Introduction to the Principles of Flow Cytometry for










- Slides: 59

Introduction to the Principles of Flow Cytometry for users of the Flow Cytometry Core Facility at BUMC 21 September 2010 Mike Xie (X 4 -5225), mike. xie@bmc. org Yan Deng (X 4 -5225), ydeng@bu. edu John Meyers (X 8 -7543), johnm@bu. edu Gerald Denis (X 4 -1371), gdenis@bu. edu

Flow Cytometry Core Facility: Personnel Director of Cell Sorting Services: Yanhui Deng Operations Manager: Mike Xie Facility Consultant: John Meyers Co-Director/Outreach: Gerald Denis Director: David Sherr http: //www. bu. edu/cores/flow-cytometry

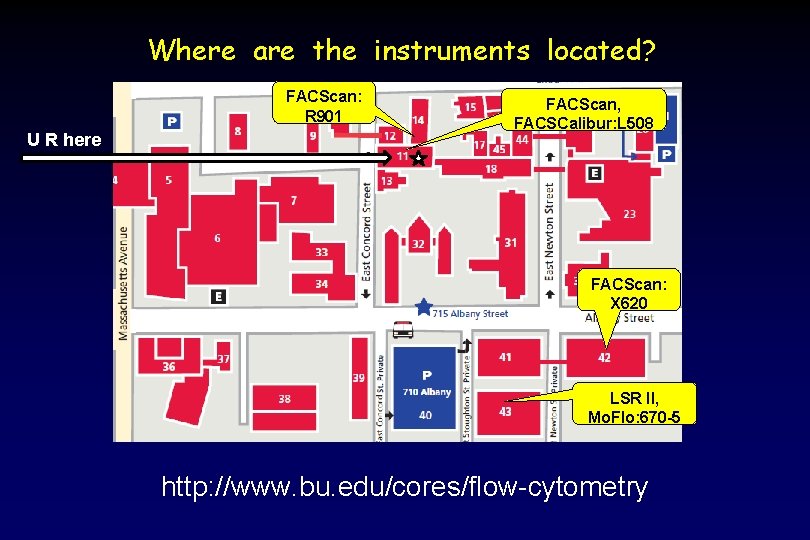
Where are the instruments located? FACScan: R 901 U R here FACScan, FACSCalibur: L 508 FACScan: X 620 LSR II, Mo. Flo: 670 -5 http: //www. bu. edu/cores/flow-cytometry


Flow cytometry was not easy in the old days ungloved hands missing PPE gendered division of labor live anthrax bacilli

But today… it’s as easy as PCR.

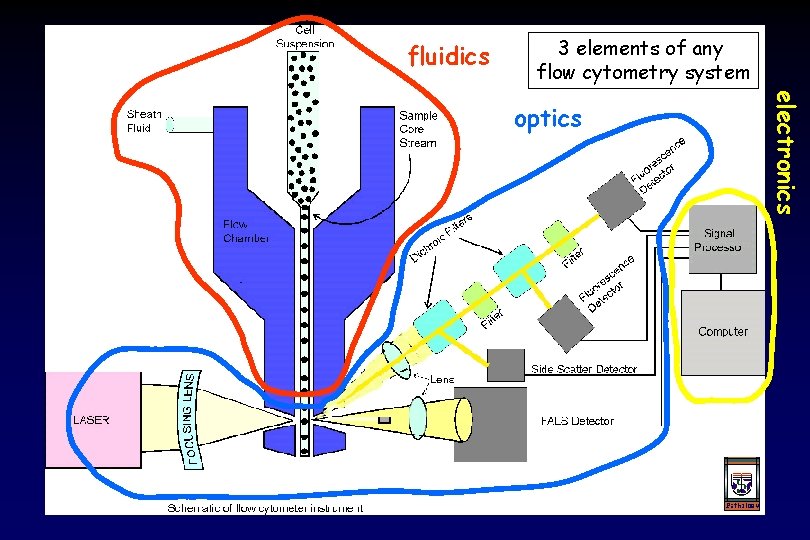
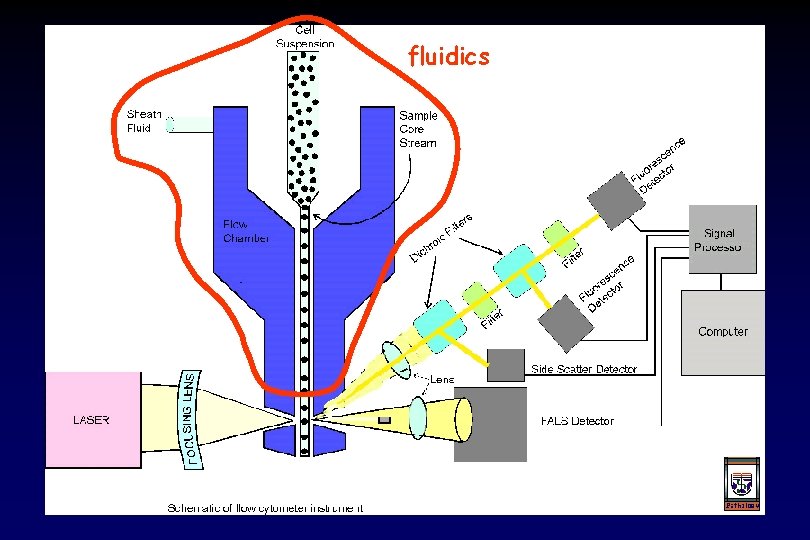
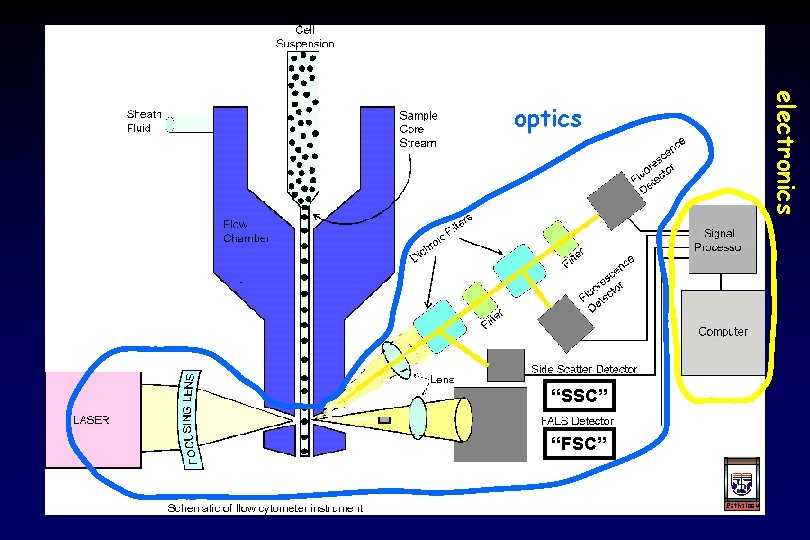
fluidics 3 elements of any flow cytometry system electronics optics

fluidics

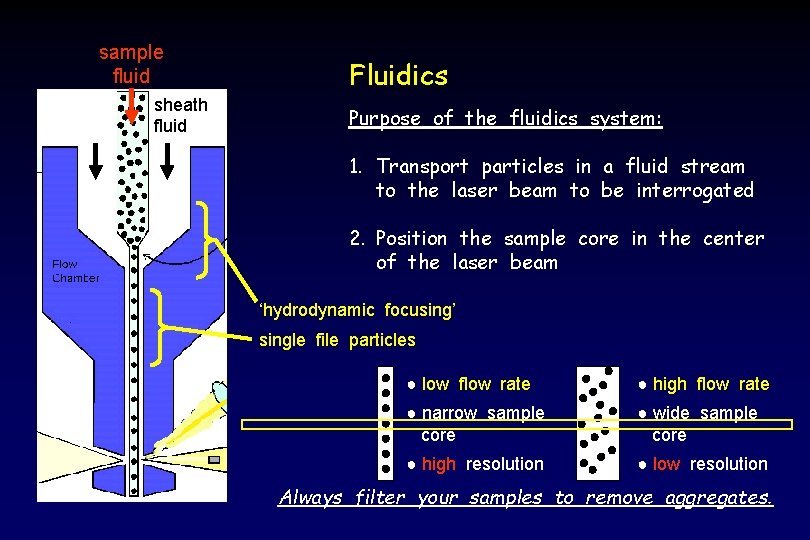
sample fluid sheath fluid Fluidics Purpose of the fluidics system: 1. Transport particles in a fluid stream to the laser beam to be interrogated 2. Position the sample core in the center of the laser beam ‘hydrodynamic focusing’ single file particles ● low flow rate ● high flow rate ● narrow sample core ● wide sample core ● high resolution ● low resolution Always filter your samples to remove aggregates.

