EMULSIONS 1 2 Emulsions encountered in everyday life


























































- Slides: 87

EMULSIONS 1

2

Emulsions encountered in everyday life! Pesticide Metal cutting oils Asphalt Skin cream Margarine Ice cream Stability of emulsions may be engineered to vary from seconds to years depending on application

EMULSIONS Definition Classification Applications Theory of emulsification Additives formulation of Formulation of emulsions Emulsification techniques Stability of emulsions Evaluation of emulsions emulsion 4

What is an emulsion ? ? An emulsion is a thermodynamically unstable system consisting of at least two immiscible liquid phases one of which is dispersed as globules in the other liquid phase stabilized by a third substance called emulsifying agent. 5

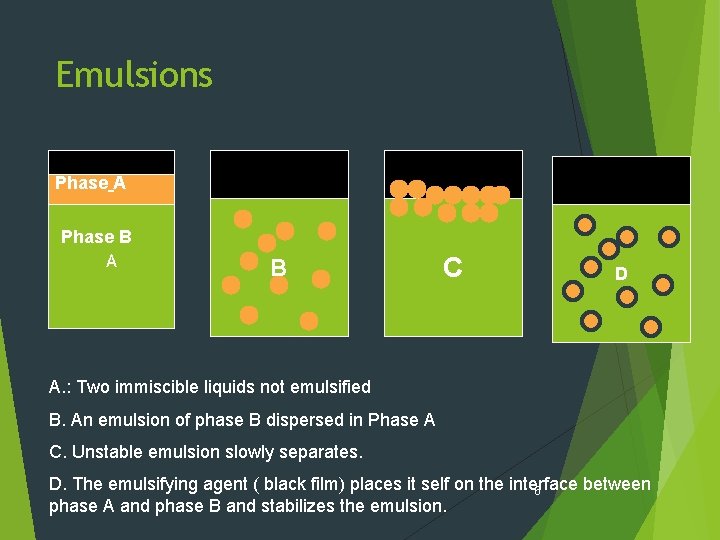
Emulsions Phase A Phase B A B C D A. : Two immiscible liquids not emulsified B. An emulsion of phase B dispersed in Phase A C. Unstable emulsion slowly separates. D. The emulsifying agent ( black film) places it self on the interface between 6 phase A and phase B and stabilizes the emulsion.

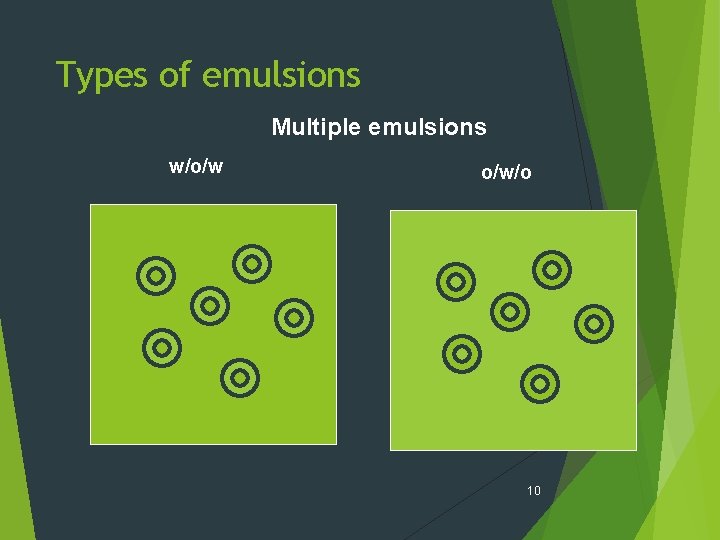
Types of emulsions Ø Simple emulsions (Macro emulsions) Oil-in-water (O/W) Water-in-oil (W/O) Ø Multiple emulsions Oil-in-water-in-oil (O/W/O) Water-in-oil-in-water (W/O/W) Ø Micro emulsions 7

Factors affecting type of emulsion Ø Type of emulsifying agent used Ø Phase volume ratio Ø Viscosity of each phase 8

Determination of type of simple emulsion Dilution test Dye solubility test Conductivity test Co. Cl 2 filter test Fluorescence test 9

Types of emulsions Multiple emulsions w/o/w o/w/o 10

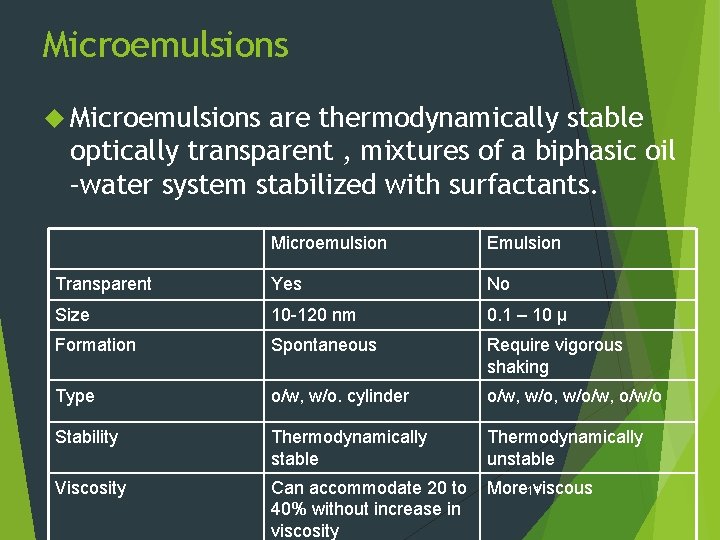
Microemulsions are thermodynamically stable optically transparent , mixtures of a biphasic oil –water system stabilized with surfactants. Microemulsion Emulsion Transparent Yes No Size 10 -120 nm 0. 1 – 10 µ Formation Spontaneous Require vigorous shaking Type o/w, w/o. cylinder o/w, w/o/w, o/w/o Stability Thermodynamically stable Thermodynamically unstable Viscosity Can accommodate 20 to 40% without increase in viscosity More 11 viscous

