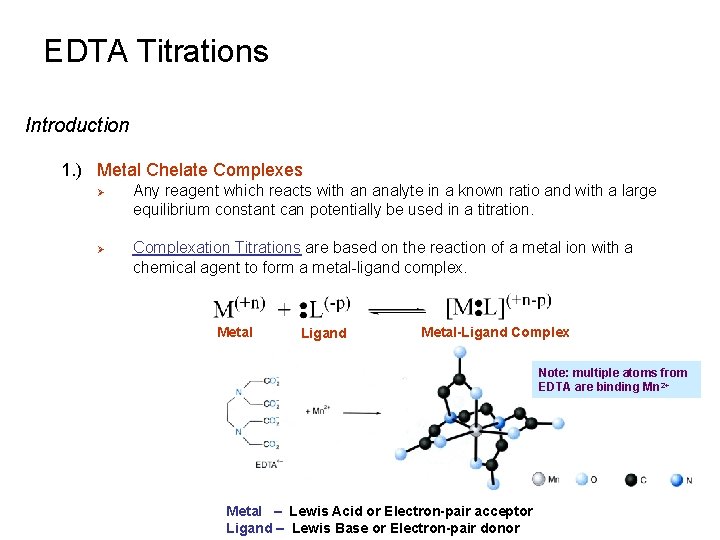
EDTA Titrations Introduction 1 Metal Chelate Complexes Any
![EDTA Titrations EDTA 3. ) EDTA Complexes Ø Substitute [Y 4 -] into Kf EDTA Titrations EDTA 3. ) EDTA Complexes Ø Substitute [Y 4 -] into Kf](https://slidetodoc.com/presentation_image_h/d4f1975318c9632badb14ee178c2aeac/image-11.jpg)


- Slides: 35

EDTA Titrations Introduction 1. ) Metal Chelate Complexes Ø Ø Any reagent which reacts with an analyte in a known ratio and with a large equilibrium constant can potentially be used in a titration. Complexation Titrations are based on the reaction of a metal ion with a chemical agent to form a metal-ligand complex. Metal Ligand Metal-Ligand Complex Note: multiple atoms from EDTA are binding Mn 2+ Metal – Lewis Acid or Electron-pair acceptor Ligand – Lewis Base or Electron-pair donor

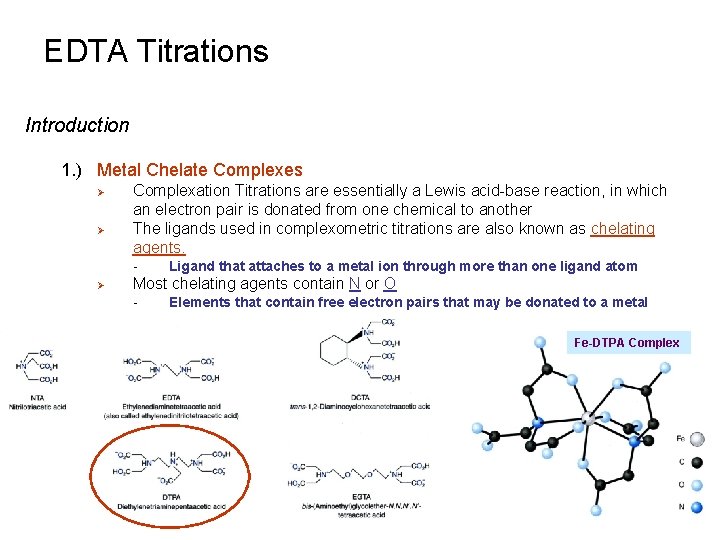
EDTA Titrations Introduction 1. ) Metal Chelate Complexes Ø Ø Complexation Titrations are essentially a Lewis acid-base reaction, in which an electron pair is donated from one chemical to another The ligands used in complexometric titrations are also known as chelating agents. - Ø Ligand that attaches to a metal ion through more than one ligand atom Most chelating agents contain N or O - Elements that contain free electron pairs that may be donated to a metal Fe-DTPA Complex

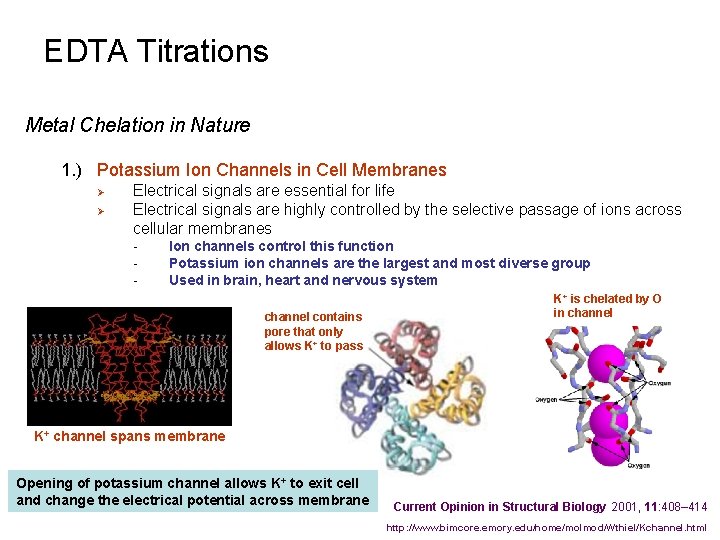
EDTA Titrations Metal Chelation in Nature 1. ) Potassium Ion Channels in Cell Membranes Ø Ø Electrical signals are essential for life Electrical signals are highly controlled by the selective passage of ions across cellular membranes - Ion channels control this function Potassium ion channels are the largest and most diverse group Used in brain, heart and nervous system channel contains pore that only allows K+ to pass K+ is chelated by O in channel K+ channel spans membrane Opening of potassium channel allows K+ to exit cell and change the electrical potential across membrane Current Opinion in Structural Biology 2001, 11: 408– 414 http: //www. bimcore. emory. edu/home/molmod/Wthiel/Kchannel. html