Fluidics When conditions are right (i. e. when turbulence is minimal): sample fluid flows in a central core does not mix with the sheath fluid This is termed ‘laminar flow’

“SSC” “FSC” electronics optics

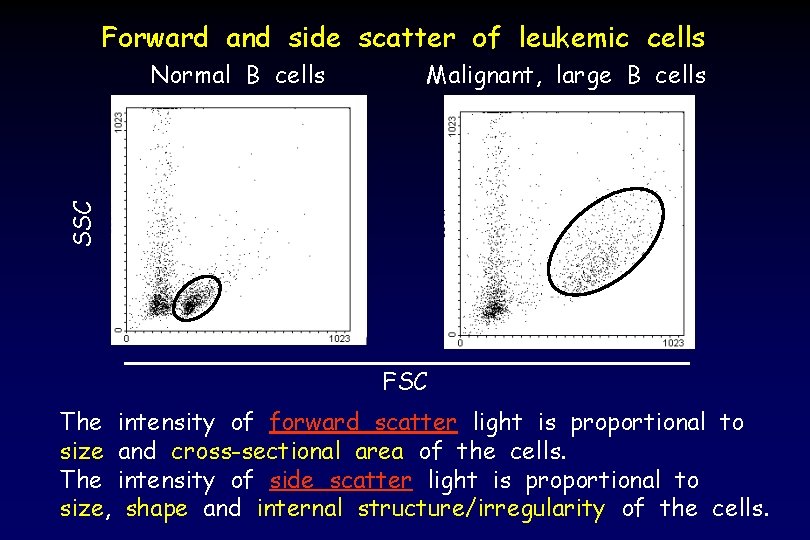
Forward and side scatter of leukemic cells Malignant, large B cells SSC Normal B cells FSC The intensity of forward scatter light is proportional to size and cross-sectional area of the cells. The intensity of side scatter light is proportional to size, shape and internal structure/irregularity of the cells.

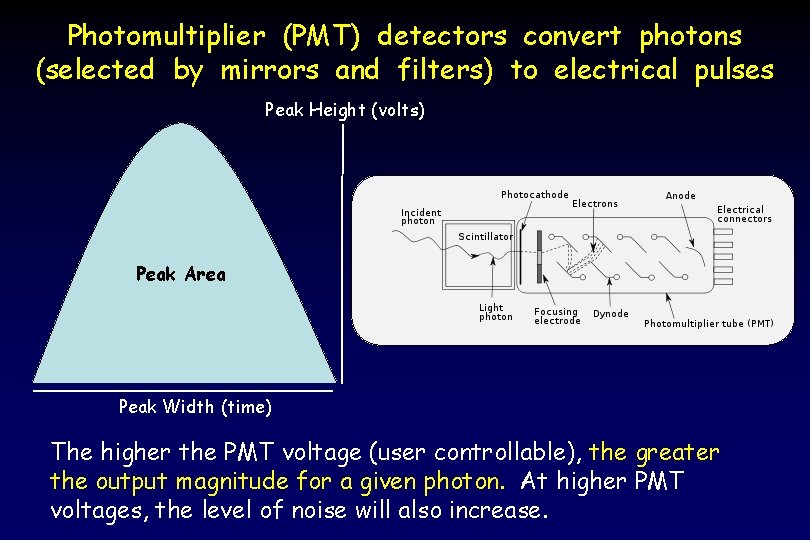
Photomultiplier (PMT) detectors convert photons (selected by mirrors and filters) to electrical pulses Peak Height (volts) Peak Area Peak Width (time) The higher the PMT voltage (user controllable), the greater the output magnitude for a given photon. At higher PMT voltages, the level of noise will also increase.

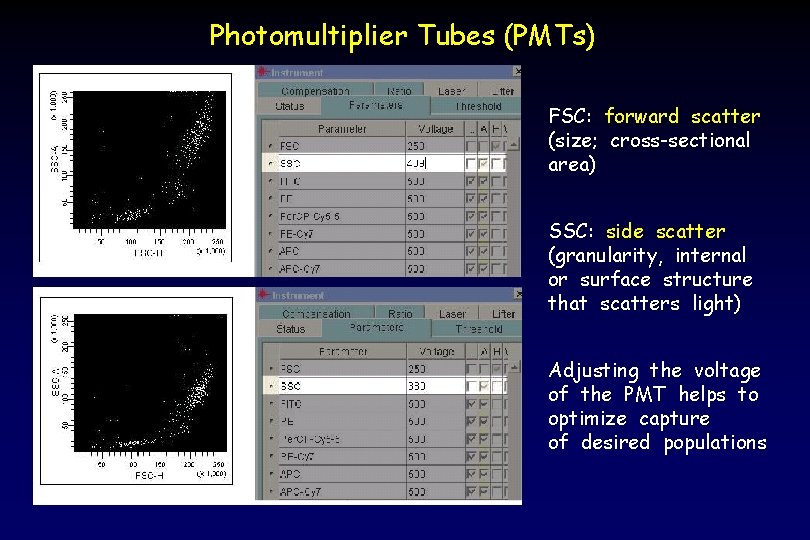
Photomultiplier Tubes (PMTs) FSC: forward scatter (size; cross-sectional area) SSC: side scatter (granularity, internal or surface structure that scatters light) Adjusting the voltage of the PMT helps to optimize capture of desired populations

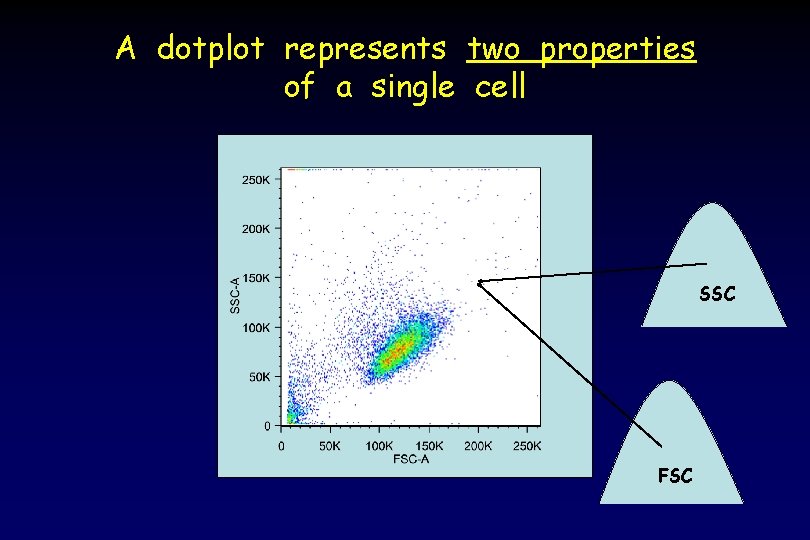
A dotplot represents two properties of a single cell SSC FSC

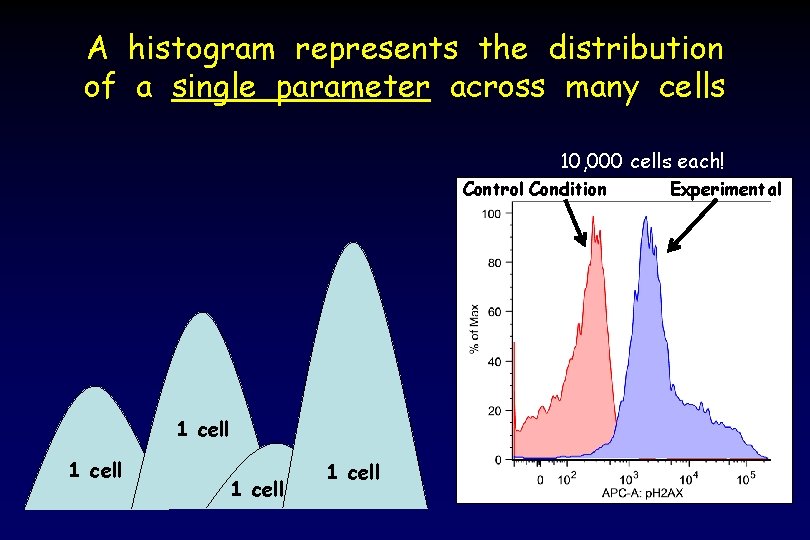
A histogram represents the distribution of a single parameter across many cells 10, 000 cells each! Control Condition 1 cell Experimental

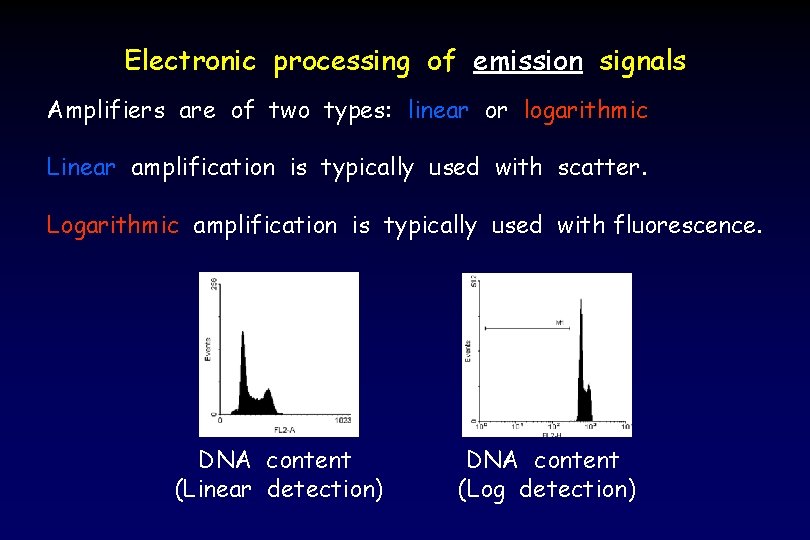
Electronic processing of emission signals Amplifiers are of two types: linear or logarithmic Linear amplification is typically used with scatter. Logarithmic amplification is typically used with fluorescence. DNA content (Linear detection) DNA content (Log detection)