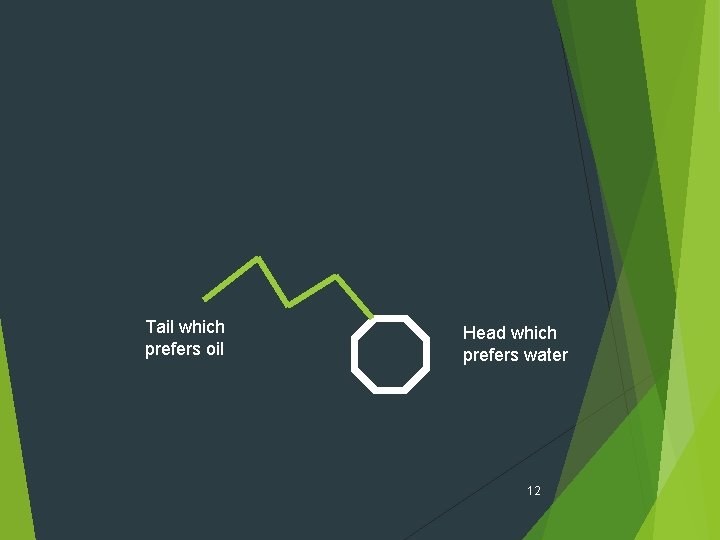
Tail which prefers oil Head which prefers water 12

Pharmaceutical applications of microemulsions Increase bioavailability of drugs poorly soluble in water. Topical drug delivery systems 13

Pharmaceutical Applications of emulsions Ø Ø Oral products It covers the unpleasant taste Increases absorption rate Ø Ø O/W Parenteral use emulsion i/v lipid nutrients i/m – depot effect fr water soluble antigenic material Ø Ø Ø Topical use : Washable Acceptable viscosity Less greasy 14

Theory of emulsification Droplets can be stabilized by three methods i. By reducing interfacial tension ii. By preventing the coalescence of droplets. a. By formation of rigid interfacial film b. By forming electrical double layer. 15

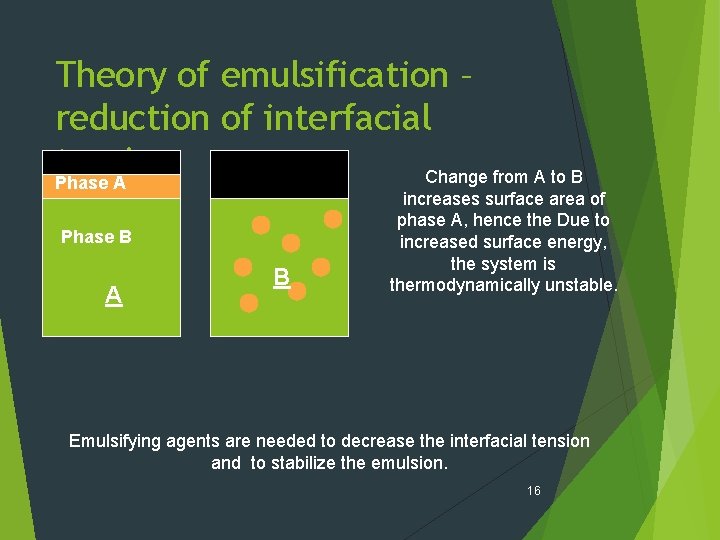
Theory of emulsification – reduction of interfacial tension Change from A to B Phase A Phase B A B increases surface area of phase A, hence the Due to increased surface energy, the system is thermodynamically unstable. Emulsifying agents are needed to decrease the interfacial tension and to stabilize the emulsion. 16

Theory of emulsification – interfacial films Mono molecular Multimolecular Solid particle films 17