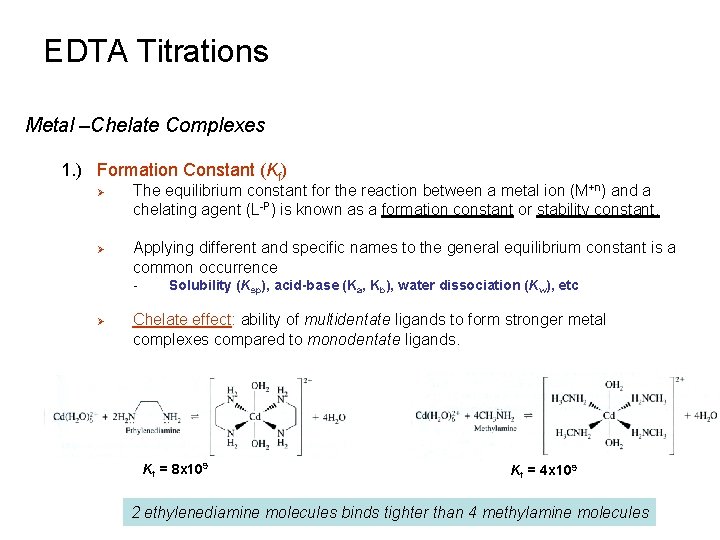
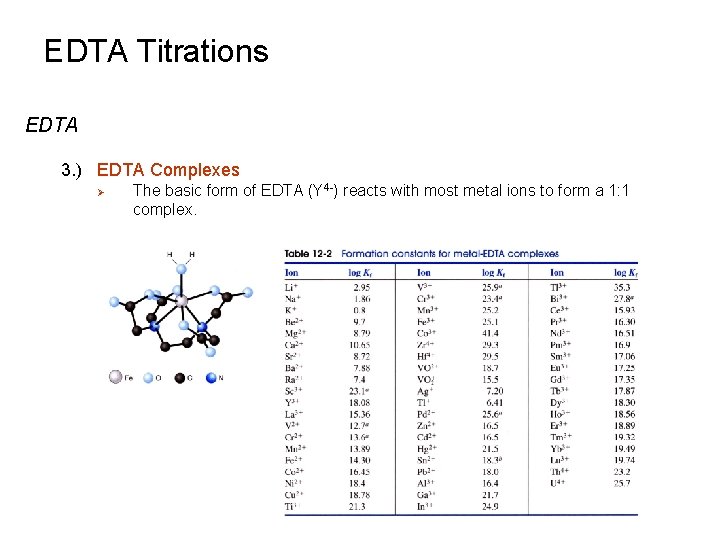
EDTA Titrations Metal –Chelate Complexes 1. ) Formation Constant (Kf) Ø Ø The equilibrium constant for the reaction between a metal ion (M+n) and a chelating agent (L-P) is known as a formation constant or stability constant. Applying different and specific names to the general equilibrium constant is a common occurrence - Ø Solubility (Ksp), acid-base (Ka, Kb), water dissociation (Kw), etc Chelate effect: ability of multidentate ligands to form stronger metal complexes compared to monodentate ligands. Kf = 8 x 109 Kf = 4 x 109 2 ethylenediamine molecules binds tighter than 4 methylamine molecules

EDTA Titrations Metal –Chelate Complexes 2. ) Chelate Effect Ø Usually chelating agents with more than one electron pair to donate will form stronger complexes with metal ions than chelating agents with only one electron pair. - Ø Multidentate ligand: a chelating agent with more than one free electron pair - Ø Typically more than one O or N Larger Kf values Stoichiometry is 1: 1 regardless of the ion charge Monodentate ligand: a chelating agent with only one pair of free electrons Multidentate ligand that binds radioactive metal attached to monoclonal antibody (m. Ab). m. Ab is a protein that binds to a specific feature on a tumor cell delivering toxic dose of radiation.

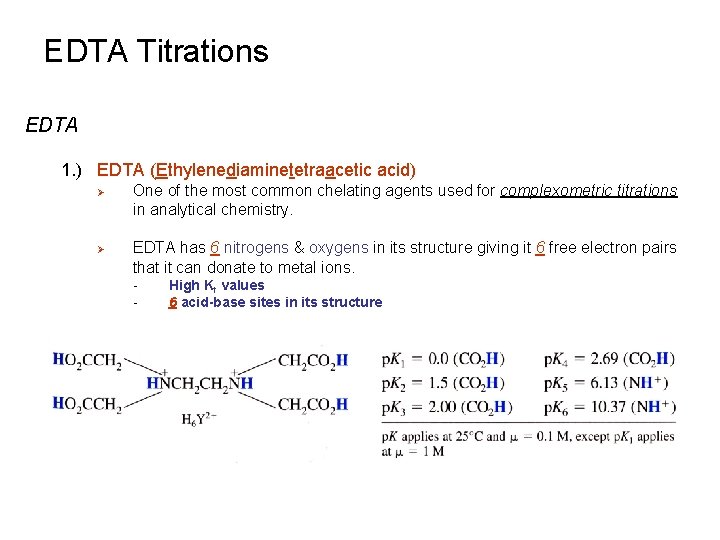
EDTA Titrations EDTA 1. ) EDTA (Ethylenediaminetetraacetic acid) Ø Ø One of the most common chelating agents used for complexometric titrations in analytical chemistry. EDTA has 6 nitrogens & oxygens in its structure giving it 6 free electron pairs that it can donate to metal ions. - High Kf values 6 acid-base sites in its structure

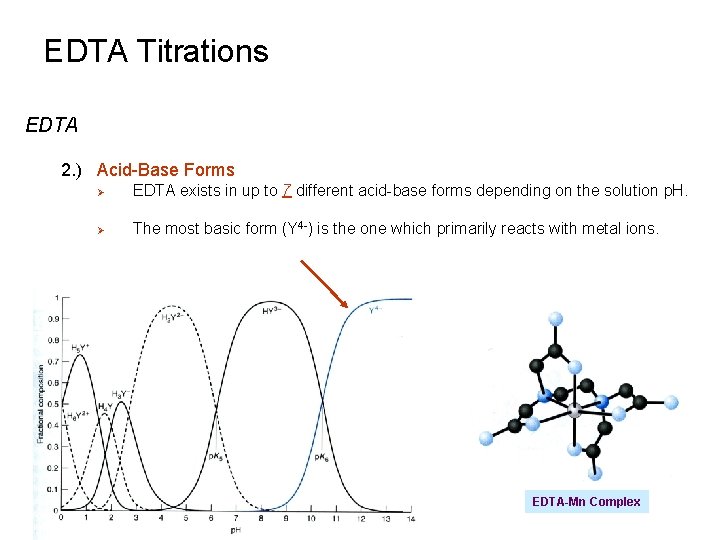
EDTA Titrations EDTA 2. ) Acid-Base Forms Ø EDTA exists in up to 7 different acid-base forms depending on the solution p. H. Ø The most basic form (Y 4 -) is the one which primarily reacts with metal ions. EDTA-Mn Complex