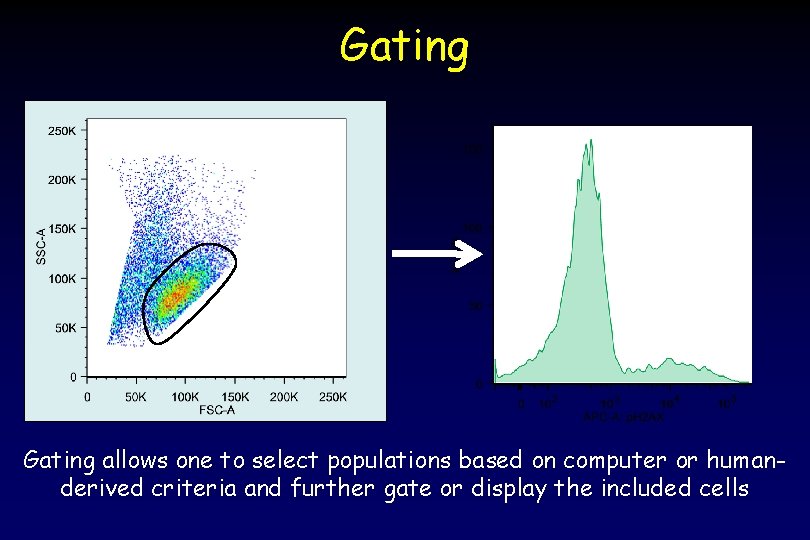
Gating allows one to select populations based on computer or humanderived criteria and further gate or display the included cells

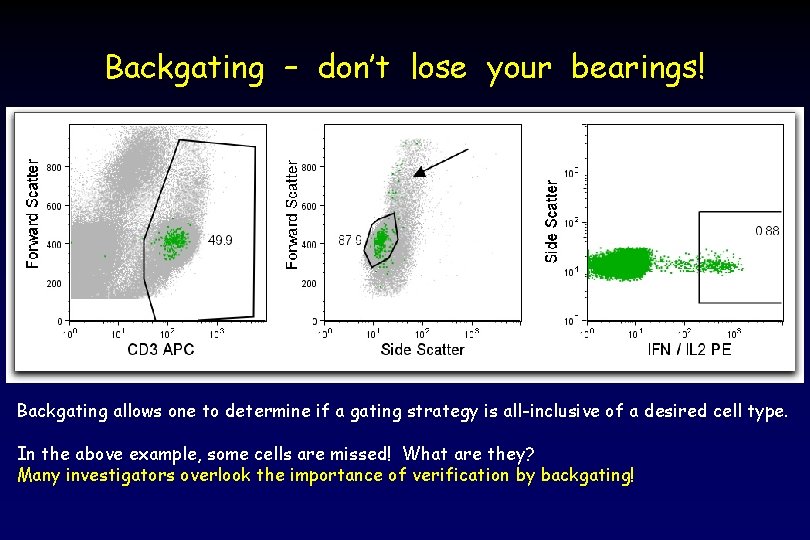
Backgating – don’t lose your bearings! Backgating allows one to determine if a gating strategy is all-inclusive of a desired cell type. In the above example, some cells are missed! What are they? Many investigators overlook the importance of verification by backgating!

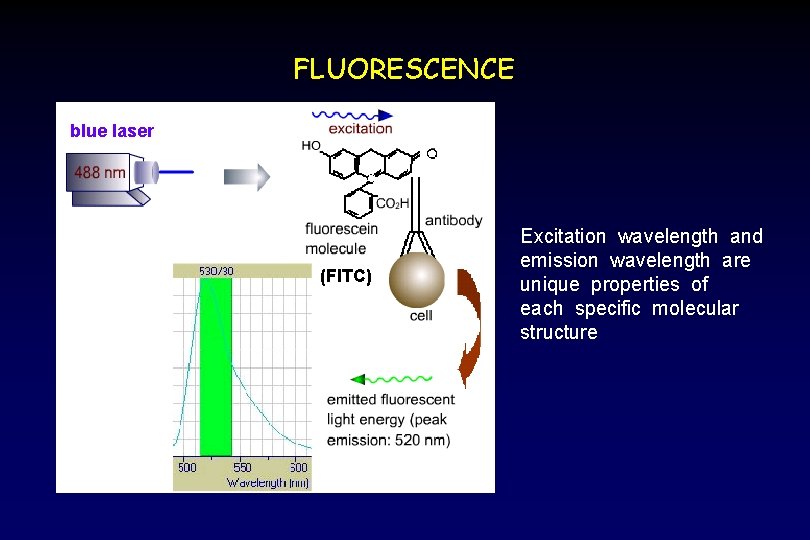
FLUORESCENCE blue laser (FITC) Excitation wavelength and emission wavelength are unique properties of each specific molecular structure

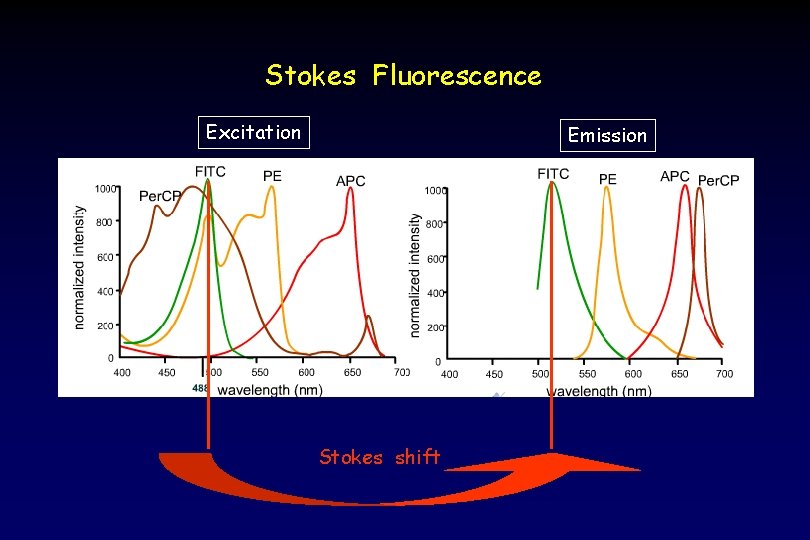
Stokes Fluorescence Excitation Emission Stokes shift

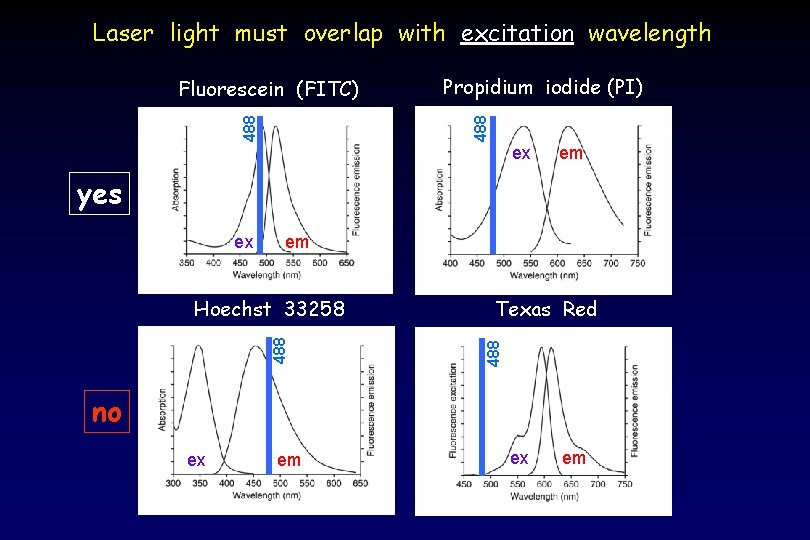
Laser light must overlap with excitation wavelength Propidium iodide (PI) 488 Fluorescein (FITC) ex em yes em 488 Hoechst 33258 Texas Red 488 ex no ex em

Widely-used molecules are excited by the 488 nm laser (FACScan) 488 nm (Blue) : FITC, GFP, PE, Per. CP, PE-Cy 5, PI, Per. CP-Cy 5. 5, PE-Cy 7 But different lasers are available to excite other molecules (LSR II) 355 405 561 633 nm nm (UV) : Indo-1, DAPI, Alexa Fluor 350, Hoechst 33258 (Violet) : Alexa Fluor 430, Alexa Fluor 405, Pacific Blue (Yellow/Green): Texas Red, Cherry Red, Tomato Red (Red): APC, APC-Cy 7, Alexa Fluor 647, Alexa 680