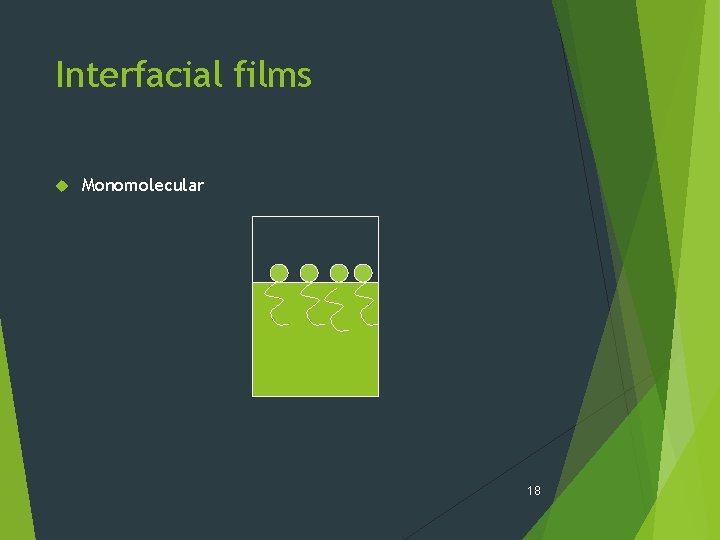
Interfacial films Monomolecular 18

Interfacial films Multimolecular films 19

Interfacial films Solid particle film 20

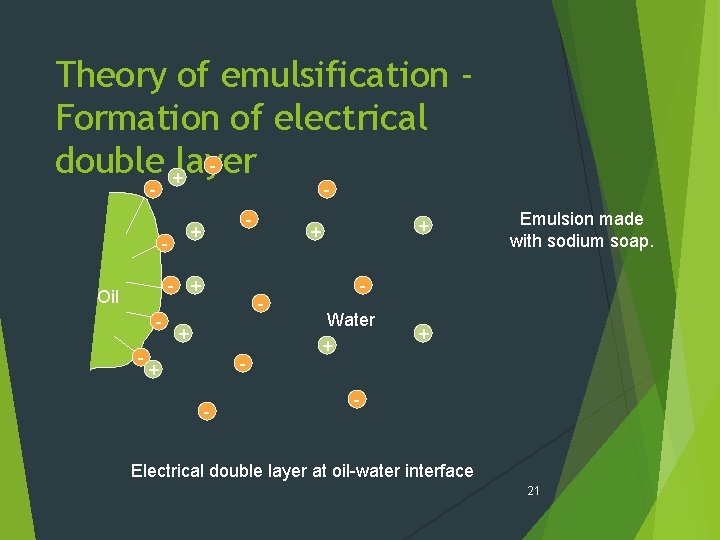
Theory of emulsification Formation of electrical double +layer - - - + Oil - - + - + + Emulsion made with sodium soap. Water + + - Electrical double layer at oil-water interface 21

ADDITIVES FORMULATION OF EMULSIONS 1. Emulsifying agents 2. Auxiliary emulsifiers. 3. Antimicrobial preservatives 4. Antioxidants 22

Emulsifying agents Added to an emulsion to prevent the coalescence of the globules of the dispersed phase. Help in emulsion formation by Ø Reduction in interfacial tension – thermodynamic stabilization Ø Formation of a rigid interfacial film – mechanical barrier to coalescence Ø Formation of an electrical double layer – electrical barrier to approach of particles 23

Classification of emulsifying agents Synthetic Surface active agents ( Monomolecular films) Semi synthetic and natural Hydrophilic colloids ( Multimolecular films) Finely divided solid particles ( Particulate film) 24

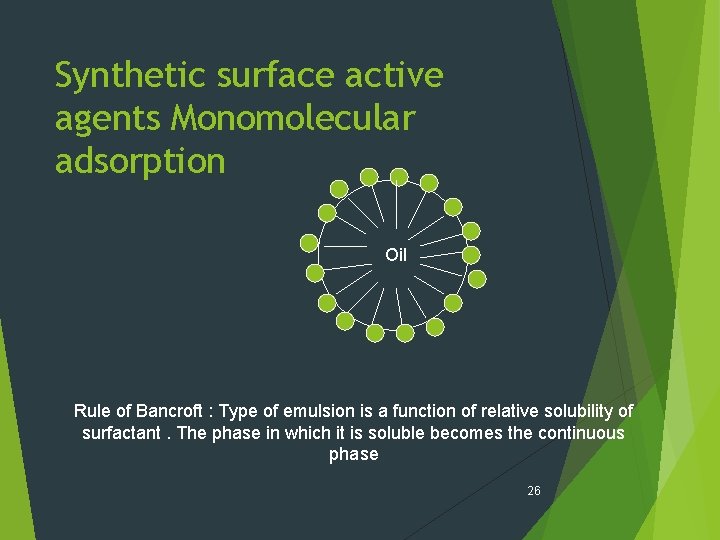
Synthetic surface active agents Description : Reduce interfacial tension and make the emulsion thermodynamically more stable. Form protective monomolecular film 25

Synthetic surface active agents Monomolecular adsorption Oil Rule of Bancroft : Type of emulsion is a function of relative solubility of surfactant. The phase in which it is soluble becomes the continuous phase 26

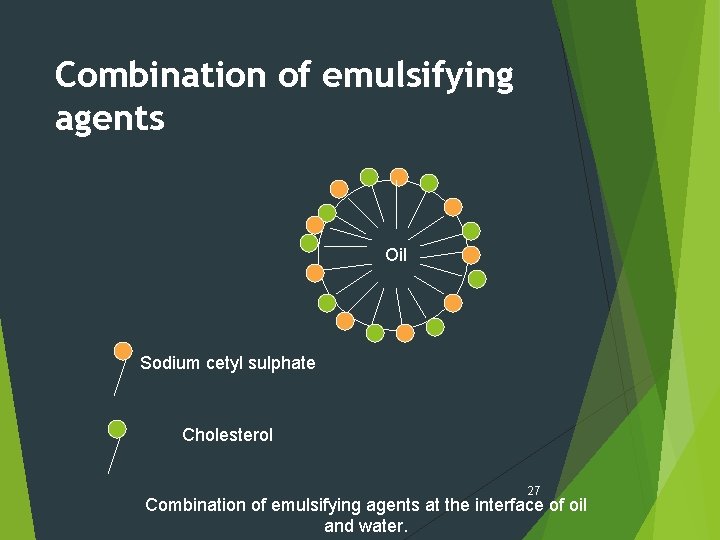
Combination of emulsifying agents Oil Sodium cetyl sulphate Cholesterol 27 Combination of emulsifying agents at the interface of oil and water.

Classification of Surfactant emulsifying agents v Synthetic Ø § § § (Surfactants) ( Monomolecular films) Anionic Soaps -Mono valent -Polyvalent -Organic Sulphates Sulphonates (CH 3(CH 2)n CH 2 SO 3 – Na 28+)

Classification of Surfactant emulsifying agents v Ø § § § Synthetic (Surfactants) ( Monomolecular films) Cationic Quaternary ammonium compounds Nonionic Polyoxy ethylene fatty alcohol ethers C 12 H 25 (OCH 2)n. OH Sorbitan fatty acid esters Polyoxyethylene sorbitan fatty acid esters Polyoxyethylene polyoxypopylene block copolymers Lanolin alcohols and ethoxylated lanolin alcohols 29

Hydrocolloid Emulsifying agents Description - Provide a protective sheath (Multimolecular films )around the droplets - Impart a charge to the dispersed droplets ( so that they repel each other - Swell to increase the viscosity of the system ( so that droplets are less likely to change. ) 30

