Radiological Terrorism Medical Response to Mass Casualties Part



















![Pharmacotherapy Filgrastim (Neupogen®) Filgrastim [Granulocyte-CSF (G-CSF)] – Genetically engineered protein – Daily IV or Pharmacotherapy Filgrastim (Neupogen®) Filgrastim [Granulocyte-CSF (G-CSF)] – Genetically engineered protein – Daily IV or](https://slidetodoc.com/presentation_image_h2/125426e86795b71ac46cfd24f991d175/image-33.jpg)


- Slides: 37

Radiological Terrorism: Medical Response to Mass Casualties Part II Jeffrey B. Nemhauser, MD Medical Officer Radiation Studies Branch National Center for Environmental Health Centers for Disease Control and Prevention

Triage Key Concepts Victim categories following radiological/nuclear incident – (Possibly) exposed • Injured and Uninjured – Unexposed • Injured and Uninjured

Triage Key Concepts Combined Injury – Physical, thermal, and/or chemical trauma – Radiation exposure in doses sufficient to threaten overall survival / recovery

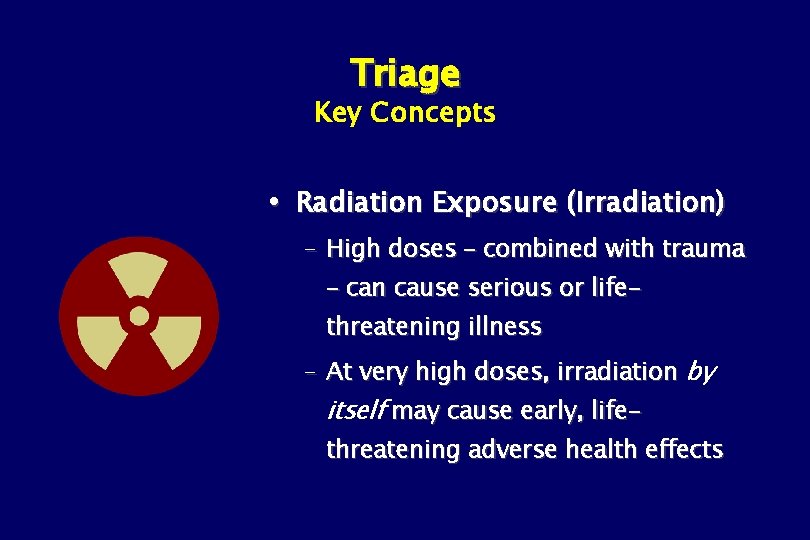
Triage Key Concepts Radiation Exposure (Irradiation) – High doses – combined with trauma – can cause serious or lifethreatening illness – At very high doses, irradiation by itself may cause early, life- threatening adverse health effects

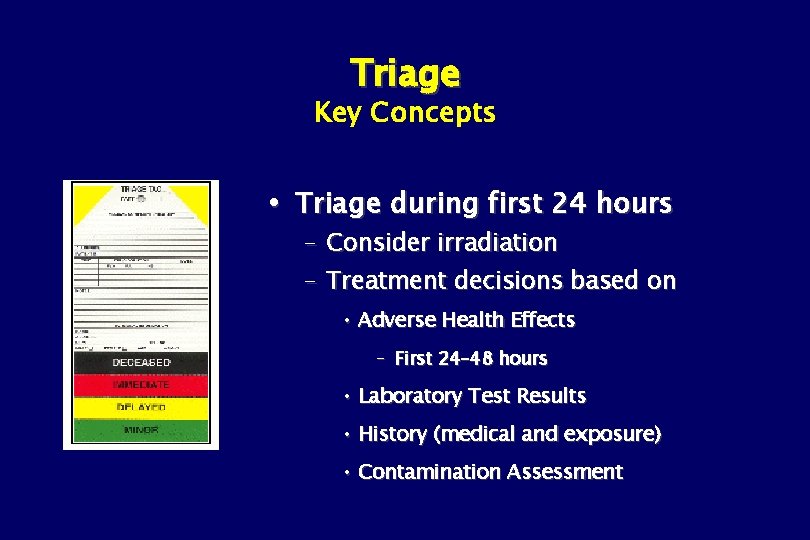
Triage Key Concepts Triage during first 24 hours – Consider irradiation – Treatment decisions based on • Adverse Health Effects – First 24– 48 hours • Laboratory Test Results • History (medical and exposure) • Contamination Assessment