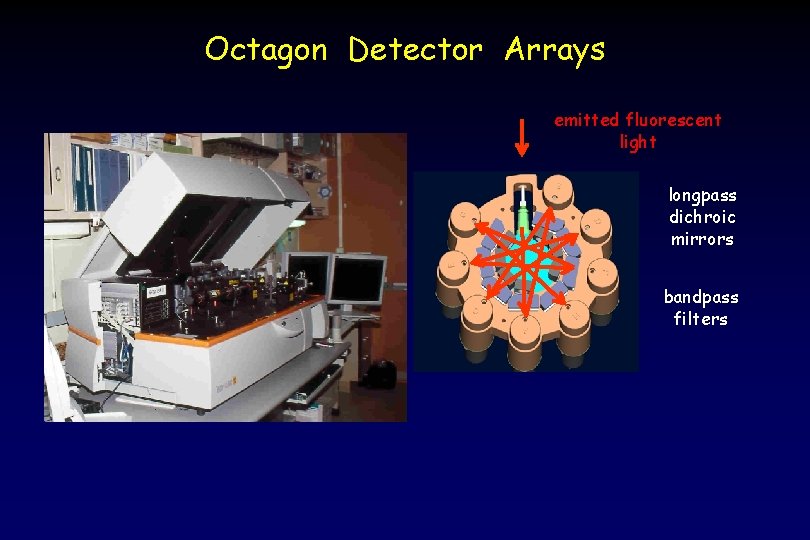
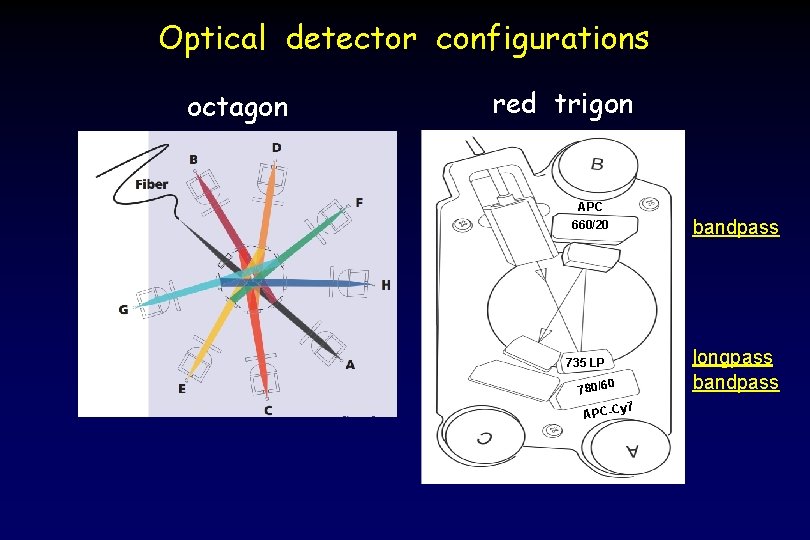
Octagon Detector Arrays emitted fluorescent light longpass dichroic mirrors bandpass filters

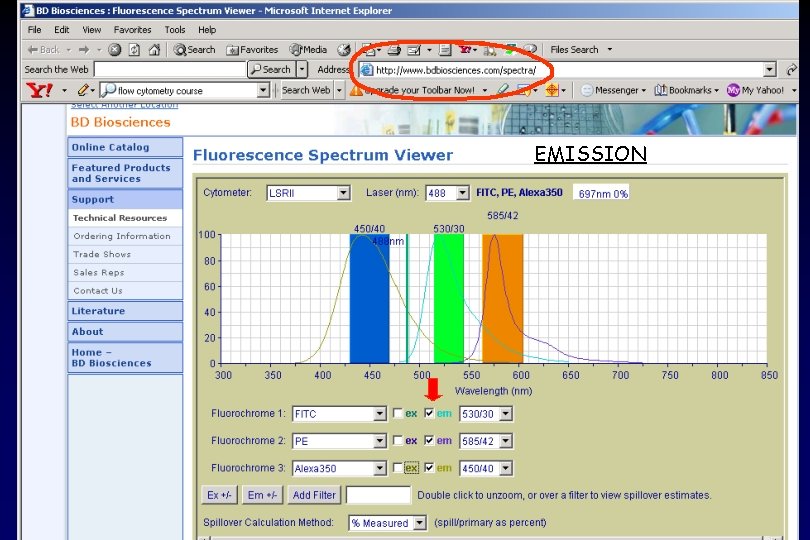
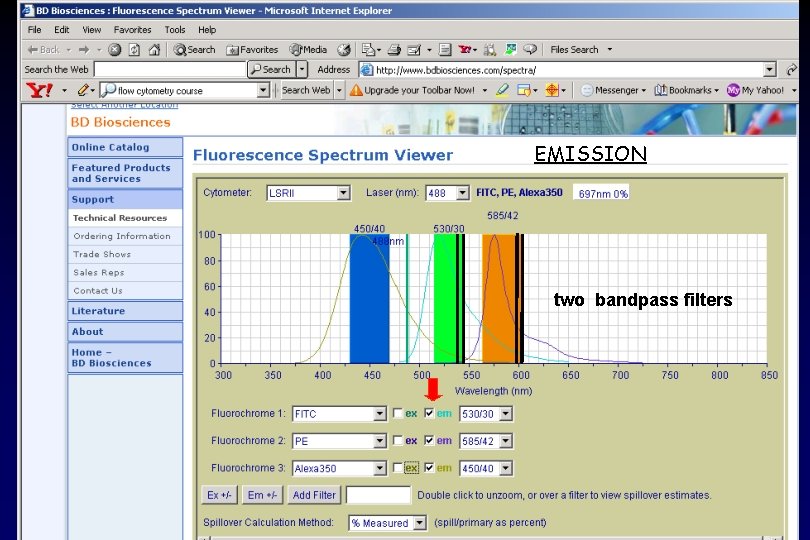
EMISSION

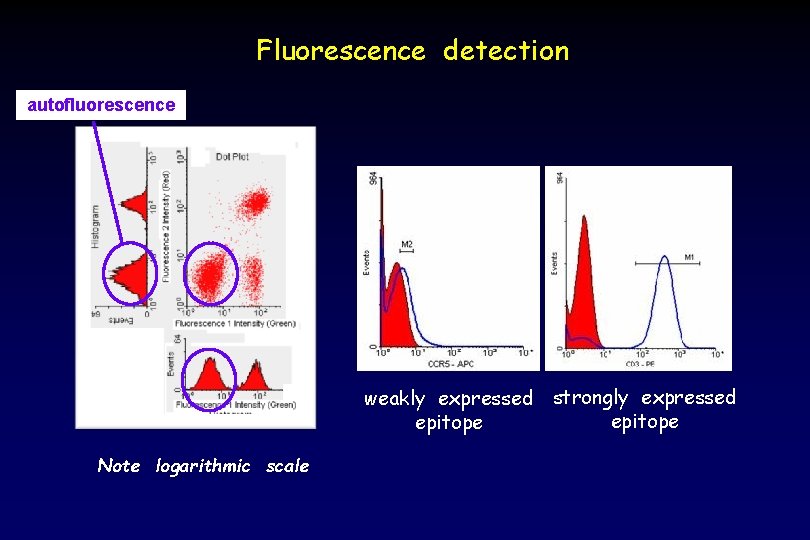
Fluorescence detection autofluorescence weakly expressed strongly expressed epitope Note logarithmic scale

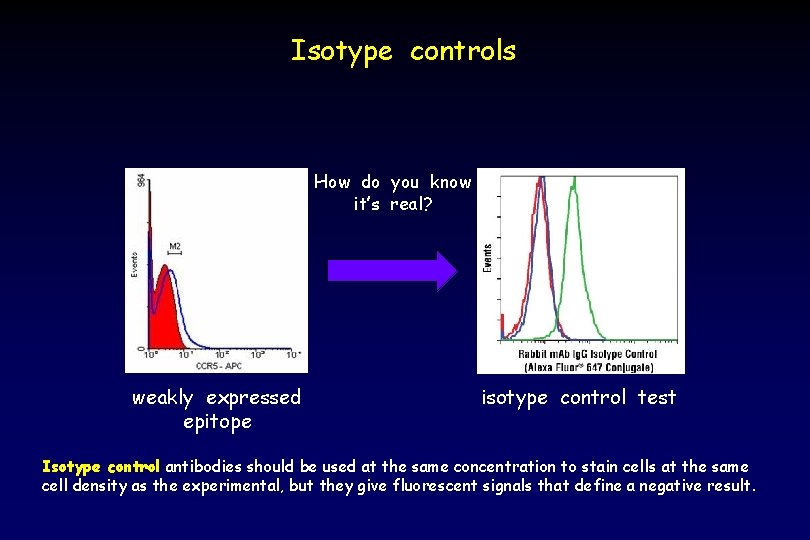
Isotype controls How do you know it’s real? weakly expressed epitope isotype control test Isotype control antibodies should be used at the same concentration to stain cells at the same cell density as the experimental, but they give fluorescent signals that define a negative result.