Classification of Hydrocolloid emulsifying agents v Semisynthetic v Natural Plant origin Animal origin 31

Classification of Hydrocolloid emulsifying agents v Semi synthetic ( Multi molecular films) § Methyl cellulose § Carboxy methyl cellulose 32

Classification of Hydrocolloid emulsifying agents v Ø § § § Natural (Multimolecular films) From plant origin Polysaccharides ( Acacia, tragacanth, agar, pectin, lecithin) From animal origin Proteins ( Gelatin) Lecithin Cholesterol Wool fat Egg yolk 33

Finely divided solids Description : Finely divided solid particles that are wetted to some degree by both oil and water act as emulsifying agents. This results from their being concentrated at interface, where they produce a particulate film around the dispersed droplets to prevent coalescence. 34

Classification of Finely divided solid emulsifying agents Finely divided solids ( Particulate film) § Colloidal Clays Bentonite, ( Al 2 O 3. 4 Si. O 2. H 2 O), Veegum ( Magnesium Aluminium silicate) , Magnesium trisilicate. § Metallic hydroxides Magnesium hydroxide, Aluminium hydroxide, v 35

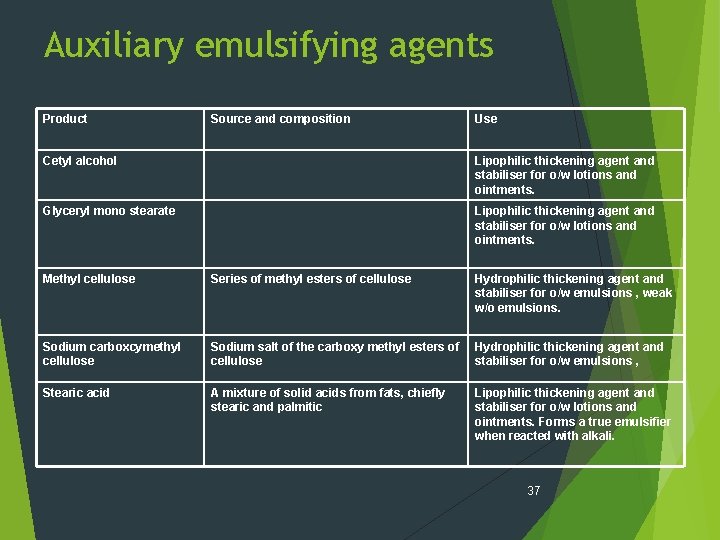
Auxiliary emulsifying agents Auxiliary (Secondary) emulsifying agents include those compounds that are normally incapable themselves of forming stable emulsion. Their main value lies in their ability to function as thickening agents and thereby help stabilize the emulsion. 36

Auxiliary emulsifying agents Product Source and composition Use Cetyl alcohol Lipophilic thickening agent and stabiliser for o/w lotions and ointments. Glyceryl mono stearate Lipophilic thickening agent and stabiliser for o/w lotions and ointments. Methyl cellulose Series of methyl esters of cellulose Hydrophilic thickening agent and stabiliser for o/w emulsions , weak w/o emulsions. Sodium carboxcymethyl cellulose Sodium salt of the carboxy methyl esters of cellulose Hydrophilic thickening agent and stabiliser for o/w emulsions , Stearic acid A mixture of solid acids from fats, chiefly stearic and palmitic Lipophilic thickening agent and stabiliser for o/w lotions and ointments. Forms a true emulsifier when reacted with alkali. 37

Preservation of emulsions Microbial contamination may occur due to: contamination during development or production of emulsion or during its use. Usage of impure raw materials Poor sanitation conditions Invasion by an opportunistic microorganisms. Contamination by the consumer during use of the product. . Precautions to prevent microbial growth ; Use of uncontaminated raw materials Careful cleaning of equipment with live straem. 38

Antimicrobial agents The preservative must be : Less toxic Stable to heat and storage Chemically compatible Reasonable cost Acceptable taste, odor and color. Effective against fungus, yeast, bacteria. Available in oil and aqueous phase at effective level concentration. Preservative should be in unionized state to penetrate the bacteria. Preservative must no bind to other components of the emulsion 39

Antimicrobial agents Acids and acid derivatives - Benzoic acid - Antifungal agent Aldehydes – Formaldehyde - Broad spectrum Phenolics - Phenol - Broad spectrum Cresol Propyl p-hydroxy benzoate Quaternaries -Chlorhexidine and salts - Broad spectrum Benzalkonium chloride Cetyl trimethyl ammonium bromide Mercurials -Phenyl mercuric acetate - Broad spectrum 40

Antioxidants Autoxidation occurs by free radical reaction Can be prevented by Ø absence of oxygen, Ø Ø a free radical chain breaker by reducing agent 41

Antioxidants Gallic acid, Propyl gallate - pharmaceuticals and cosmetics - Bitter taste Ascorbic acid – Suitable for oral use products Sulphites - Suitable for oral use products L-tocopherol - pharmaceuticals and cosmetics Suitable for oral preparations e. g. those containing vit A Butylated hydroxyl toluene - pharmaceuticals and cosmetics - Pronounced odor, to be used at low conc. Butylated hydroxylanisol - pharmaceuticals and cosmetics 42

Formulation of emulsions – Chemical factors Factors affecting the choice of materials Purpose for which emulsion is to be used. Chemical stability Inertness Safety 43

Formulation of emulsions – Chemical factors Selection of liquid phase Phase ratio Selection of emulsifying agent Selection of preservative Selection of antioxidant 44

Selection of liquid phase: Choose from Lipids of natural or synthetic origin depends upon the release rate needed For topical pre[arations – feel of the product Phase ratio Depends upon the solubility of the active ingredient Desired consistency 45