Adverse Health Effects Acute Radiation Syndrome (ARS) ARS caused by irradiation of whole body or significant portion thereof Adverse health effects – Type, severity, and time to onset dependent on radiation dose (i. e. , amount of energy deposited in the body)

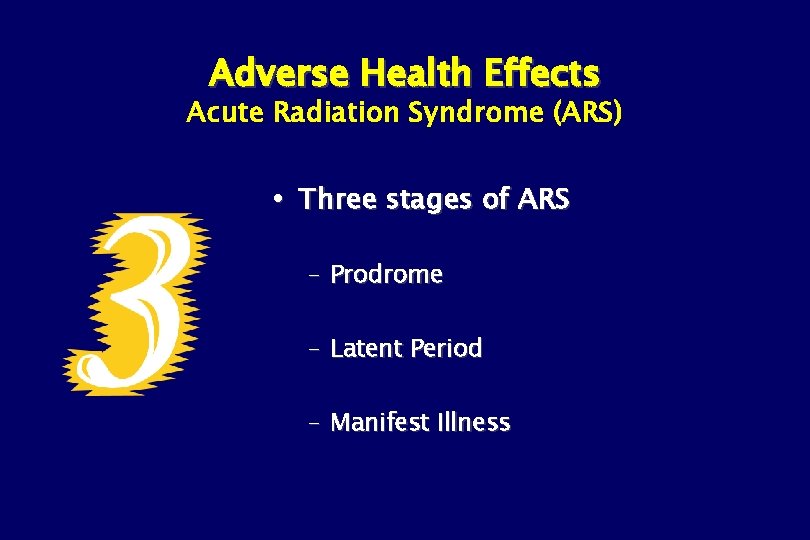
Adverse Health Effects Acute Radiation Syndrome (ARS) Three stages of ARS – Prodrome – Latent Period – Manifest Illness

Adverse Health Effects Acute Radiation Syndrome: Prodrome Begins after exposure Lasts 24– 48 hours Adverse health effects – Nausea/vomiting/diarrhea, fatigue, headache, parotitis, erythema, fever

Adverse Health Effects Acute Radiation Syndrome: Prodrome Prodromal adverse health effects – Onset occurs more rapidly with severe ARS than with mild ARS – Nausea and vomiting are hallmark • Time to emesis may be used as rough estimate of exposure and outcome

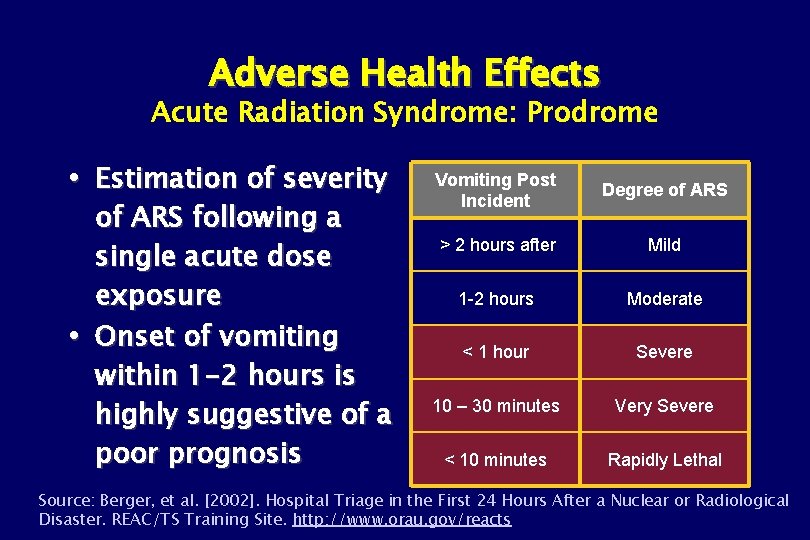
Adverse Health Effects Acute Radiation Syndrome: Prodrome Estimation of severity of ARS following a single acute dose exposure Onset of vomiting within 1 -2 hours is highly suggestive of a poor prognosis Vomiting Post Incident Degree of ARS > 2 hours after Mild 1 -2 hours Moderate < 1 hour Severe 10 – 30 minutes Very Severe < 10 minutes Rapidly Lethal Source: Berger, et al. [2002]. Hospital Triage in the First 24 Hours After a Nuclear or Radiological Disaster. REAC/TS Training Site. http: //www. orau. gov/reacts