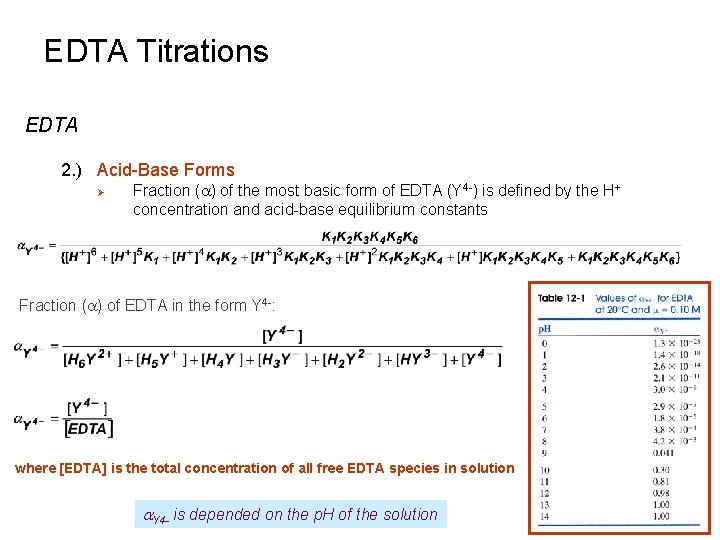
EDTA Titrations EDTA 2. ) Acid-Base Forms Ø Fraction (a) of the most basic form of EDTA (Y 4 -) is defined by the H+ concentration and acid-base equilibrium constants Fraction (a) of EDTA in the form Y 4 -: where [EDTA] is the total concentration of all free EDTA species in solution a. Y 4 - is depended on the p. H of the solution

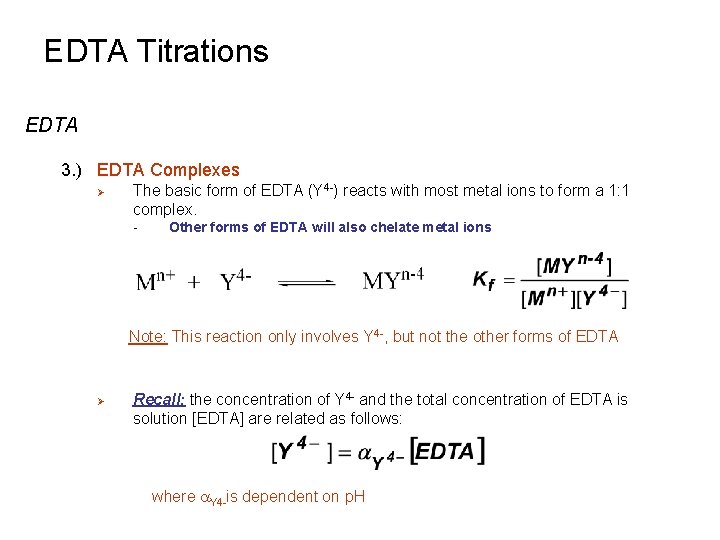
EDTA Titrations EDTA 3. ) EDTA Complexes Ø The basic form of EDTA (Y 4 -) reacts with most metal ions to form a 1: 1 complex. - Other forms of EDTA will also chelate metal ions Note: This reaction only involves Y 4 -, but not the other forms of EDTA Ø Recall: the concentration of Y 4 - and the total concentration of EDTA is solution [EDTA] are related as follows: where a. Y 4 -is dependent on p. H

EDTA Titrations EDTA 3. ) EDTA Complexes Ø The basic form of EDTA (Y 4 -) reacts with most metal ions to form a 1: 1 complex.
![EDTA Titrations EDTA 3 EDTA Complexes Ø Substitute Y 4 into Kf EDTA Titrations EDTA 3. ) EDTA Complexes Ø Substitute [Y 4 -] into Kf](https://slidetodoc.com/presentation_image_h/d4f1975318c9632badb14ee178c2aeac/image-11.jpg)
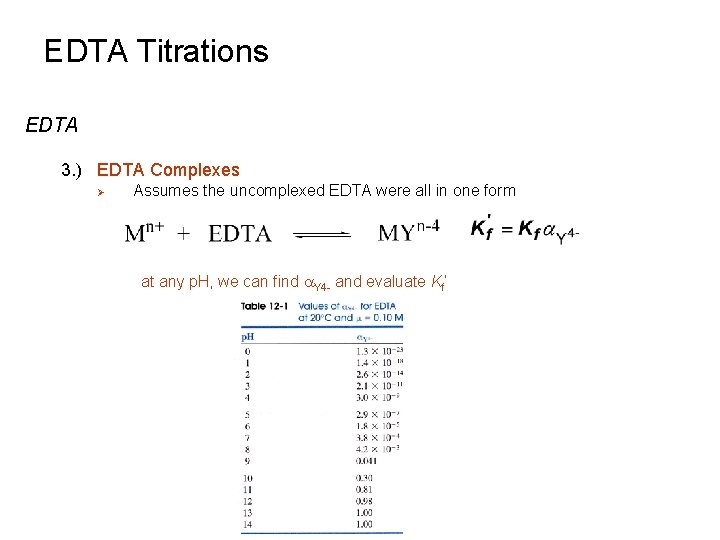
EDTA Titrations EDTA 3. ) EDTA Complexes Ø Substitute [Y 4 -] into Kf equation where [EDTA] is the total concentration of EDTA added to the solution not bound to metal ions Ø If p. H is fixed by a buffer, then a. Y 4 - is a constant that can be combined with Kf Conditional or effective formation constant: (at a given p. H)

EDTA Titrations EDTA 3. ) EDTA Complexes Ø Assumes the uncomplexed EDTA were all in one form at any p. H, we can find a. Y 4 - and evaluate Kf’

EDTA Titrations EDTA 4. ) Example: Ø What is the concentration of free Fe 3+ in a solution of 0. 10 M Fe(EDTA)- at p. H 8. 00?