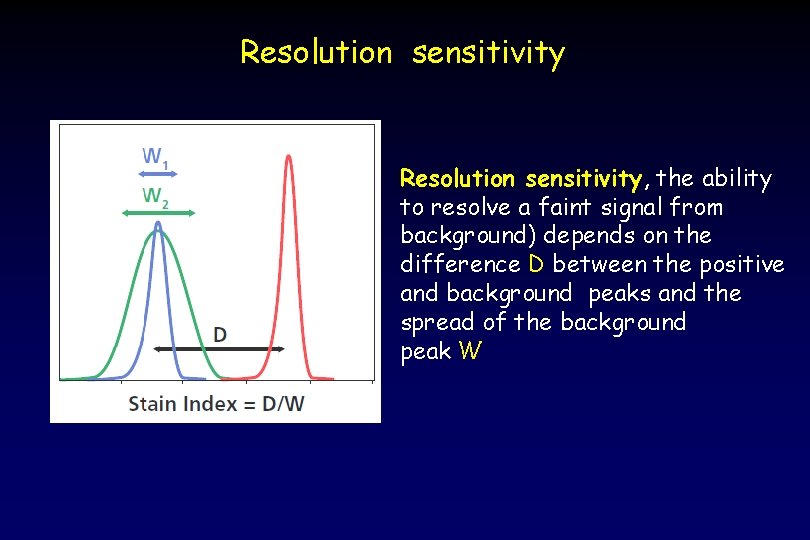
Resolution sensitivity, the ability to resolve a faint signal from background) depends on the difference D between the positive and background peaks and the spread of the background peak W

Choose the right fluor for the job! Reagent Stain Index Phycoerythrin (PE) Alexa Fluor 647 356. 3 313. 1 APC PE-Cy 7 PE-Cy 5 279. 2 278. 5 222. 1 Per. CP-Cy 5. 5 PE-Alexa Fluor 610 Alexa Fluor 488 FITC Per. CP APC-Cy 7 Alexa Fluor 700 Pacific Blue Am. Cyan 92. 7 80. 4 75. 4 68. 9 64. 4 42. 2 39. 9 22. 5 20. 2 i. e. pick a bright fluor for a dim epitope and avoid spillover of bright cell populations into detector channels that require high sensitivity for rare signals

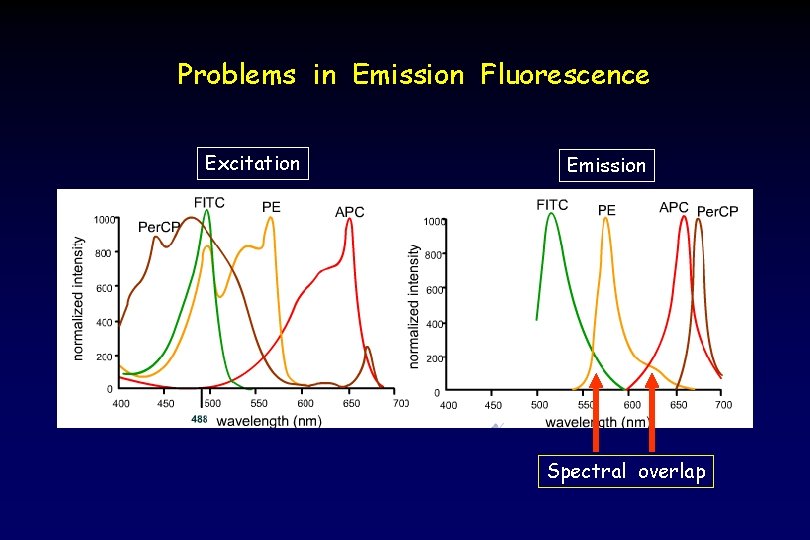
Problems in Emission Fluorescence Excitation Emission Spectral overlap

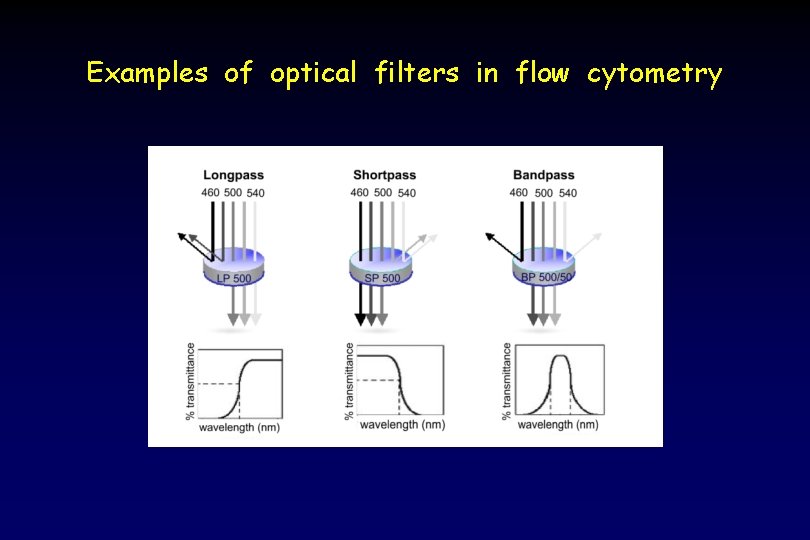
Optical solutions to spectral overlap: Filters resolve overlapping wavelengths of emitted light Longpass filter: transmits light of longer than or equal to a specific wavelength Shortpass filter: transmits light of shorter than or equal to a specific wavelength Bandpass filter: transmits light only within a narrow range of wavelengths

Examples of optical filters in flow cytometry

Optical detector configurations octagon red trigon APC 660/20 735 LP 0 780/6 y 7 APC-C bandpass longpass bandpass

EMISSION two bandpass filters

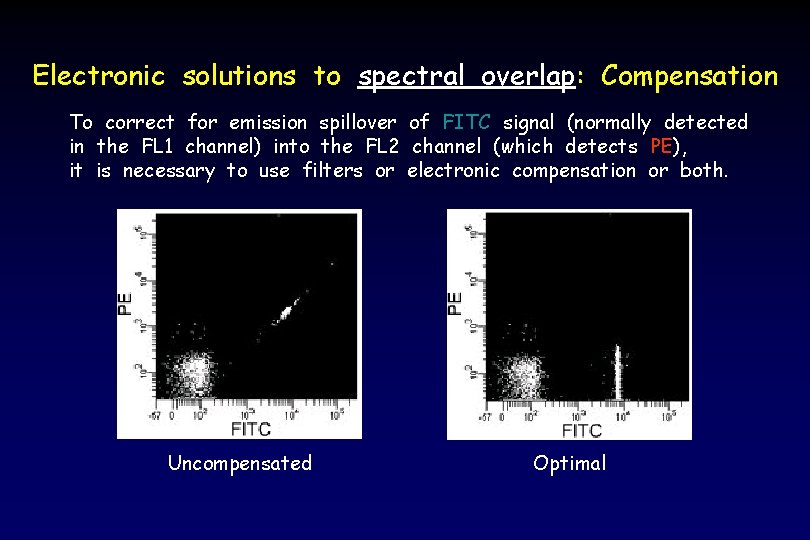
Electronic solutions to spectral overlap: Compensation To correct for emission spillover of FITC signal (normally detected in the FL 1 channel) into the FL 2 channel (which detects PE), it is necessary to use filters or electronic compensation or both. Uncompensated Optimal

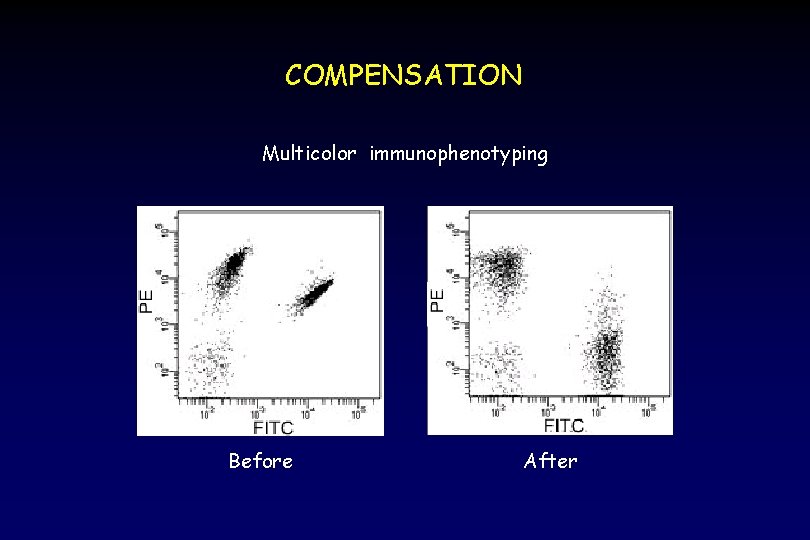
COMPENSATION Multicolor immunophenotyping Before After

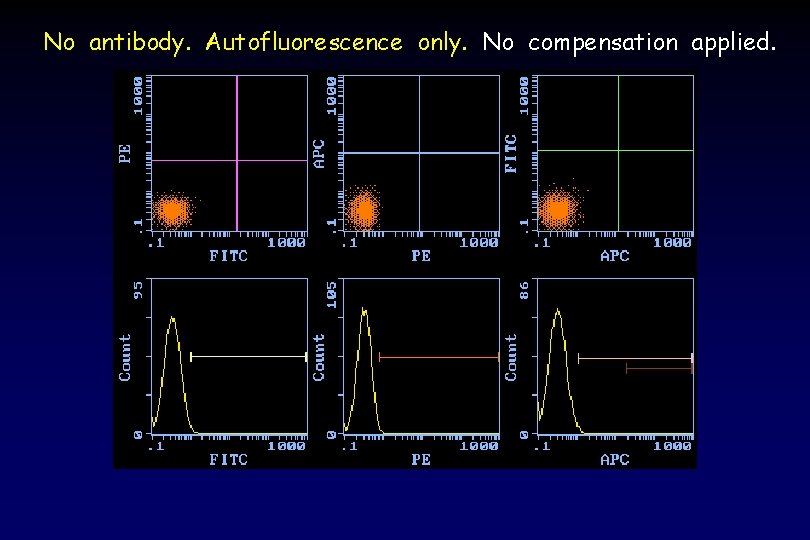
No antibody. Autofluorescence only. No compensation applied.