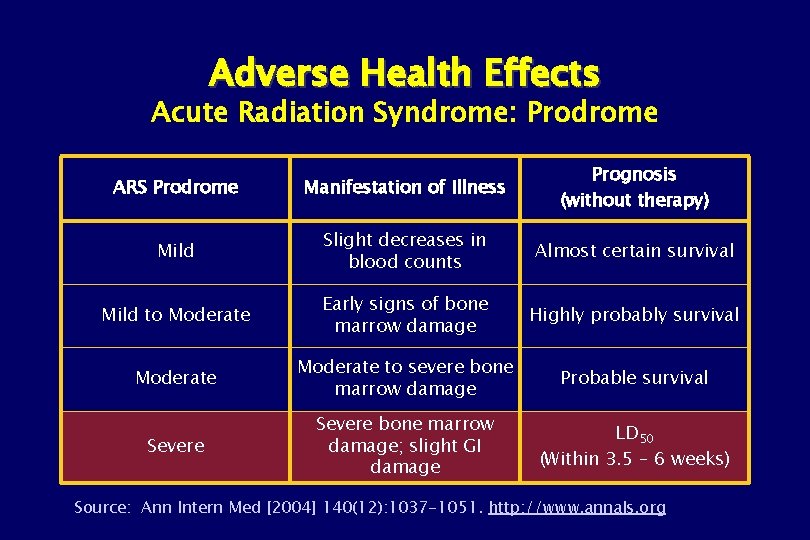
Adverse Health Effects Acute Radiation Syndrome: Prodrome ARS Prodrome Manifestation of Illness Prognosis (without therapy) Mild Slight decreases in blood counts Almost certain survival Mild to Moderate Early signs of bone marrow damage Highly probably survival Moderate to severe bone marrow damage Probable survival Severe bone marrow damage; slight GI damage LD 50 (Within 3. 5 – 6 weeks) Source: Ann Intern Med [2004] 140(12): 1037 -1051. http: //www. annals. org

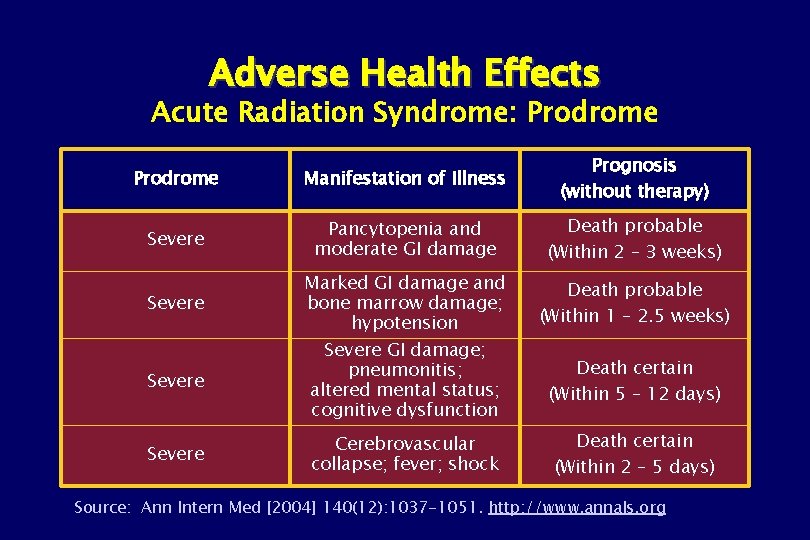
Adverse Health Effects Acute Radiation Syndrome: Prodrome Manifestation of Illness Prognosis (without therapy) Severe Pancytopenia and moderate GI damage Death probable (Within 2 – 3 weeks) Severe Marked GI damage and bone marrow damage; hypotension Death probable (Within 1 – 2. 5 weeks) Severe GI damage; pneumonitis; altered mental status; cognitive dysfunction Death certain (Within 5 – 12 days) Severe Cerebrovascular collapse; fever; shock Death certain (Within 2 – 5 days) Source: Ann Intern Med [2004] 140(12): 1037 -1051. http: //www. annals. org