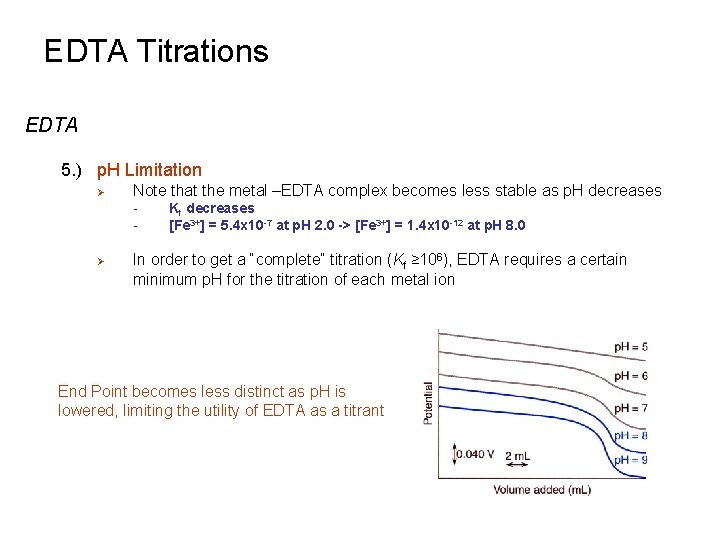
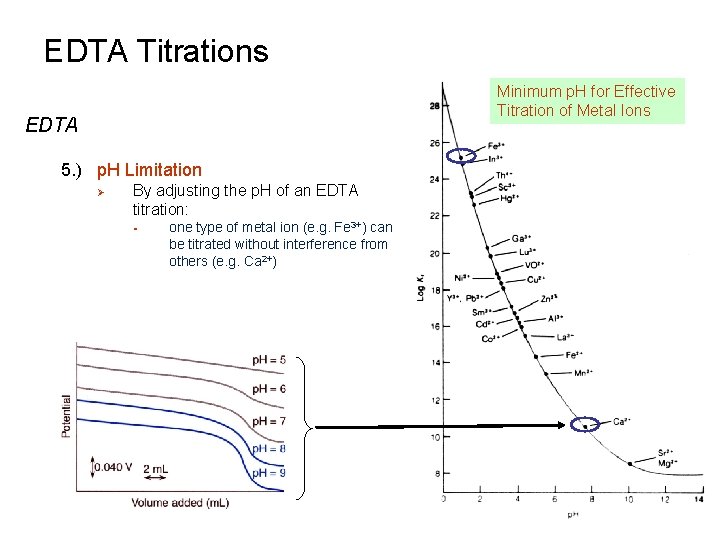
EDTA Titrations EDTA 5. ) p. H Limitation Ø Note that the metal –EDTA complex becomes less stable as p. H decreases - Ø Kf decreases [Fe 3+] = 5. 4 x 10 -7 at p. H 2. 0 -> [Fe 3+] = 1. 4 x 10 -12 at p. H 8. 0 In order to get a “complete” titration (Kf ≥ 106), EDTA requires a certain minimum p. H for the titration of each metal ion End Point becomes less distinct as p. H is lowered, limiting the utility of EDTA as a titrant

EDTA Titrations Minimum p. H for Effective Titration of Metal Ions EDTA 5. ) p. H Limitation Ø By adjusting the p. H of an EDTA titration: § one type of metal ion (e. g. Fe 3+) can be titrated without interference from others (e. g. Ca 2+)

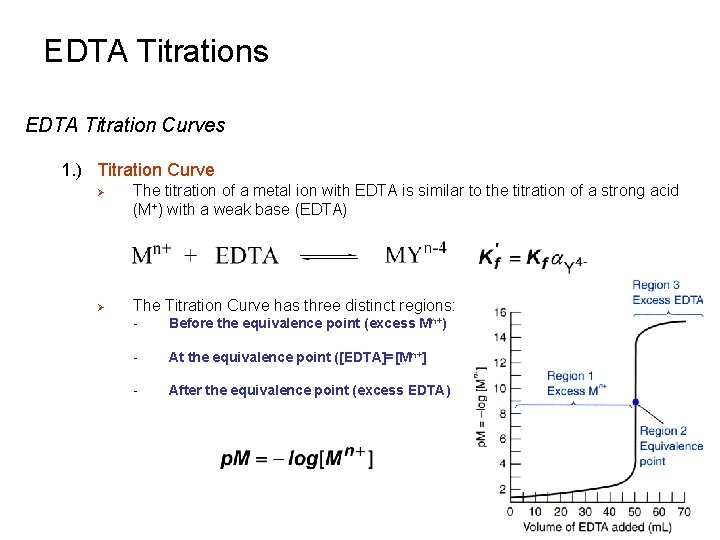
EDTA Titrations EDTA Titration Curves 1. ) Titration Curve Ø Ø The titration of a metal ion with EDTA is similar to the titration of a strong acid (M+) with a weak base (EDTA) The Titration Curve has three distinct regions: - Before the equivalence point (excess Mn+) - At the equivalence point ([EDTA]=[Mn+] - After the equivalence point (excess EDTA)

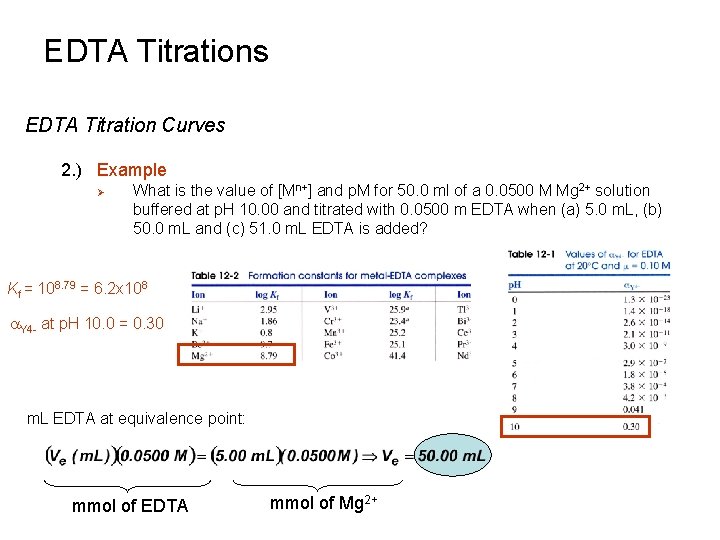
EDTA Titrations EDTA Titration Curves 2. ) Example Ø What is the value of [Mn+] and p. M for 50. 0 ml of a 0. 0500 M Mg 2+ solution buffered at p. H 10. 00 and titrated with 0. 0500 m EDTA when (a) 5. 0 m. L, (b) 50. 0 m. L and (c) 51. 0 m. L EDTA is added? Kf = 108. 79 = 6. 2 x 108 a. Y 4 - at p. H 10. 0 = 0. 30 m. L EDTA at equivalence point: mmol of EDTA mmol of Mg 2+

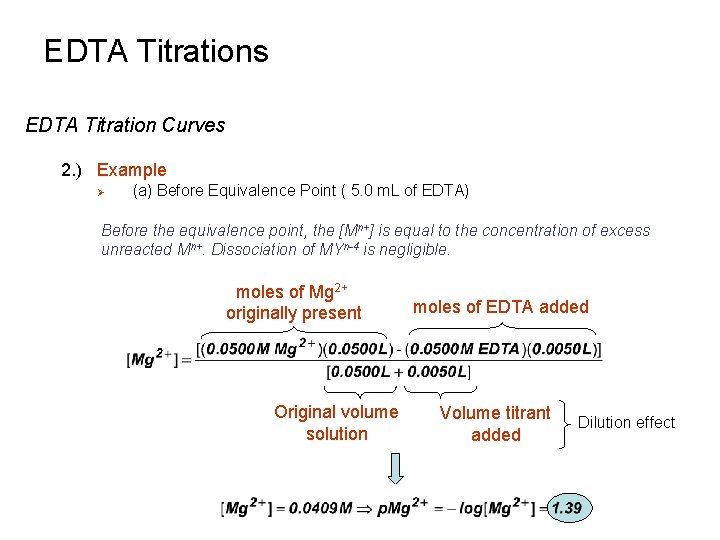
EDTA Titrations EDTA Titration Curves 2. ) Example Ø (a) Before Equivalence Point ( 5. 0 m. L of EDTA) Before the equivalence point, the [Mn+] is equal to the concentration of excess unreacted Mn+. Dissociation of MYn-4 is negligible. moles of Mg 2+ originally present Original volume solution moles of EDTA added Volume titrant added Dilution effect

