Topical Agents Definition Topical Place the use of



































- Slides: 40

Topical Agents

Definition • Topical : Place the use of these drugs or compounds on the surface • Ex: Antiseptic • Systemic: The drugs are absorbed into circulatory system and distributed to various organs or tissues.

Types of inorganic topical agents • The compounds used topically will be divided into broad categories based on their usual action or uses. • There are TWO types: • 1 - Protective agents. • 2 - Antimicrobial agents.

Protective agents • Are substances which may be applied to skin to protect certain areas from irritation. • Properties of protective agents are insoluble in water ( H 2 O), and chemically inert ( unreactive), in order to prevent interactions between the protective substance and the tissue. • Also, small fine partials(large surface area).

Classification of protective agents • 1 - Dusting powders. • 2 -Suspensions. • 3 - Ointments

Talc • Mg. O. 4 Si. O 2. H 2 O • Hydrous magnesium silicate, very fine white powder, smoothly, greasy feeling to the touch( soapstone). • Talc is characterized as fine powder ( 80/100) mesh particle size, odorless, insoluble in water , dil HCl, bases.


Uses of Talc • 1 - It is useful in lubricating, protective dusting, • Can be prevent any friction. • 2 -Used for wound & surgical incision because can produce sterile abscesses • 3 - Used in medical gloves. ( plastic disposable) • 4 - Used in cosmetic may be perfumed • Medical Talc by mixing boric acid with talc as antimicrobial agents.

Zinc Oxide • The chemical formula for Zinc oxide is Zn. O • Is a very fine, oderless, amorphous , white or fait yellow powdwer, can heated 400 -500 ∙C • Zno is insoluble in water, alcohol, but react with dil HCl • Zn. O + HCl ----- Zn. Cl 2 + H 2 O

Uses of Zinc Oxide • 1 - Is a mild astringent and weak antimicrobial agents. Used as powder, ointments to protect the skin. • 2 - Dusting powder used in the treatment of skin ulceration & other dermatological problems. • Medicated zinc oxide by mixing with boric acid as antimicrobial agents.

Calamine • The formula is Zn. O. x. Fe 2 O 3 • Can be synthesized by mixing Zn. O with ferric oxide. • It is a fine powder, water insoluble, alcohols , adhering to the skin.

Uses of Calamine • Calamine can be used as topical protective agent. • 1 - It is USP product used as dust powder, calamine lotion ( applied to skin )pink color. • Calamine is applied to the skin for its adsorbent, protective properties, used in dermatological problems.

• The calamine lotion contain Zn. O and ferric oxide equally mixed Bentonite magma in the solution of Calcium hydroxide. • Phenolated calamine lotion( USP ) contains 1% liquid which provide a local anesthetic and anti- itching action.

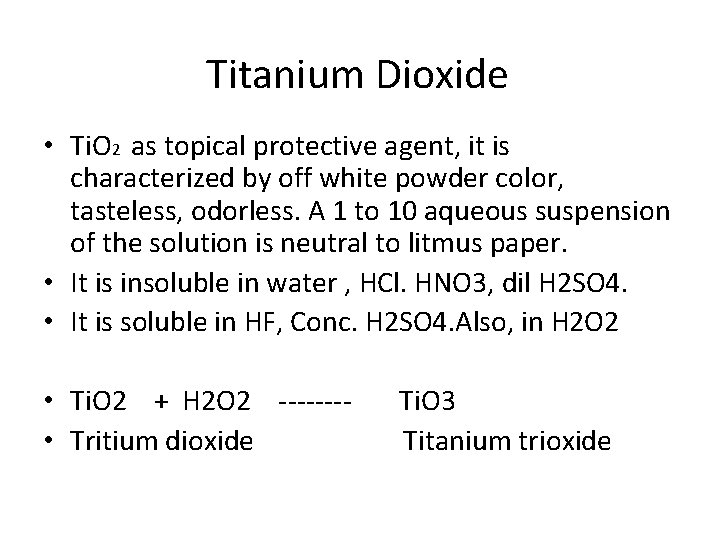
Titanium Dioxide • Ti. O 2 as topical protective agent, it is characterized by off white powder color, tasteless, odorless. A 1 to 10 aqueous suspension of the solution is neutral to litmus paper. • It is insoluble in water , HCl. HNO 3, dil H 2 SO 4. • It is soluble in HF, Conc. H 2 SO 4. Also, in H 2 O 2 • Ti. O 2 + H 2 O 2 ---- Ti. O 3 • Tritium dioxide Titanium trioxide

Uses of Ti. O 2 • Ti. O 2 is a protective agent, topically, used as Sun screen light ( UV light radiation) due to its highly refractive index. • Many pharmaceutical preparation as Ti. O 2 cream, 5, 10, . . 25% ointments. • Other organic sun screen agent p- aminobenzoic acid ( PABA). • Ti. O 2 used in cosmetic and paints.