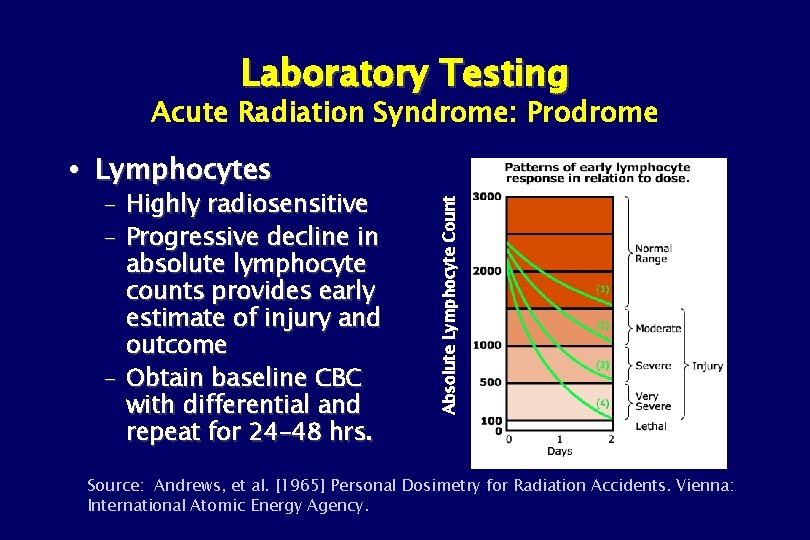
Laboratory Testing Acute Radiation Syndrome: Prodrome – Highly radiosensitive – Progressive decline in absolute lymphocyte counts provides early estimate of injury and outcome – Obtain baseline CBC with differential and repeat for 24– 48 hrs. Absolute Lymphocyte Count Lymphocytes Source: Andrews, et al. [1965] Personal Dosimetry for Radiation Accidents. Vienna: International Atomic Energy Agency.

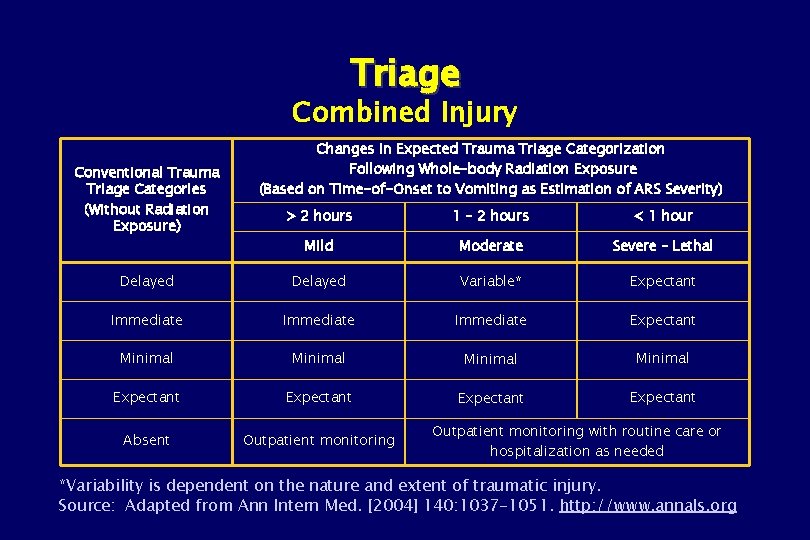
Triage Combined Injury Conventional Trauma Triage Categories (Without Radiation Exposure) Changes in Expected Trauma Triage Categorization Following Whole-body Radiation Exposure (Based on Time-of-Onset to Vomiting as Estimation of ARS Severity) > 2 hours 1 – 2 hours < 1 hour Mild Moderate Severe – Lethal Delayed Variable* Expectant Immediate Expectant Minimal Expectant Absent Outpatient monitoring with routine care or hospitalization as needed *Variability is dependent on the nature and extent of traumatic injury. Source: Adapted from Ann Intern Med. [2004] 140: 1037 -1051. http: //www. annals. org

Triage Wrap-Up Acute Radiation Syndrome – 3 stages – Prodome is triage key – Clinical guides to victim triage • Time to prodrome onset (vomiting) • Total lymphocyte count – Combined injury generally means a worse prognosis

Radiological Terrorism Internal Contamination Time-dependent phenomenon Related to physical properties of isotope Incorporation can occur rapidly

Radiological Terrorism Internal Contamination: Diagnosis Exposure history – Potential for inhalation or ingestion Open wounds containing shrapnel Bioassay – Complete Blood Count – Urinalysis for radiation

Radiological Terrorism Internal Contamination: Treatment most effective when administered early – Decision to treat may be based on preliminary data Block or decorporate and treat effects of exposure

Radiological Terrorism Internal Contamination: Treatment Anti-emetics as necessary – Ondansetron, granisetron, or other serotonin receptor antagonists Anti-diarrheals – Loperamide hydrochloride, Lonox (diphenoxylate/atropine) Replace fluids and electrolytes as necessary