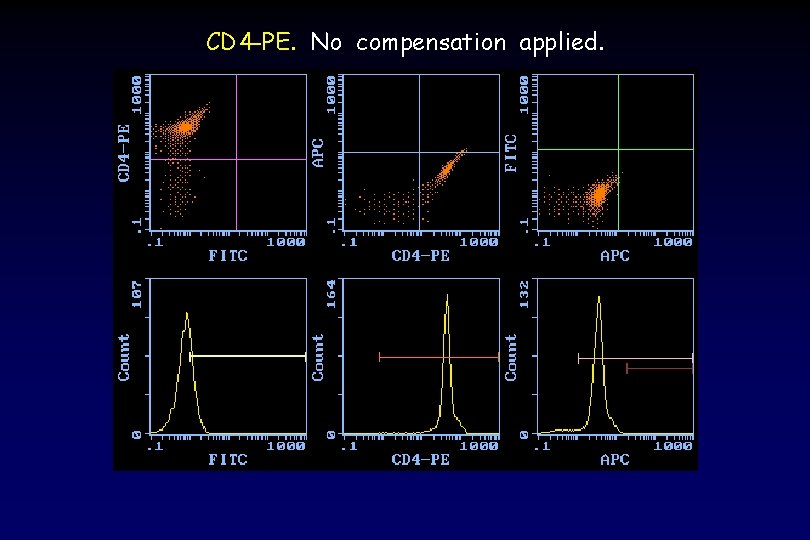
CD 4 -PE. No compensation applied.

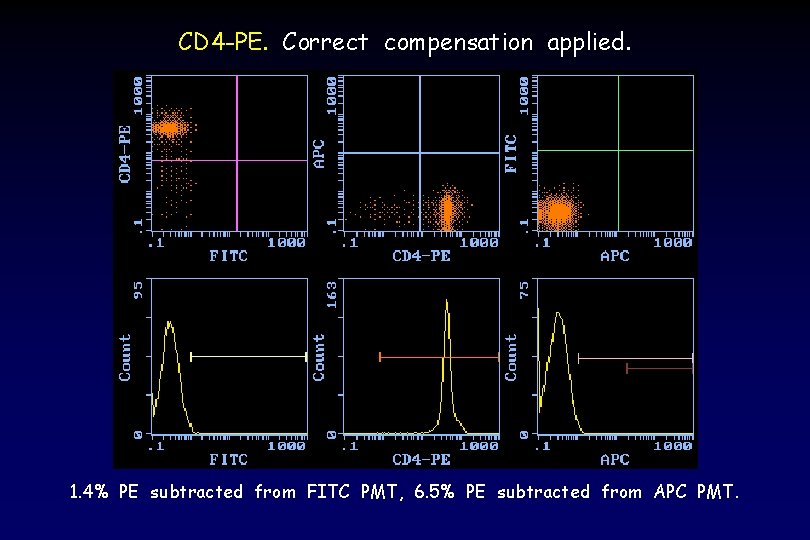
CD 4 -PE. Correct compensation applied. 1. 4% PE subtracted from FITC PMT, 6. 5% PE subtracted from APC PMT.

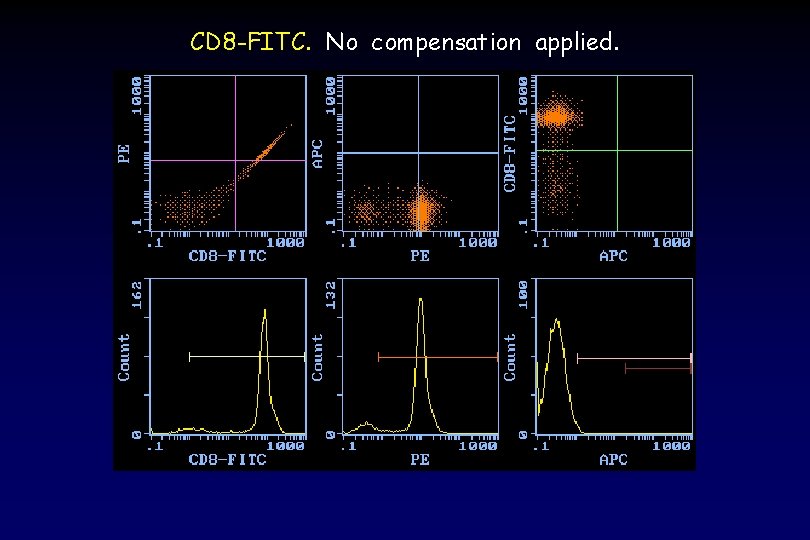
CD 8 -FITC. No compensation applied.

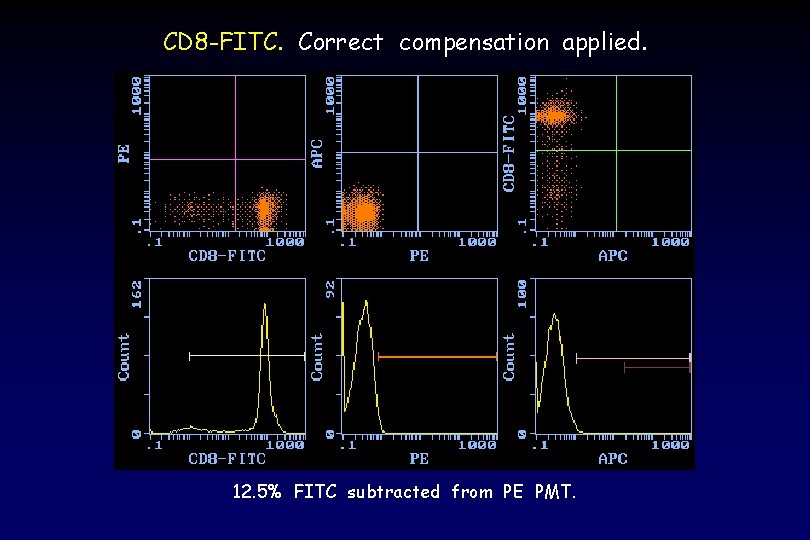
CD 8 -FITC. Correct compensation applied. 12. 5% FITC subtracted from PE PMT.

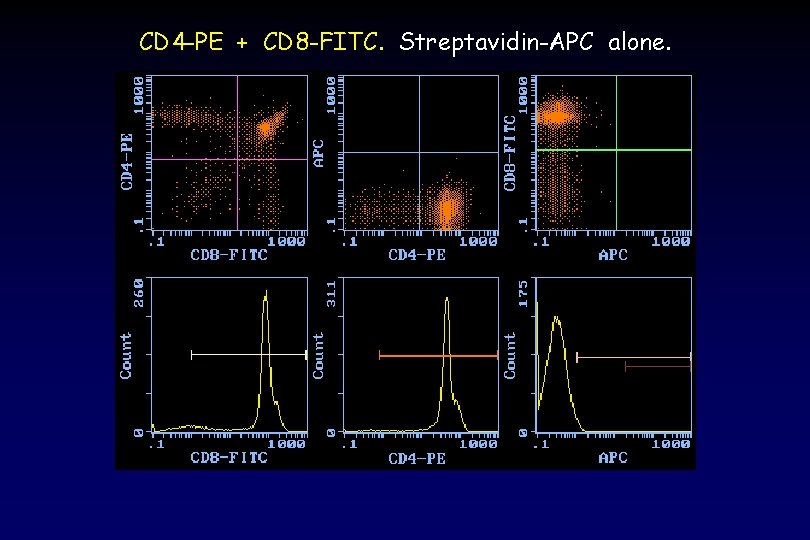
CD 4 -PE + CD 8 -FITC. Streptavidin-APC alone.

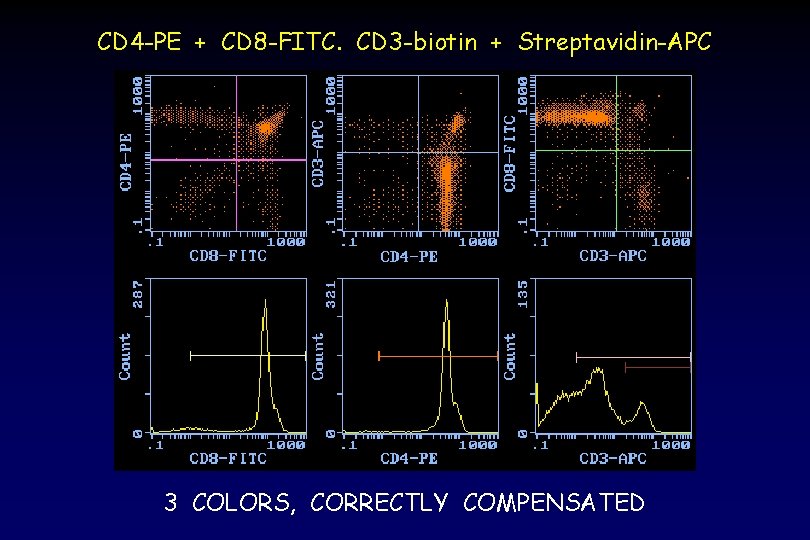
CD 4 -PE + CD 8 -FITC. CD 3 -biotin + Streptavidin-APC 3 COLORS, CORRECTLY COMPENSATED