Selection of emulsifying agent Properties of an ideal emulsifying agent Ø reduce the interfacial tension between the two immiscible liquids. Ø physically and chemically stable, inert and compatible with the other ingredients of the formulation. Ø completely non irritant and non toxic in the concentrations used. Ø organoleptically inert i. e. should not impart any colour, odour or taste to the preparation. Ø form a coherent film around the globules of the dispersed phase and should prevent the coalescence of the droplets of the dispersed phase. Ø produce and maintain the required viscosity of the preparation. 46

Selection of emulsifying agent Factors Ø Ø Ø Ø affecting choice of emulsifying agent : Shelf life of the product Type of emulsion desired Cost of emulsifier. Compatibility Non toxicity Taste Chemical stability. 47

Method for Selection of emulsifying agent HLB method for selection of emulsifying agent HLB blend = f x HLB (A) + (1 -f) x HLB ( B) f = fraction of surfactant (A) in the blend 48

Specific considerations formulation of emulsions Consistency ( viscosity) a consistency that provided the desired stability and yet has the appropriate flow characteristics must be attained. Can be changed by addition of auxiliary emulsifying agents. 49

Emulsification techniques Two steps for emulsification : i. Breaking of internal phase into droplets By putting energy into the system ii. Stabilization of droplets 50

Emulsification techniques Laboratory scale preparation techniques Large scale preparation techniques 51

Extemporaneous (Laboratory scale ) method of preparation Continental or dry gum method Wet gum method Bottle or Forbes bottle method Auxiliary method In situ soap method 52

Dry gum method ( Continental method) The continental method is used to prepare the initial or primary emulsion from oil , water and a hydrocolloid or “gum” type emulsifier ( usually acacia). The primary emulsion or emulsion nucleus is formed from 4 parts of oil, 2 parts of water and one part of gum. The 4 parts of oil and 1 part of gum represent their total amount for the fianl emulsion. In a mortar the 1 part of gum ( acacia) is levigated with 4 parts of oil until the powder is thoroughly wetted; then the 2 parts water is added all at once and the mixture is vigorously and continuously trituarted until the primary emulsion formed is craemy white. Additional water or aqueous soltions may be incorporated after the primary emulsion is formed. Slid substances ( e. g. active ingredients, preservatives , color, flavors) are generally dissolved and added as a solution to the primary emulsion , oil soluble substancs in small amounts may be incorporated directly into the primary emulsion. Any substance which might reduce the physical stability of the emulsion, such as alcohohol ( which may precipitate the gum) should be added as near to the end of the process as possible to avoid breaking th emulsion. When all agents have been incorporated , the emulsion sholud be transferred to a caliberated vessel, brought to fianl volume with water, then homogenised or blended to ensure unifrom distribution of ingredients. 53

Dry gum method ( Continental method) used to prepare the initial or primary emulsion from oil , water and a hydrocolloid or “gum” type emulsifier ( usually acacia). Ratio of oil : gum : water in primary emulsion Fixed oil = 4: 1: 2 Mineral oil = 3: 1: 2 Volatile oil = 2: 1; 2 Oleo gum resin = 1: 1: 2 54

Dry gum method ( Continental method) In a mortar gum ( acacia) is levigated with oil until the powder is thoroughly wetted; Then water is added all at once and the mixture is vigorously and continuously triturated until the primary emulsion formed is creamy white. Additional water or aqueous solutions may be incorporated after the primary emulsion is formed. Solid substances ( e. g. active ingredients, preservatives , color, flavors) are generally dissolved and added as a solution to the primary emulsion , 55

Dry gum method ( Continental method) oil soluble substances in small amounts may be incorporated directly into the primary emulsion. Any substance which might reduce the physical stability of the emulsion, such as alcohol ( which may precipitate the gum) should be added as near to the end of the process as possible to avoid breaking the emulsion. When all agents have been incorporated , the emulsion should be transferred to a calibrated vessel, brought to final volume with water, then homogenized or blended to ensure uniform distribution of ingredients. 56

Preparing emulsion by dry gum method - example Cod liver oil 50 ml Acacia 12. 5 gm Syrup 10 ml Flavor oil 0. 4 ml Purified oil up to 100 ml 1. Accurately weigh or measure each ingredient Place cod liver oil in dry mortar Add acacia and give it a very quick mix. Add 25 ml of water and immediately triturate to form thick white , homogenous primary emulsion. Add flaor and mix. Add syrup and mix. Add sufficient water to total 100 ml. 57

Wet gum method (English method) The proportion of oil and water and emulsifier ( gum) are the same as in dry gum method , but the order and technique of mixing are different. The gum is triturated with water to form a mucilage; Then oil is slowly added in portions , while triturating. After all the oil is added , the mixture is triturated for several minutes to form the primary emulsion. Then other ingredients are added as in continental method. Generally speaking, the English method is more difficult to perform successfully , especially with more viscous oils, but may result in a more stable emulsion. 58

Bottle method Used to prepare emulsions of volatile oils, or oligeneous substances of vary low viscosities. Acacia ( or other gum) is placed in a dry bottle and oil are added, The bottle is capped and thoroughly shaken. To this the required volume of water is added all at once and the mixture is shaken thoroughly until the primary emulsion is formed. It is important to minimise the initial amount of time the gum and oil are mxed. The gum will tend to imbibe the oil and will become water proof. 59

Auxiliary method An emulsion prepared by other methods can also be improved by passing it through a hand homogenizer, which forces the emulsion through a very small orifice, reducing the dispersed droplet size to about 5 microns or less. 60

In situ soap method Calcium Soaps : w/o emulsions contain oils such as oleic acid , in combination with lime water ( calcium hydroxide solution, USP). Prepared by mixing equal volumes of oil and lime water. 61