Aluminum • Aluminum ( Al ) is a silver- white metal as protective agents , highly affinity to O 2 forming Al 2 O 3 as protective layer. • It is insoluble in water, alcohols, and uncreative towards HNO 3, H 2 SO 4 • It react rapidly with dil HCl • HCl + Al ------ Al. Cl 3. 6 H 2 O • Aluminum, paste ( Zn. O with base ) used for prevent irritation.

Silicon Polymer • There are inert protective substances occurring in liquid form known as Silicon oils; e. g. dimethyl silicon ( Dimethicon, or Simethicone ) • CH 3 • CH 3 Si-O- ( - Si - O ) ------Si - CH 3 • CH 3 • Very well adhere to skin, but not wound, be avoided contact to the eyes.


Topical Agents 3 rd Year Pharmacy 2016 - 2017

Outline • Classification a- protective b- antimicrobial agents • A- protective products • Talc , Zinc oxide Zn. O, Calamine, Titinum oxide Ti. O 2, Al products& silicon polymer • Antimicrobials • Mechanism 1 -oxidation H 2 O 2, Zn. O, KMn. O 4, Iodine • 2 - Halogenations Na. OCl • 3 - protein ppt Ag. NO 3.

Protective Agents • Protective • Any agent that isolates the exposed surface from harmful or annoying stimuli • Properties of protective agents: • Insoluble in water Chemical Inertness Adsorbent

• Deeper penetration of topical agents are also seen in many cases which are beneficial. (Penetration of antiseptics into the tissue • below the skin prevents the possibility of deeper in fections-( •

Protective Agents Talc • Talc (Mg 3 Si 4 O 10(OH)2 • protective: native hydrous magnesium silicate, common name and chemical formula. • Soapstone can be grinding in 80/100 mesh particle size. • As grease touch. , insoluble in water, • Uses as protective agents , as lubricants and in pharmaceuticals, as cosmetics, ceramics, for plastic cloves,

Zinc Oxide ( Zn. O ) • White or fait yellow color powder, insoluble in water. Other formulation as Zinc Oxide ointment for adult, & Zinc Oxide in Glycerin for children. • Dusting powder, used astringent and protective topical agent, mild antiseptic, used in the acne preparation, eczema, & psoriasis • It is usually used in the manufacture of plasters.

Calamine Zn. O and Fe 2 O 3

• It used as dusting powder, lotions, ointments, as smoothing agents, for sunburn, eczema, urticaria. Calamine lotion is very popular.

Boric Acid H 3 BO 3 • Is a weak acid, used as antiseptic, and as chemical precursor for many reactions. • Uses: • 1 - Antiseptic • 2 - dilute solution for eye wash • 3 -Acne • 4 - Vaginal douche against bacteria. • 5 -It used as protective against athlete foot.

Titanium dioxide Ti. O 2 • Topical protective agents as cream is used against sunscreen to protect the skin aganist ultra-violet light ( U. V. ). • The organic topical protective agents is • P-aminobenzoic acid ( PABA).

Antimicrobial Agents • Antiseptic: • Any agent which kills or inhibits the growth of microorganisms found in living tissue. • Disinfectant: • Any agent which kills or inhibits the growth of microorganisms found in inanimate objects. • Germicide: • General term for fungicide, bactericide, amoebicide

Mechanisms of action of antimicrobials • There are THREE proposal mechanisms: • 1 -Oxidation 2 -Halogenation Protein Precipitation 3 -

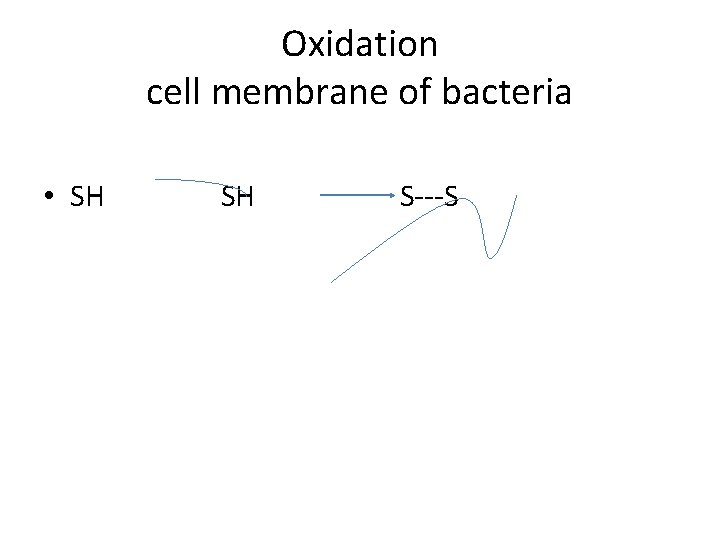
Oxidation cell membrane of bacteria • SH S---S