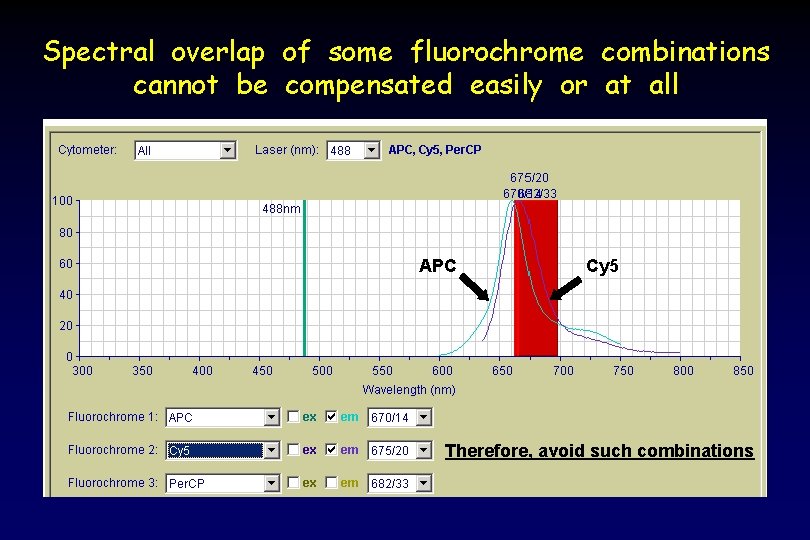
Spectral overlap of some fluorochrome combinations cannot be compensated easily or at all APC Cy 5 Therefore, avoid such combinations

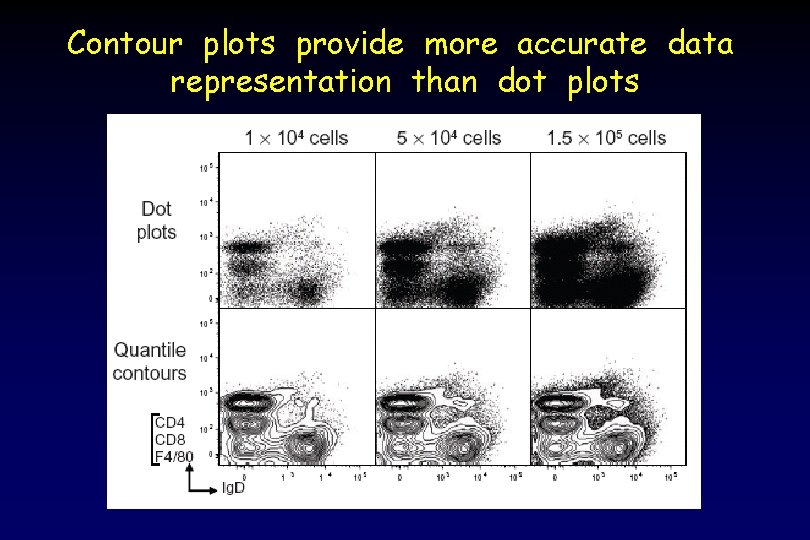
Contour plots provide more accurate data representation than dot plots

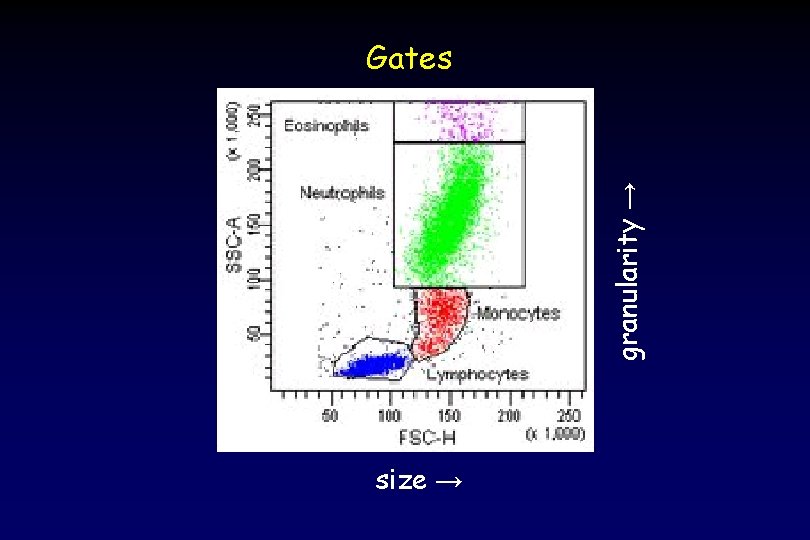
granularity → Gates size →

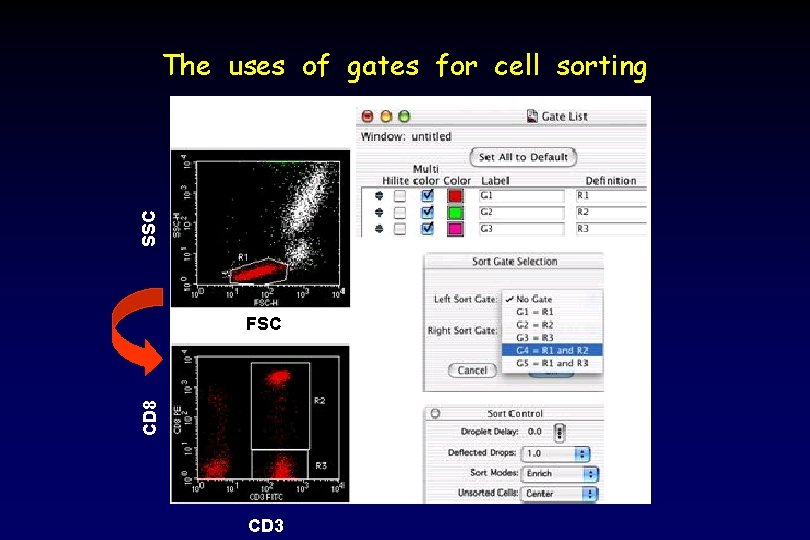
SSC The uses of gates for cell sorting CD 8 FSC CD 3

Four Applications Cell cycle analysis Multicolor immunophenotyping Phosphoprotein and kinase signaling Stem cell sorting by the “side population” method

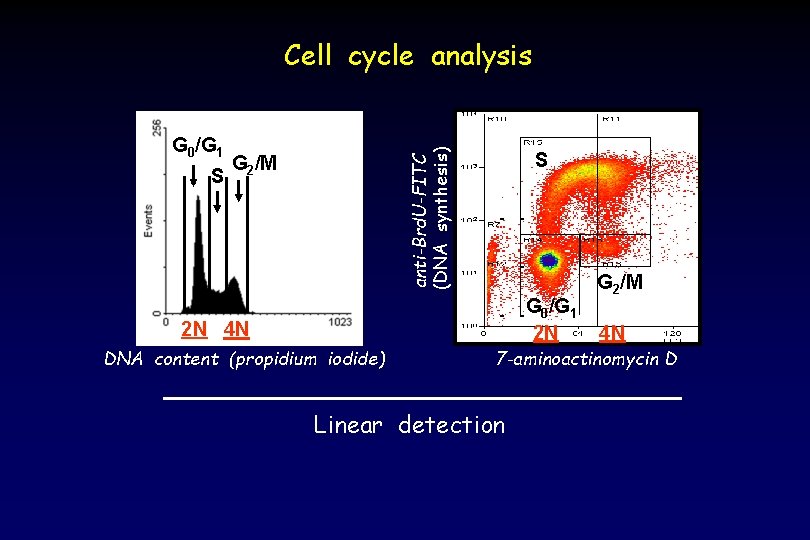
G 0/G 1 S anti-Brd. U-FITC (DNA synthesis) Cell cycle analysis G 2/M S G 0/G 1 2 N 2 N 4 N DNA content (propidium iodide) G 2/M 4 N 7 -aminoactinomycin D Linear detection

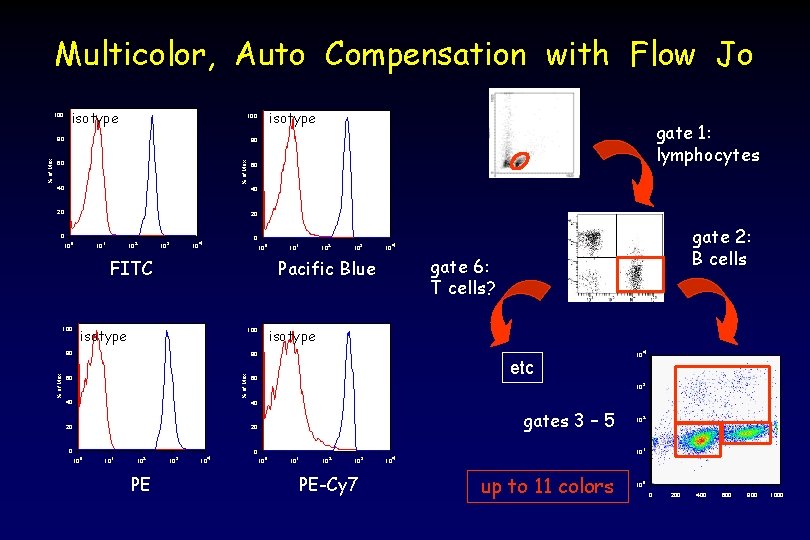
Multicolor, Auto Compensation with Flow Jo 100 isotype 80 gate 1: lymphocytes 80 % of Max 60 40 20 20 0 10 1 10 2 10 3 10 4 gate 6: T cells? Pacific Blue isotype 100 gate 2: B cells 0 10 4 FITC isotype 100 80 80 % of Max isotype 100 60 40 etc 60 10 4 10 3 40 20 gates 3 – 5 20 0 10 1 10 2 PE 10 3 10 4 10 2 10 0 10 1 10 2 10 PE-Cy 7 3 10 4 up to 11 colors 10 0 0 200 400 600 800 1000

