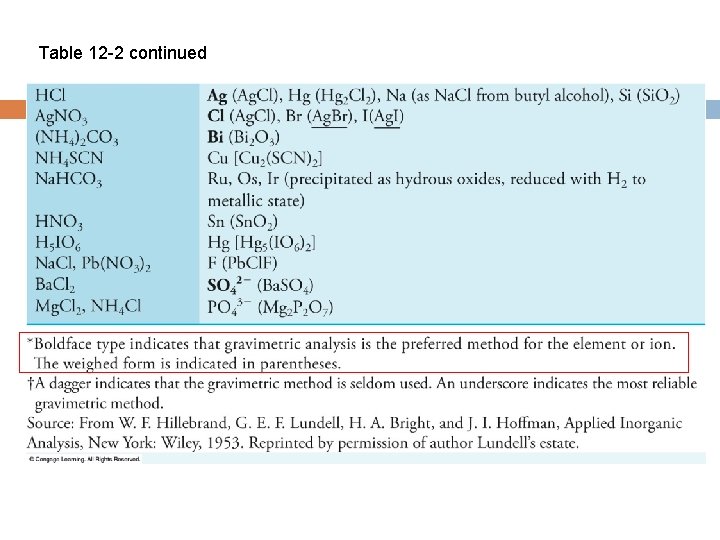
Table 12 -2 continued

12 C-3 Organic Precipitating Agents Numerous organic reagents have been developed for the gravimetric determination of inorganic species. One forms slightly soluble nonionic products called coordination compounds (配位化合物,錯合物); the other forms products in which the bonding between the inorganic species and the reagent is largely ionic. Chelates (螯合物) are cyclical metal-organic compounds in which the metal is a part of one or more five- or six-membered rings. 8 -Hydroxyquinoline and dimethylglyoxime are two widely used chelating reagent (螯合劑). Sodium tetraphenyl borate is a nearspecific precipitation agent for potassium and ammonium.

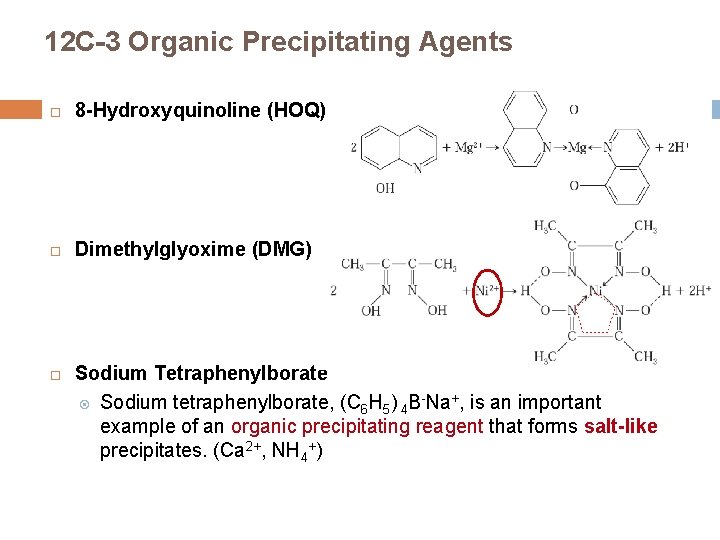
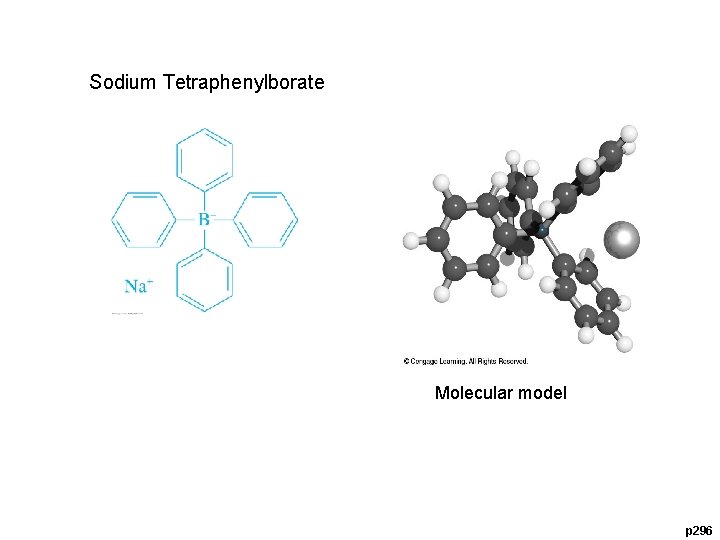
12 C-3 Organic Precipitating Agents 8 -Hydroxyquinoline (HOQ) Dimethylglyoxime (DMG) Sodium Tetraphenylborate Sodium tetraphenylborate, (C 6 H 5) 4 B-Na+, is an important example of an organic precipitating reagent that forms salt-like precipitates. (Ca 2+, NH 4+)