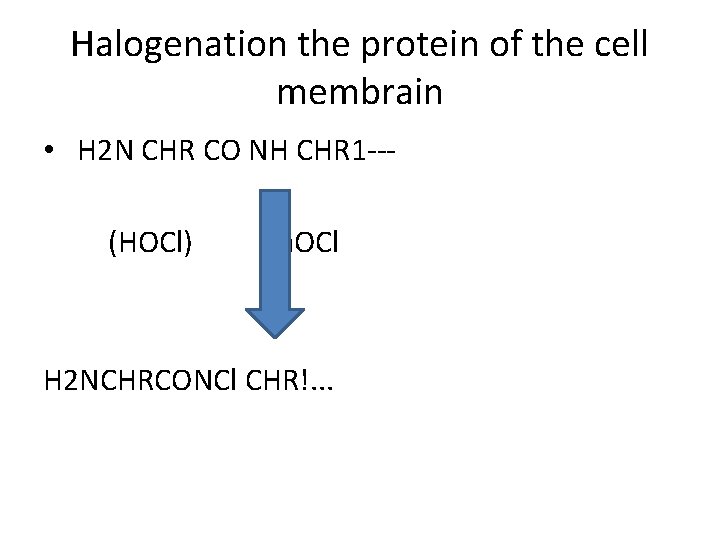
Halogenation the protein of the cell membrain • H 2 N CHR CO NH CHR 1 -- (HOCl) Na. OCl H 2 NCHRCONCl CHR!. . .

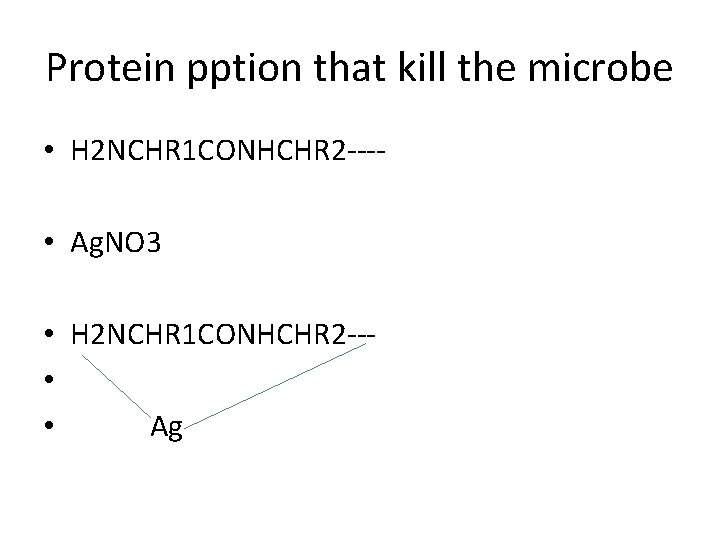
Protein pption that kill the microbe • H 2 NCHR 1 CONHCHR 2 --- • Ag. NO 3 • H 2 NCHR 1 CONHCHR 2 -- • Ag

Hydrogen Peroxide H 2 O 2 • liquid not store in glass bottles because it decompose , it store in white plastic container, keep in cold dark place to prevent decomposition. • There are many uses : • 1 - Antiseptic for wash wound , teeth and ear • 2 -Bleaching color ( hair ). • 3 - Laundry for cloths.

• Sodium Perborate • Antimicrobial : Composite of H 2 O 2, Na. BO 2 and Na. OH Used as bleaching agent in non-chlorine laundry bleaches

Potassium Permanganate KMn. O 4 • Chemically is a strong oxidizing agent, solid purple crystalline , odorless. • Store in well closed container. • Uses: • 1 - Oxidizing agent for skin disease in different concentrations 1: 5000, 1: 15000 • 2 - used in swimming pool for athlete foot. • 3 - Strong oxidizing for many organic reactions.

Iodine I 2 • It is dark violet , insoluble in water , but the • I 2 + KI = I 3 – Complex soluble in water as iodine solution, soluble in alcohol known as tincture iodine • Iodine solution weak solution ( 25 g I 2 + 25 g KI ) in 100 ml • And strong solution ( 50 g I 2 + 50 g KI ) in 100 ml. • Iodine preparations and compounds • Antimicrobial property due to oxidation and Iodination disinfectants • A chemical reagents in analytical reagent. • In drug synthesis. • In Povidin iodine as new product.

Povidone Iodine ( PVP – I) • is a stable chemical complex of poly vinyl pyrrolidine iodide. It is broad spectrum antiseptic for wounds. It is used disinfectant.

2 - Halogenation • Dakin's Solution • Sodium hypochlorite solution, (usp) Contains 4 -6% of Na. OCl, • It is made from bleach that has been diluted and treated to decrease irritation. Chlorine, the active ingredient in Dakin's solution, is a strong antiseptic that kills most forms of bacteria and viruses. .

3 - Protein ppt (Silver Nitrate Ag. NO 3) • Prepare as 1% Ag. NO 3 , protective from light due to oxidation. Effective against gonococcal organism. • Antimicrobial activity of these compounds is due to protein precipitant action. • 1% used eye newborn babies.