Pharmacotherapy Potassium Iodide IOSAT™; Thyro. Safe™; Thyro-Block™ Thyro. Shield™ solution for children Orally administered radioactive iodine blocking agent Prevents radioactive iodine uptake in thyroid gland by competing for binding sites

Pharmacotherapy Potassium Iodide Radioactive iodine release scenarios – Nuclear power plant incident – Detonation of improvised nuclear device – “Dirty bomb” is unlikely source

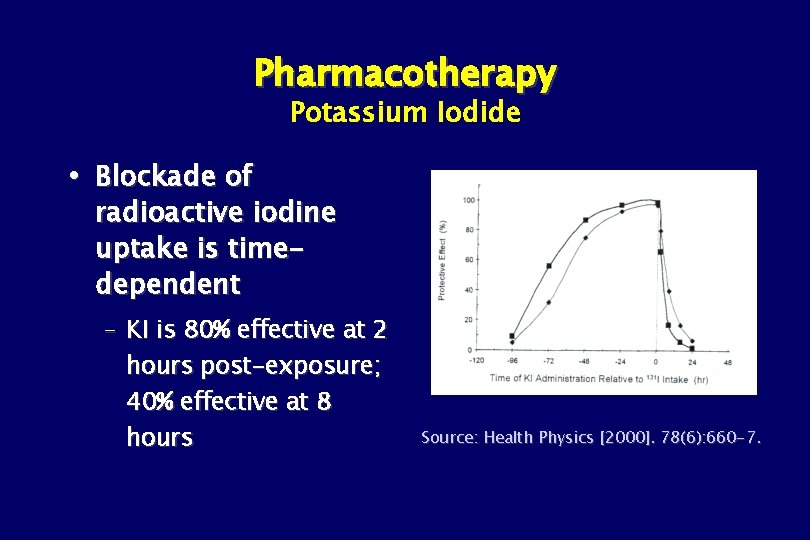
Pharmacotherapy Potassium Iodide Blockade of radioactive iodine uptake is timedependent – KI is 80% effective at 2 hours post-exposure; 40% effective at 8 hours Source: Health Physics [2000]. 78(6): 660 -7.

Pharmacotherapy Potassium Iodide Thyroid of fetus and young children more sensitive to carcinogenic effects of radioiodine – Radioiodine uptake inversely proportional to thyroid size

Pharmacotherapy Potassium Iodide Administration guidance available from US Food and Drug Administration (FDA) Guidance based on prevention of thyroid cancer Source: Potassium Iodide as a Thyroid Blocking Agent in Radiation Emergencies [2001]. http: //www. fda. gov/cder/guidance/4825 fnl. pdf

Pharmacotherapy Prussian Blue Ferric (III) hexacyanoferrate (II) (Radiogardase®) Orally administered decorporation agent (capsules) Promotes fecal excretion of radioactive cesium and thallium

Pharmacotherapy Prussian Blue Binds isotopes in gastrointestinal tract via ion-exchange, adsorption, and mechanical trapping; limits entero-hepatic recirculation Does not treat complications of radiation exposure – Need concomitant supportive care

Pharmacotherapy Prussian Blue Initiate treatment as soon as possible Treat for minimum of 30 days – Duration based on level of contamination Additional guidance – http: //www. fda. gov/cder/drug/infopage/prussian_blue/default. htm – http: //www. bt. cdc. gov/radiation/prussianblue. asp – http: //www. nlm. nih. gov/medlineplus/druginfo/uspdi/202737. html – http: //www. orau. gov/reacts/prussian. htm

Pharmacotherapy DTPA Diethylenetriaminepentaacetate – Calcium (Ca) and zinc (Zn) salts – Intravenous chelating agents for • Plutonium, Americium, Curium – DO NOT USE for • Uranium, Neptunium

Pharmacotherapy DTPA Ca-DTPA – Administration within 6 hours of exposure is most effective – Initially 10 x more effective than Zn. DTPA • 24 hours post-exposure Ca-DTPA and Zn-DTPA have equivalent efficacy – Causes depletion of zinc and magnesium

Pharmacotherapy DTPA Ca-DTPA contraindications – Minors, pregnant women, serious kidney disease, bone marrow suppression – Check renal function prior to each administration