In situ soap method – Example Nascent soap Oil Phase : Olive oil / oleic acid ; olive oil may be replaces by other oils but oleic acid must be added. Lime water : Ca(OH)2 should be freshly prepared. The emulsin formed is w/o Method of preparation : Bottle method Mortar method : When the formulatio contains solid insoluble such as zinc oxide and calamine. 62

Emulsification techniques – large scale Physical parameters affecting the droplet size distribution , viscosity, and stability of emulsion. Location of the emulsifier, method of incorporation of the phases, the rates of addition , the temperature of each phase and the rate of cooling after mixing of the phases considerably 63

Preparation of emulsionslarge scale Energy may be supplied in the form of Heat Homogenization Agitation 64

Preparation of emulsionslarge scale Heat : Emulsification by vaporization Emulsification by phase inversion Low energy emulsification 65

Preparation of emulsions Mechanical equipment for emulsification (Agitation) Mechanical stirrers Propeller type mixers -Turbine mixers - Homogenizers Colloid mills Ultrasonifiers 66

Mechanical stirrers 67

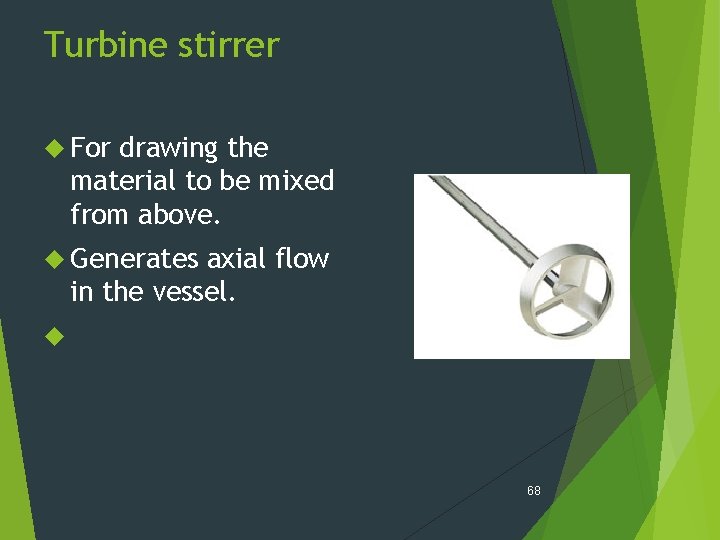
Turbine stirrer For drawing the material to be mixed from above. Generates axial flow in the vessel. 68

Propeller stirrers Standard stirring element. For drawing the material to be mixed from the top to the bottom. Local shearing forces. Generates axial flow in the vessel. Used at medium to high speeds. . 69

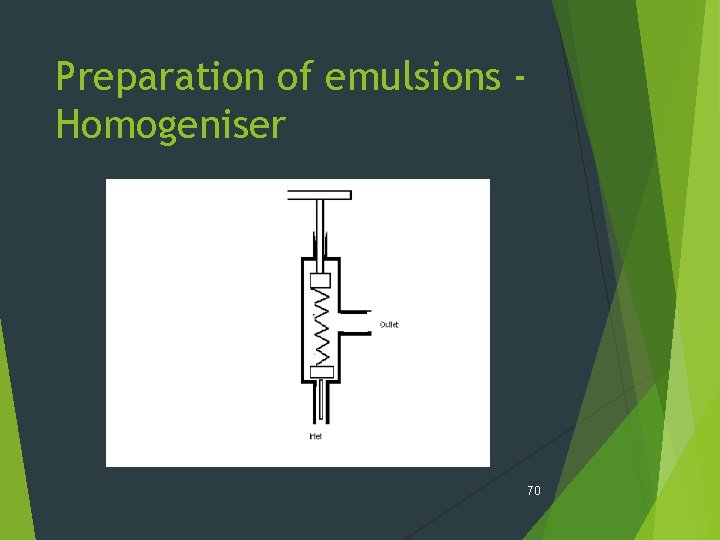
Preparation of emulsions Homogeniser 70

Colloidal mill 71

Colloidal mill rotor and stator 72

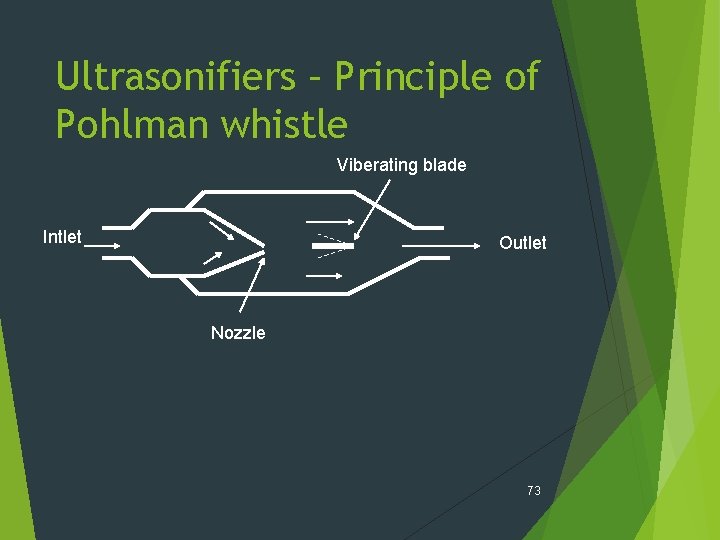
Ultrasonifiers – Principle of Pohlman whistle Viberating blade Intlet Outlet Nozzle 73

Incorporation of medicinal agents Addition of drug during emulsion formation Addition of drugs to a preformed emulsion 1. Addition of materials into w/o emulsion 2. Addition of oleaginous material to o/w emulsion 3. Addition of water soluble materials to a w/o emulsion 4. Addition of water soluble materials to an o/w emulsion 74