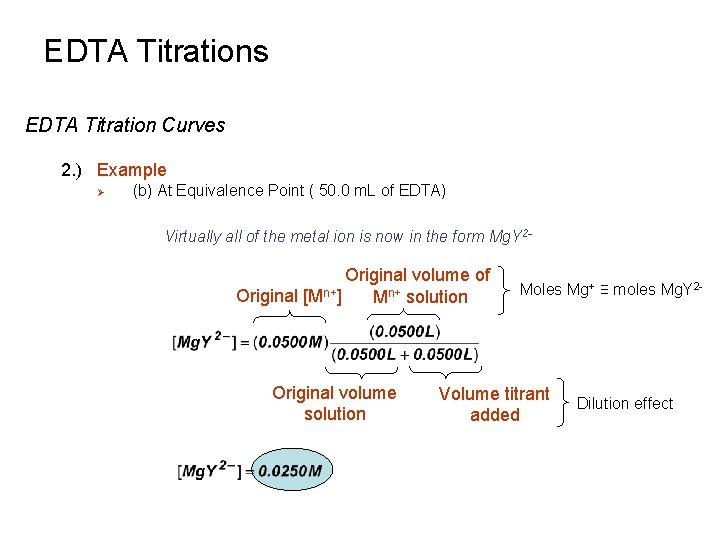
EDTA Titrations EDTA Titration Curves 2. ) Example Ø (b) At Equivalence Point ( 50. 0 m. L of EDTA) Virtually all of the metal ion is now in the form Mg. Y 2 - Original volume of Original [Mn+] Mn+ solution Original volume solution Moles Mg+ ≡ moles Mg. Y 2 - Volume titrant added Dilution effect

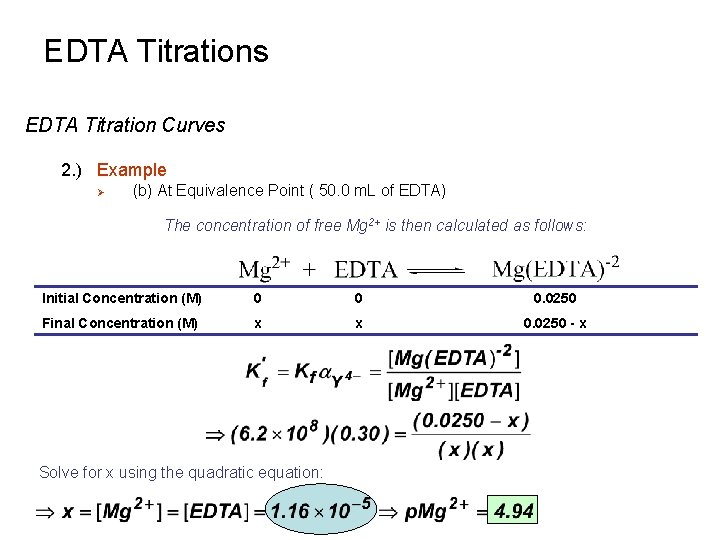
EDTA Titrations EDTA Titration Curves 2. ) Example Ø (b) At Equivalence Point ( 50. 0 m. L of EDTA) The concentration of free Mg 2+ is then calculated as follows: Initial Concentration (M) 0 0 0. 0250 Final Concentration (M) x x 0. 0250 - x Solve for x using the quadratic equation:

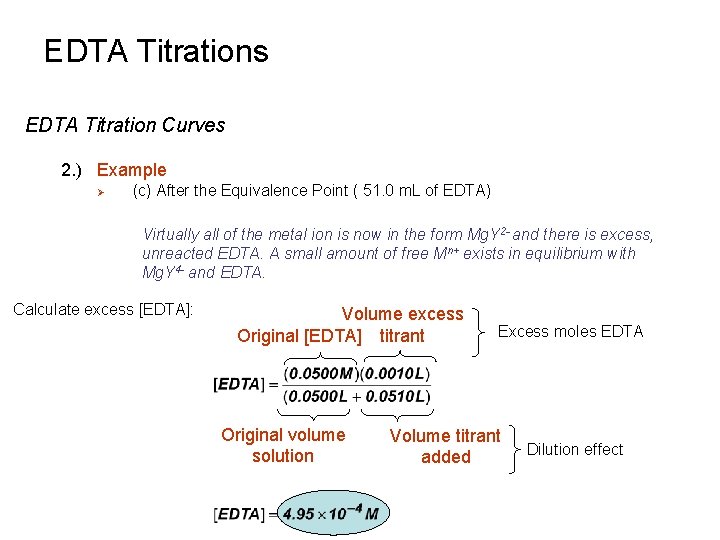
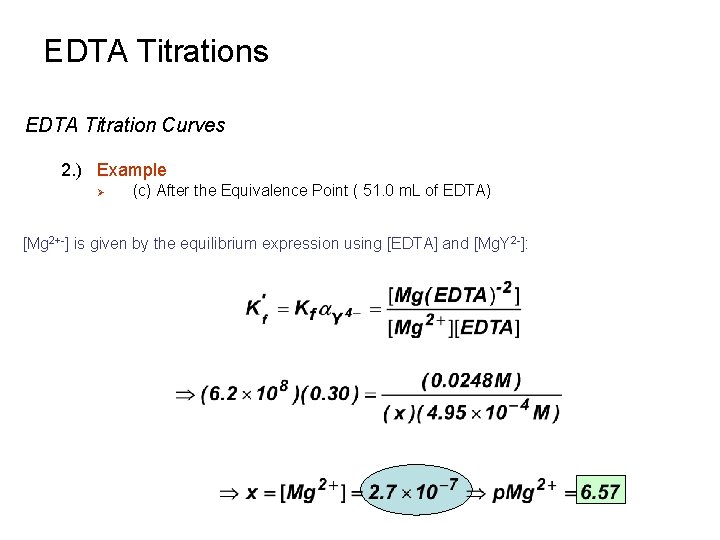
EDTA Titrations EDTA Titration Curves 2. ) Example Ø (c) After the Equivalence Point ( 51. 0 m. L of EDTA) Virtually all of the metal ion is now in the form Mg. Y 2 - and there is excess, unreacted EDTA. A small amount of free Mn+ exists in equilibrium with Mg. Y 4 - and EDTA. Calculate excess [EDTA]: Volume excess Original [EDTA] titrant Original volume solution Excess moles EDTA Volume titrant added Dilution effect