Sodium Tetraphenylborate Molecular model p 296

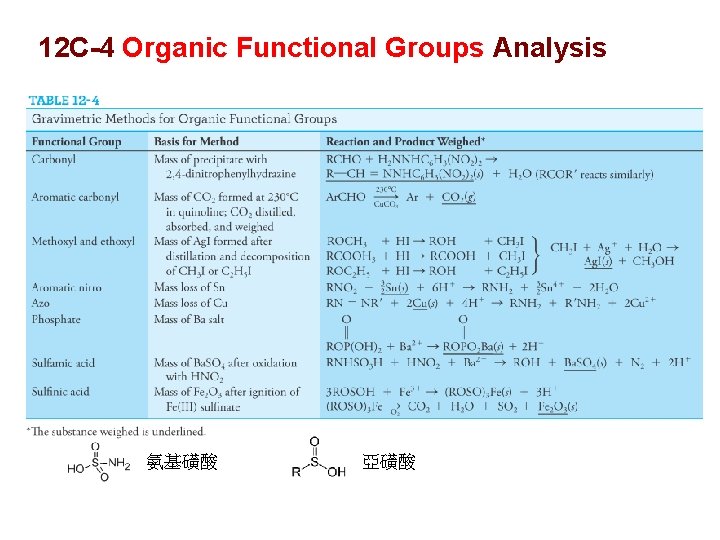
12 C-4 Organic Functional Groups Analysis 氨基磺酸 亞磺酸