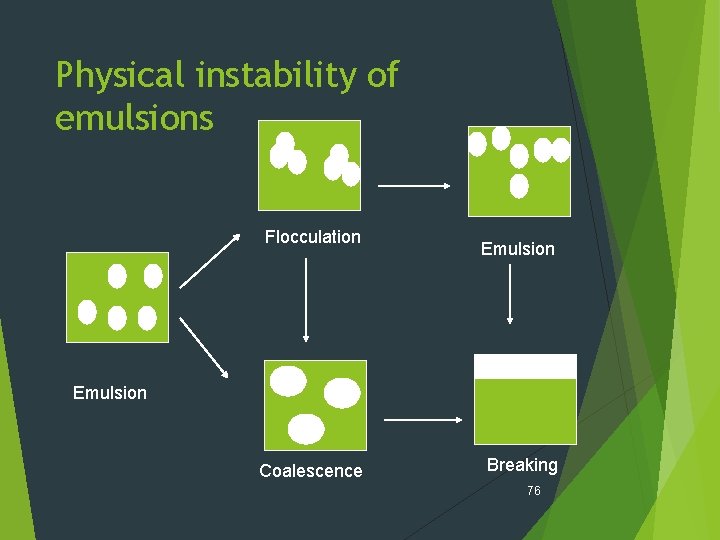
Emulsion stability ( Instability) - Types Physical instability i. Flocculation Ii. Creaming or sedimentation iii. Aggregation or coalescence Iv. Phase inversion 75

Physical instability of emulsions Flocculation Emulsion Coalescence Breaking 76

Emulsion stability ( Instability) - Types Flocculation : Redispersible association of particle within an emulsion to form large aggregates. precursor to the irreversible coalescence. differs from coalescence mainly in that interfacial film and individual droplets remain intact. influenced by the charges on the surface of the emulsified globules. 77

Emulsion stability ( Instability) - Types Flocculation : The reversibility of flocculation depends upon strength of interaction between particles as determined by a the chemical nature of emulsifier, b the phase volume ratio, c. the concentration of dissolved substances, specially electrolytes and ionic emulsifiers. 78

Physical stability of emulsion Creaming : Creaming is the upward movement of dispersed droplets of emulsion relative to the continuous phase ( due to the density difference between two phases). Stoke’s law : dx / dt = d 2 (ρi- ρe)g/18η Dx/dt = rate of setting D = diameter of particles ρi and ρe density of internal nd external phase g = gravitational constant η =viscosity of medium 79

Physical stability of emulsion Aggregation, Coalescence, Breaking Aggregation : Dispersed particles come together but do not fuse. Coalescence is the process by which emulsified particles merge with each to form large particles. Breaking is the destroying of the film surrounding the particles. The major factor to prevent coalescence is the mechanical strength of the interfacial film. 80

Physical stability of emulsion Phase inversion An emulsion is said to invert when it changes from an o/w to w/o or vice versa. Addition of electrolyte : Addition of Ca. Cl 2 into o/w emulsion by sodium soaps can be inverted to w/o. Changing the phase volume ratio : 81

Evaluation of emulsion stability Final evaluation to be done in final container As : - The ingredients may interact with the container, - Some material may leach out from the container - Loss of water and volatile ingredients may occur through the container or closures. 82

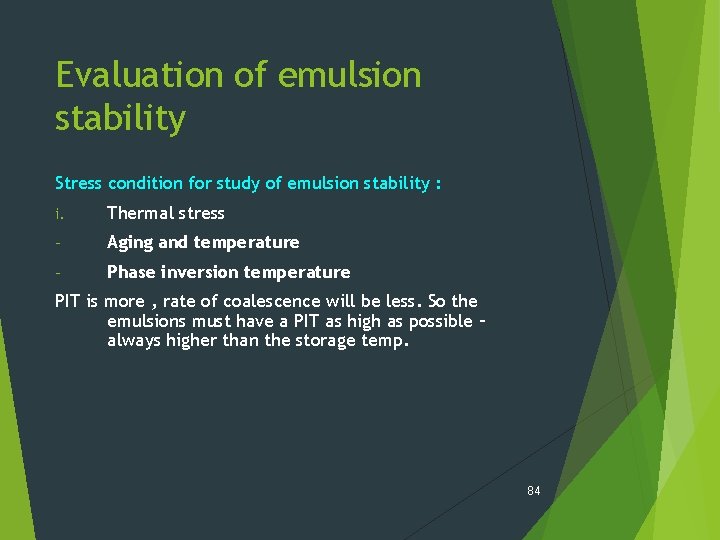
Evaluation of emulsion stability Stress condition for study of emulsion stability : i. Thermal stress - Aging and temperature - Phase inversion temperature ii. Gravitational stress iii: Agitation 83

Evaluation of emulsion stability Stress condition for study of emulsion stability : i. Thermal stress - Aging and temperature - Phase inversion temperature PIT is more , rate of coalescence will be less. So the emulsions must have a PIT as high as possible – always higher than the storage temp. 84

Evaluation of emulsion stability Stress condition for study of emulsion stability : ii. Gravitational stress centrifugation at 3750 rpm in a 10 cm radius centrifuge for a period of 5 hrs is equivalent to the effect of gravity for about one year iii: Agitation 85