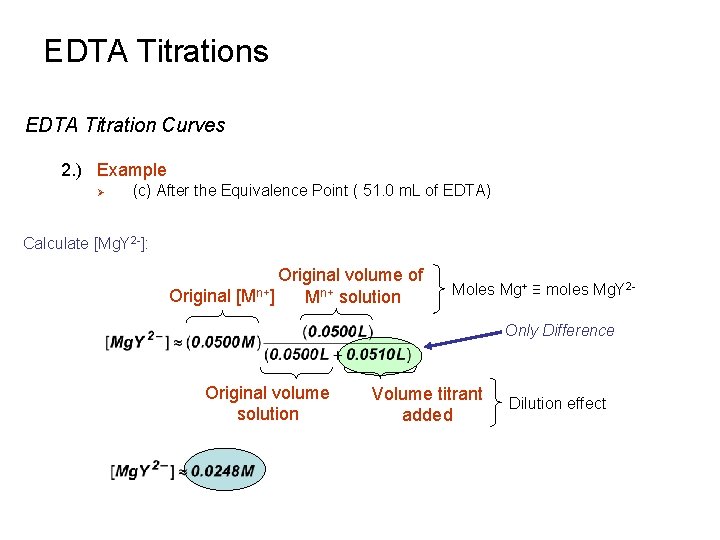
EDTA Titrations EDTA Titration Curves 2. ) Example Ø (c) After the Equivalence Point ( 51. 0 m. L of EDTA) Calculate [Mg. Y 2 -]: Original volume of Original [Mn+] Mn+ solution Moles Mg+ ≡ moles Mg. Y 2 Only Difference Original volume solution Volume titrant added Dilution effect

EDTA Titrations EDTA Titration Curves 2. ) Example Ø (c) After the Equivalence Point ( 51. 0 m. L of EDTA) [Mg 2+-] is given by the equilibrium expression using [EDTA] and [Mg. Y 2 -]:

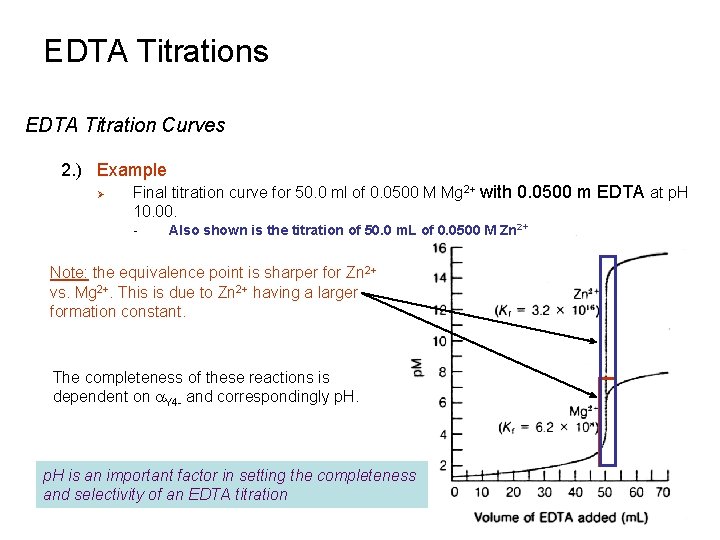
EDTA Titrations EDTA Titration Curves 2. ) Example Ø Final titration curve for 50. 0 ml of 0. 0500 M Mg 2+ with 0. 0500 m EDTA at p. H 10. 00. - Also shown is the titration of 50. 0 m. L of 0. 0500 M Zn 2+ Note: the equivalence point is sharper for Zn 2+ vs. Mg 2+. This is due to Zn 2+ having a larger formation constant. The completeness of these reactions is dependent on a. Y 4 - and correspondingly p. H is an important factor in setting the completeness and selectivity of an EDTA titration

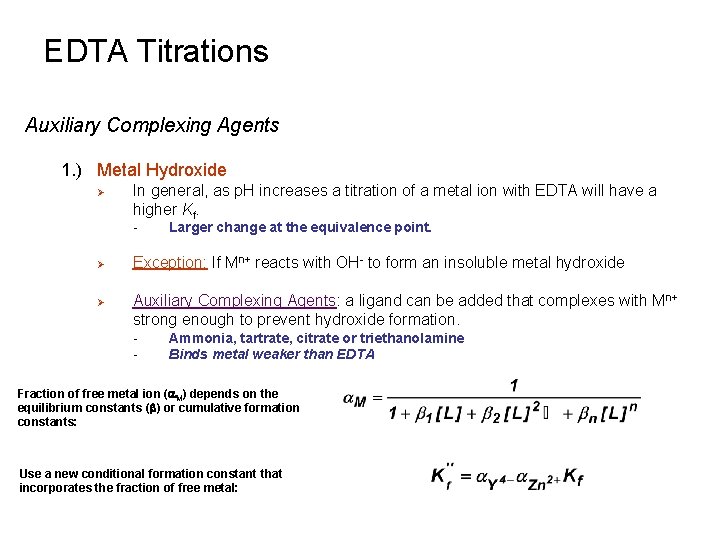
EDTA Titrations Auxiliary Complexing Agents 1. ) Metal Hydroxide Ø In general, as p. H increases a titration of a metal ion with EDTA will have a higher Kf. - Ø Ø Larger change at the equivalence point. Exception: If Mn+ reacts with OH- to form an insoluble metal hydroxide Auxiliary Complexing Agents: a ligand can be added that complexes with Mn+ strong enough to prevent hydroxide formation. - Ammonia, tartrate, citrate or triethanolamine Binds metal weaker than EDTA Fraction of free metal ion (a. M) depends on the equilibrium constants (b) or cumulative formation constants: Use a new conditional formation constant that incorporates the fraction of free metal:

EDTA Titrations Auxiliary Complexing Agents 2. ) Illustration: Ø Ø Titration of Cu+2 (Cu. SO 4) with EDTA Addition of Ammonia Buffer results in a dark blue solution - Ø Cu(II)-ammonia complex is formed Addition of EDTA displaces ammonia with corresponding color change Cu. SO 4 Cu-ammonia Cu-EDTA