Pharmacotherapy DTPA Additional Guidance – http: //www. fda. gov/cder/drug/infopage/dtpa/default. htm – http: //www. orau. gov/reacts/calcium. htm – http: //www. orau. gov/reacts/zinc. htm – http: //www. bt. cdc. gov/radiation/dtpa. asp

Pharmacotherapy Filgrastim (Neupogen®) Colony Stimulating Factors (CSF) – Endogenous glycoproteins – Induce hematopoietic progenitor cells of bone marrow to proliferate and differentiate into specific mature blood cell types
![Pharmacotherapy Filgrastim Neupogen Filgrastim GranulocyteCSF GCSF Genetically engineered protein Daily IV or Pharmacotherapy Filgrastim (Neupogen®) Filgrastim [Granulocyte-CSF (G-CSF)] – Genetically engineered protein – Daily IV or](https://slidetodoc.com/presentation_image_h2/125426e86795b71ac46cfd24f991d175/image-33.jpg)
Pharmacotherapy Filgrastim (Neupogen®) Filgrastim [Granulocyte-CSF (G-CSF)] – Genetically engineered protein – Daily IV or IM administration – Minimal effect on hematopoietic cell types other than neutrophil progenitors

Pharmacotherapy Filgrastim (Neupogen®) FDA-approved for treatment of neutropenia resulting from myelosuppressive cancer therapy Not approved for treatment of ARS – Used as investigational drug for persons with unintentional radiological exposures

Pharmacotherapy Filgrastim (Neupogen®) Side effects – Allergic reactions (< 1 in 4000 patients) • Good response to antihistamines, steroids, bronchodilators and/or epinephrine • ~50% have recurrence on re-exposure – Fatal and non-fatal splenic rupture – Severe sickle cell crises in patients with sickle cell disease

Pharmacotherapy Filgrastim (Neupogen®) Additional Guidance – http: //www. bt. cdc. gov/radiation/neupogenfacts. asp – http: //www. accessdata. fda. gov/scripts/cder/onctools/labels. cfm? GN=Filgrastim

Treatment Wrap-Up Pharmacotherapy must be administered quickly after exposure Match the treatment to the exposure Guidance is available from the FDA and CDC concerning appropriate administration
 Suzanne meade
Suzanne meade Casualties
Casualties World war 2 countries
World war 2 countries Center for devices and radiological health
Center for devices and radiological health National radiological emergency preparedness conference
National radiological emergency preparedness conference Radiological dispersal device
Radiological dispersal device Tennessee division of radiological health
Tennessee division of radiological health Terrorism definition
Terrorism definition Terrorism
Terrorism Terrorism
Terrorism Nato definition of terrorism
Nato definition of terrorism Arizona counter terrorism information center
Arizona counter terrorism information center Liberals vs conservatives apush
Liberals vs conservatives apush Natural response and forced response
Natural response and forced response Natural response and forced response example
Natural response and forced response example A subsequent
A subsequent Ela regents rubric
Ela regents rubric As part of a project about response bias ellery
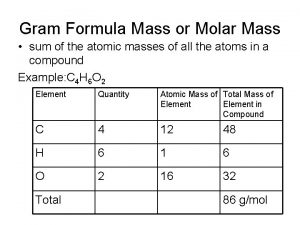
As part of a project about response bias ellery Mass to mass formula
Mass to mass formula Atomic mass and mass number
Atomic mass and mass number Relative atomic mass of beryllium
Relative atomic mass of beryllium How to find percent mass
How to find percent mass Inertial mass vs gravitational mass
Inertial mass vs gravitational mass Mass/molar mass
Mass/molar mass Molecules to mass
Molecules to mass Molar mass units
Molar mass units Example of mole
Example of mole What are the units of molar mass
What are the units of molar mass Mass/mass problems
Mass/mass problems Difference between atomic mass and atomic number
Difference between atomic mass and atomic number Gravitational mass vs inertial mass
Gravitational mass vs inertial mass Gram formula mass
Gram formula mass Formula mass vs molecular mass
Formula mass vs molecular mass How to find the atomicity of a molecule
How to find the atomicity of a molecule Mass number formula
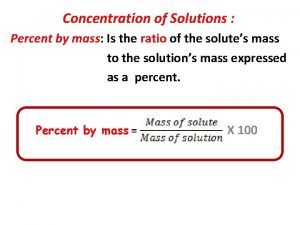
Mass number formula Mass to mass percent
Mass to mass percent Inertial mass vs gravitational mass
Inertial mass vs gravitational mass Co2 relative molecular mass
Co2 relative molecular mass