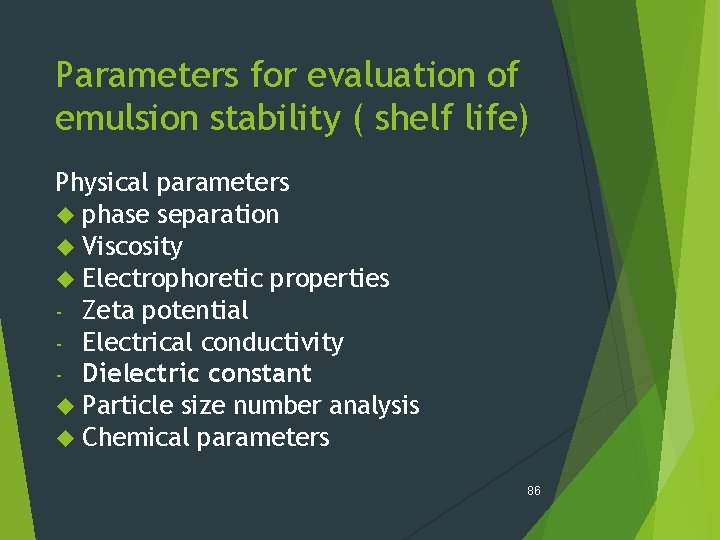
Parameters for evaluation of emulsion stability ( shelf life) Physical parameters phase separation Viscosity Electrophoretic properties - Zeta potential - Electrical conductivity - Dielectric constant Particle size number analysis Chemical parameters 86

Phase diagram Surfactant Water oil 87
 Divided powders examples
Divided powders examples Evacl
Evacl Classification of emulsifying agents
Classification of emulsifying agents Emulsion classification
Emulsion classification Oriented wedge theory of emulsification
Oriented wedge theory of emulsification Inorganic emulsifying agent example
Inorganic emulsifying agent example Critical thinking examples in real life
Critical thinking examples in real life Inertia in daily life
Inertia in daily life Examples of social interaction in everyday life
Examples of social interaction in everyday life Examples of complex machines used in everyday life
Examples of complex machines used in everyday life Example of projectile motion
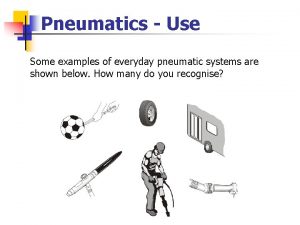
Example of projectile motion Pneumatics examples
Pneumatics examples Cool physical changes
Cool physical changes Example of logos in literature
Example of logos in literature Where do we see percentages in everyday life
Where do we see percentages in everyday life Parabola in eiffel tower
Parabola in eiffel tower Chapter 6 section 1 price supply and demand together
Chapter 6 section 1 price supply and demand together Examples of chemical reactions in everyday life
Examples of chemical reactions in everyday life Examples of bases in everyday life
Examples of bases in everyday life Vital information for your everyday life
Vital information for your everyday life Practical applications of polynomials
Practical applications of polynomials Associative learning vs non associative learning
Associative learning vs non associative learning What is an example of an economic enigma
What is an example of an economic enigma Measurement in everyday life
Measurement in everyday life Annoying things essay
Annoying things essay What is algebra used for in everyday life
What is algebra used for in everyday life Simon the zealot death
Simon the zealot death Status set
Status set Scientific notation facts
Scientific notation facts 10 benedictine values
10 benedictine values Ethos pathos logos in everyday life
Ethos pathos logos in everyday life Information technology in everyday life
Information technology in everyday life What happens in evaporation
What happens in evaporation How does biotechnology impact an individual?
How does biotechnology impact an individual? How is pascal's principle used in everyday life?
How is pascal's principle used in everyday life? The practice of everyday life
The practice of everyday life Examples of simple machines used in everyday life
Examples of simple machines used in everyday life Newton's 3 laws
Newton's 3 laws Psychology in everyday life myers
Psychology in everyday life myers Place value in everyday life
Place value in everyday life Newtons second law of motion
Newtons second law of motion Examples of levers in everyday life
Examples of levers in everyday life Total internal reflection in daily life
Total internal reflection in daily life Daily life in colonial virginia
Daily life in colonial virginia Unbalanced force
Unbalanced force Everyday signs and symbols
Everyday signs and symbols The psychology of everyday things
The psychology of everyday things Dangers seen and unseen
Dangers seen and unseen I study english everyday simple present tense
I study english everyday simple present tense He plays football
He plays football Cargill everyday performance management
Cargill everyday performance management They football yesterday
They football yesterday Passive voice wash
Passive voice wash Make everyday mediterranean
Make everyday mediterranean Everyday use for your grandmama
Everyday use for your grandmama Everyday use by alice walker summary
Everyday use by alice walker summary Everyday use steal chart
Everyday use steal chart How to do partial quotient division
How to do partial quotient division ü10ü
ü10ü Everyday activities vocabulary
Everyday activities vocabulary Calendar math kit
Calendar math kit The type of menu that offers the same dishes everyday
The type of menu that offers the same dishes everyday Design of everyday things summary
Design of everyday things summary Real life examples of charles law
Real life examples of charles law Defensive line get off drills
Defensive line get off drills Everyday use by alice walker audio
Everyday use by alice walker audio Everyday use for your grandmama
Everyday use for your grandmama Greenberg
Greenberg Tukey pairwise comparison minitab
Tukey pairwise comparison minitab Everyday objects from unusual angles quiz
Everyday objects from unusual angles quiz We make choices everyday
We make choices everyday Everyday acids and alkalis
Everyday acids and alkalis Excuses excuses are all that i hear
Excuses excuses are all that i hear Mandy can i help you
Mandy can i help you Simple present always
Simple present always When was everyday use written
When was everyday use written Every day edit
Every day edit Talk about your favourite food and drink
Talk about your favourite food and drink Everyday it's you i live for
Everyday it's you i live for Everyday choices we make
Everyday choices we make Summary of the flowers by alice walker
Summary of the flowers by alice walker Swim present continuous tense
Swim present continuous tense Somebody cleans the room everyday
Somebody cleans the room everyday Journey everyday
Journey everyday Teach test of everyday attention
Teach test of everyday attention Everyday hero donation
Everyday hero donation How everyday things are made
How everyday things are made When was everyday use published
When was everyday use published