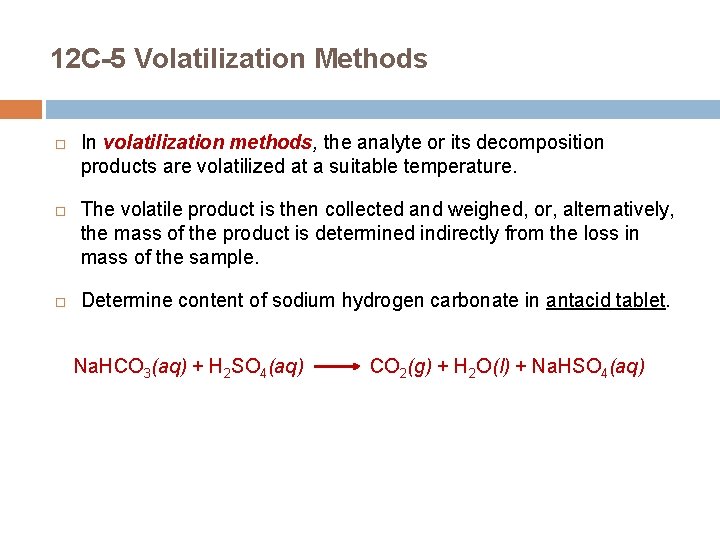
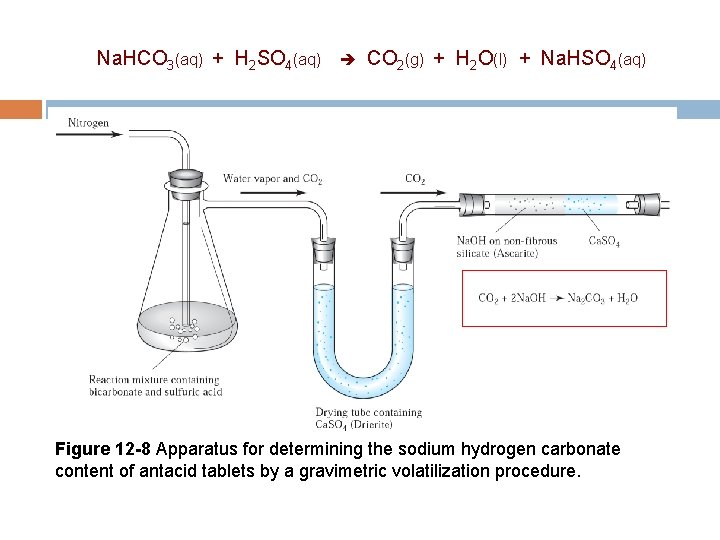
12 C-5 Volatilization Methods In volatilization methods, the analyte or its decomposition products are volatilized at a suitable temperature. The volatile product is then collected and weighed, or, alternatively, the mass of the product is determined indirectly from the loss in mass of the sample. Determine content of sodium hydrogen carbonate in antacid tablet. Na. HCO 3(aq) + H 2 SO 4(aq) CO 2(g) + H 2 O(l) + Na. HSO 4(aq)

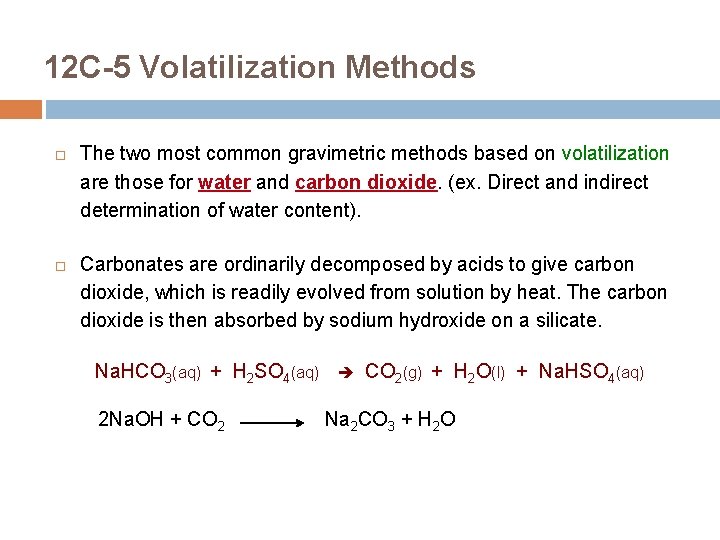
12 C-5 Volatilization Methods The two most common gravimetric methods based on volatilization are those for water and carbon dioxide. (ex. Direct and indirect determination of water content). Carbonates are ordinarily decomposed by acids to give carbon dioxide, which is readily evolved from solution by heat. The carbon dioxide is then absorbed by sodium hydroxide on a silicate. Na. HCO 3(aq) + H 2 SO 4(aq) CO 2(g) + H 2 O(l) + Na. HSO 4(aq) 2 Na. OH + CO 2 Na 2 CO 3 + H 2 O

Na. HCO 3(aq) + H 2 SO 4(aq) CO 2(g) + H 2 O(l) + Na. HSO 4(aq) Figure 12 -8 Apparatus for determining the sodium hydrogen carbonate content of antacid tablets by a gravimetric volatilization procedure.

Review 1) What factors determine particle size of precipitates? How to control particle size? () 2) Why colloidal suspensions are stable and do not coagulate spontaneously? ()