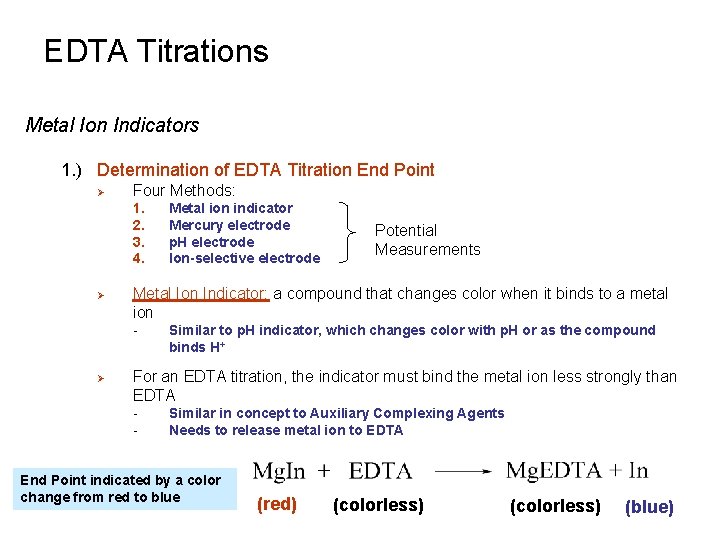
EDTA Titrations Metal Ion Indicators 1. ) Determination of EDTA Titration End Point Ø Four Methods: 1. 2. 3. 4. Ø Potential Measurements Metal Ion Indicator: a compound that changes color when it binds to a metal ion - Ø Metal ion indicator Mercury electrode p. H electrode Ion-selective electrode Similar to p. H indicator, which changes color with p. H or as the compound binds H+ For an EDTA titration, the indicator must bind the metal ion less strongly than EDTA - Similar in concept to Auxiliary Complexing Agents Needs to release metal ion to EDTA End Point indicated by a color change from red to blue (red) (colorless) (blue)

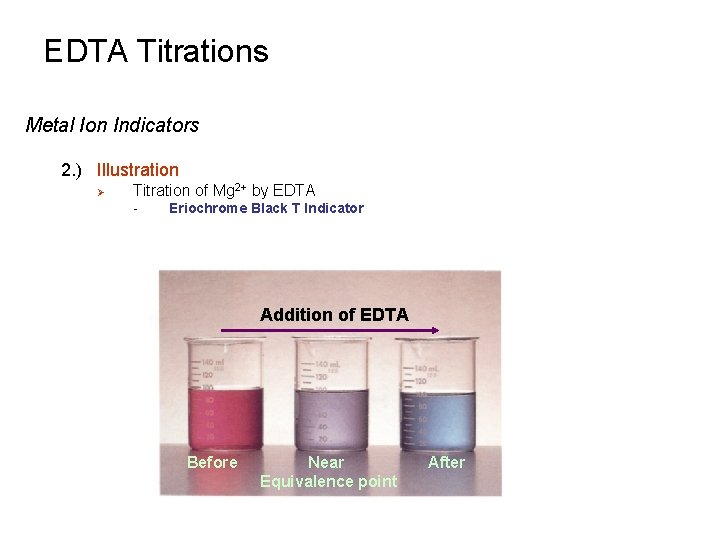
EDTA Titrations Metal Ion Indicators 2. ) Illustration Ø Titration of Mg 2+ by EDTA - Eriochrome Black T Indicator Addition of EDTA Before Near Equivalence point After

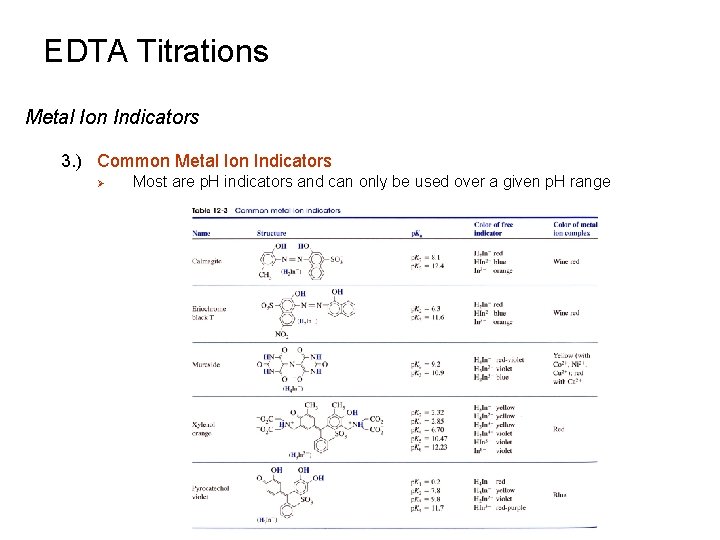
EDTA Titrations Metal Ion Indicators 3. ) Common Metal Ion Indicators Ø Most are p. H indicators and can only be used over a given p. H range

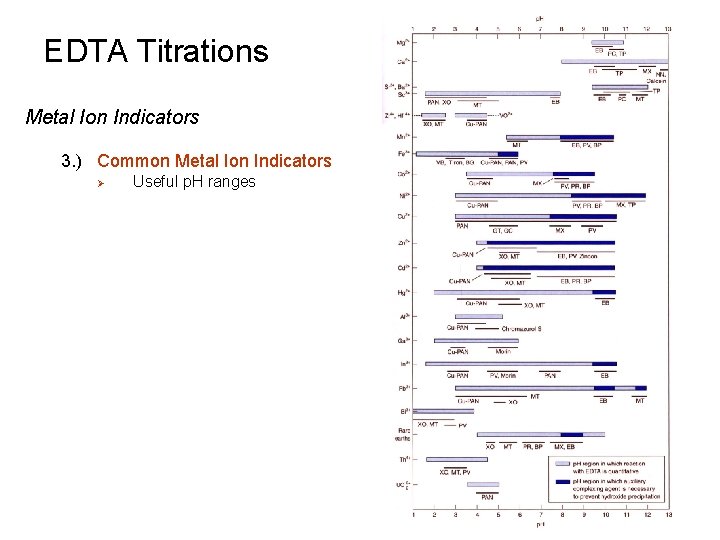
EDTA Titrations Metal Ion Indicators 3. ) Common Metal Ion Indicators Ø Useful p. H ranges

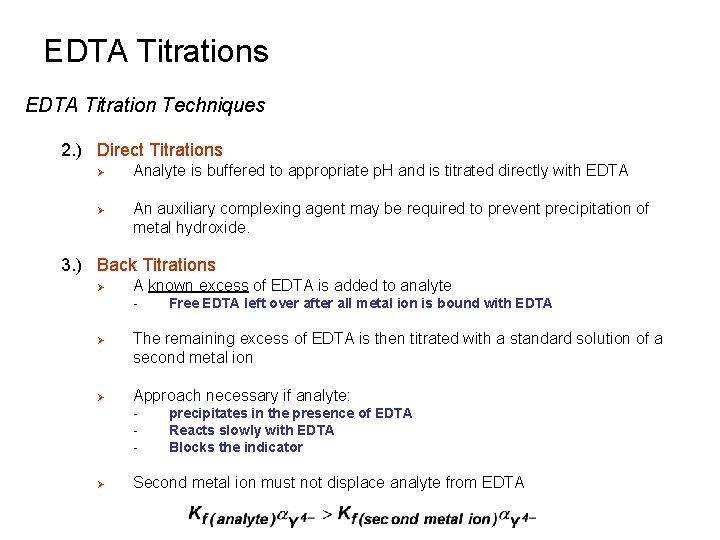
EDTA Titrations EDTA Titration Techniques 1. ) Almost all elements can be determined by EDTA titration Ø Needs to be present at sufficient concentrations Ø Extensive Literature where techniques are listed in: 1) 2) 3) Ø G. Schwarzenbach and H. Flaschka, “Complexometric Titrations”, Methuen: London, 1969. H. A. Flaschka, “EDTA Titrations”, Pergamon Press: New York, 1959 C. N. Reilley, A. J. Bernard, Jr. , and R. Puschel, In: L. Meites (ed. ) “Handbook of Analytical Chemistry”, Mc. Graw-Hill: New York, 1963; pp. 3 -76 to 3 -234. Some Common Techniques used in these titrations include: a) b) c) d) e) Direct Titrations Back Titrations Displacement Titrations Indirect Titrations Masking Agents

EDTA Titrations EDTA Titration Techniques 2. ) Direct Titrations Ø Ø Analyte is buffered to appropriate p. H and is titrated directly with EDTA An auxiliary complexing agent may be required to prevent precipitation of metal hydroxide. 3. ) Back Titrations Ø A known excess of EDTA is added to analyte - Ø Ø The remaining excess of EDTA is then titrated with a standard solution of a second metal ion Approach necessary if analyte: - Ø Free EDTA left over after all metal ion is bound with EDTA precipitates in the presence of EDTA Reacts slowly with EDTA Blocks the indicator Second metal ion must not displace analyte from EDTA

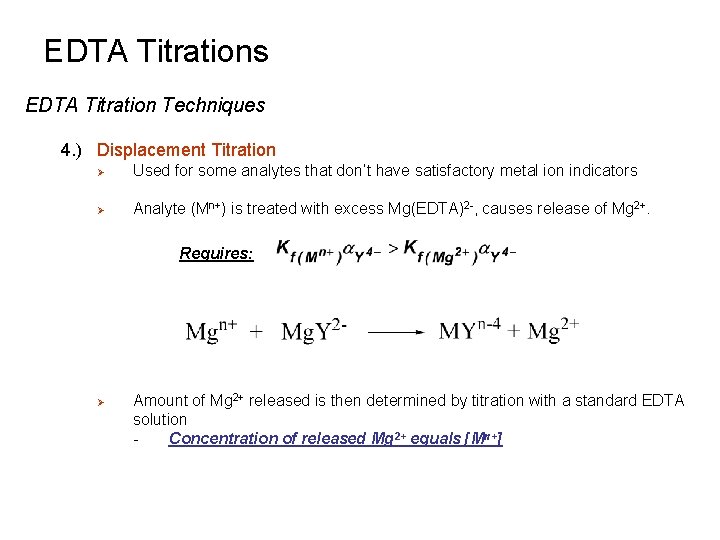
EDTA Titrations EDTA Titration Techniques 4. ) Displacement Titration Ø Used for some analytes that don’t have satisfactory metal ion indicators Ø Analyte (Mn+) is treated with excess Mg(EDTA)2 -, causes release of Mg 2+. Requires: Ø Amount of Mg 2+ released is then determined by titration with a standard EDTA solution Concentration of released Mg 2+ equals [Mn+]

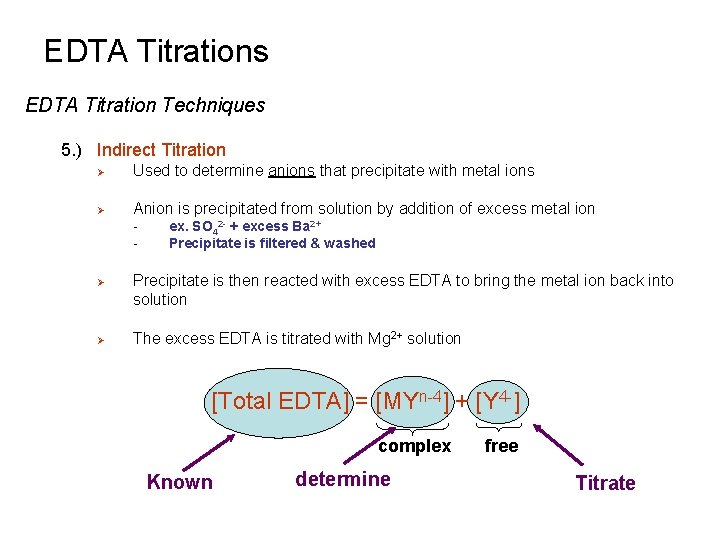
EDTA Titrations EDTA Titration Techniques 5. ) Indirect Titration Ø Used to determine anions that precipitate with metal ions Ø Anion is precipitated from solution by addition of excess metal ion - Ø Ø ex. SO 42 - + excess Ba 2+ Precipitate is filtered & washed Precipitate is then reacted with excess EDTA to bring the metal ion back into solution The excess EDTA is titrated with Mg 2+ solution [Total EDTA] = [MYn-4] + [Y 4 -] complex Known determine free Titrate

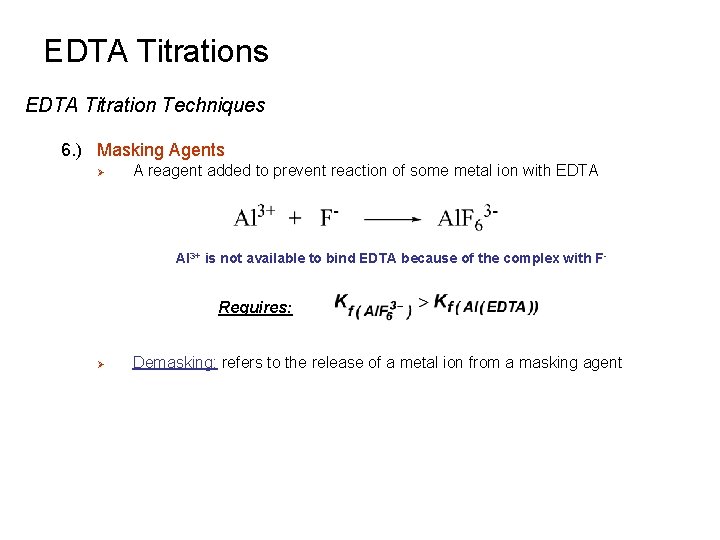
EDTA Titrations EDTA Titration Techniques 6. ) Masking Agents Ø A reagent added to prevent reaction of some metal ion with EDTA Al 3+ is not available to bind EDTA because of the complex with F - Requires: Ø Demasking: refers to the release of a metal ion from a masking agent