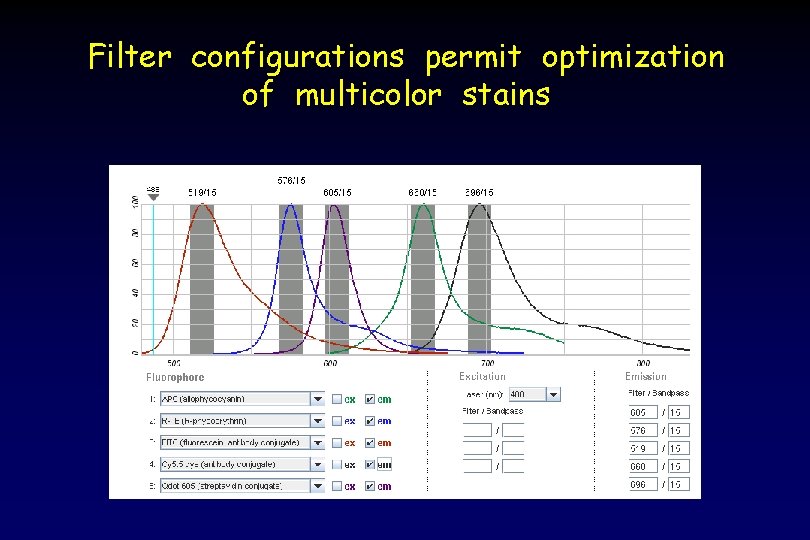
Filter configurations permit optimization of multicolor stains

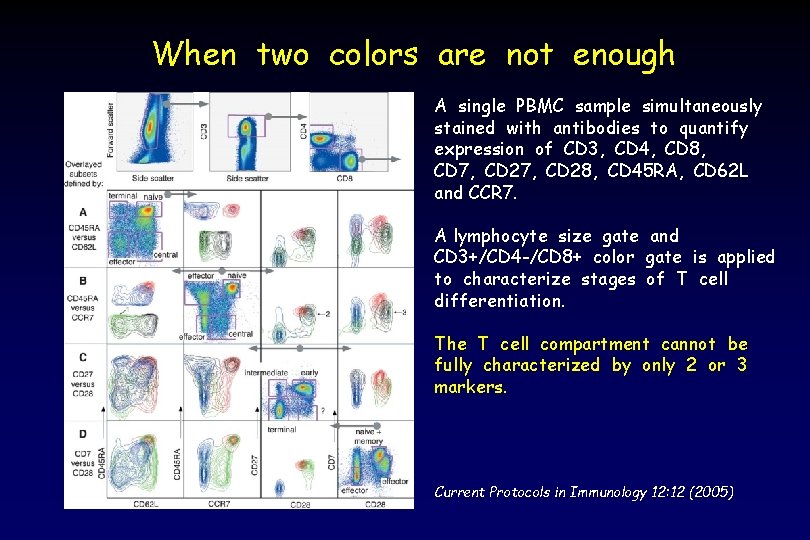
When two colors are not enough A single PBMC sample simultaneously stained with antibodies to quantify expression of CD 3, CD 4, CD 8, CD 7, CD 28, CD 45 RA, CD 62 L and CCR 7. A lymphocyte size gate and CD 3+/CD 4 -/CD 8+ color gate is applied to characterize stages of T cell differentiation. The T cell compartment cannot be fully characterized by only 2 or 3 markers. Current Protocols in Immunology 12: 12 (2005)

Example of a 10 -color experiment on our LSR II Marker Fluorochrome B 220: B lineage Pacific Blue Ig. M: mature B cells PE-Cy 7 Ig. D: mature B cells PE CD 23: transitional B cells FITC AA 4. 1: transitional B cells APC Other markers CD 45. 1: donor phenotype Per. CP-Cy 5. 5 CD 45. 2 -Biotin: recipient phenotype Streptavidin Alexa 350 CD 11 b/Mac-1: myeloid lineage APC-Cy 7 CD 3: T lineage Qdot 605 live/dead discrimination* Invitrogen UV-activated vital dye

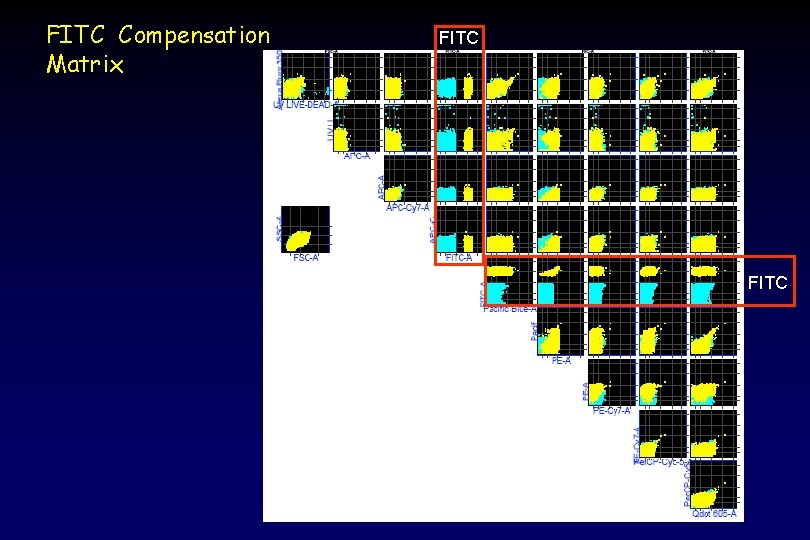
FITC Compensation Matrix FITC

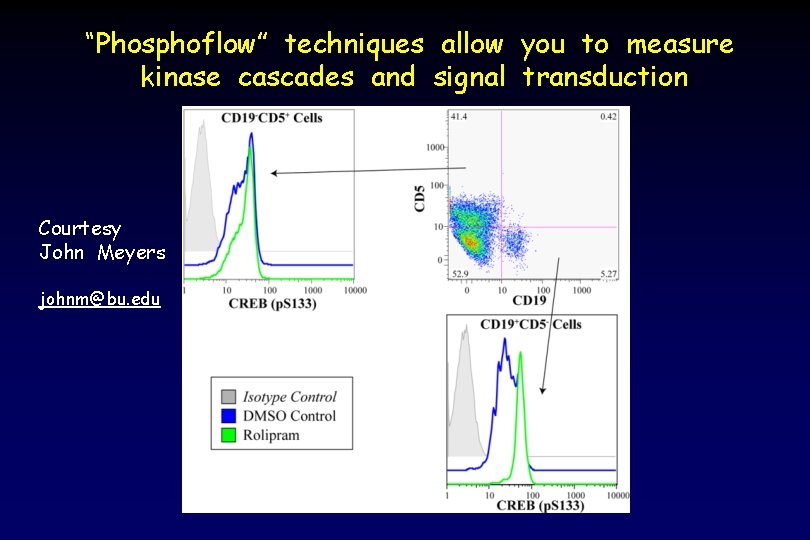
“Phosphoflow” techniques allow you to measure kinase cascades and signal transduction Courtesy John Meyers johnm@bu. edu

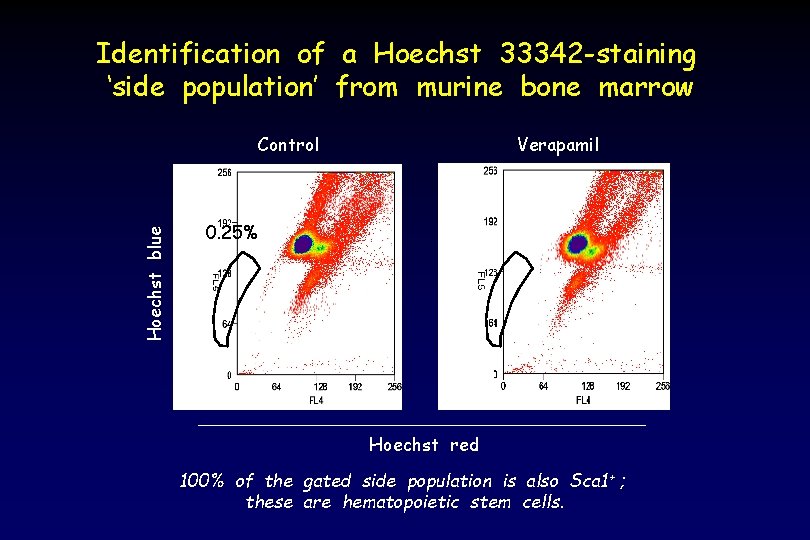
Identification of a Hoechst 33342 -staining ‘side population’ from murine bone marrow Hoechst blue Control Verapamil 0. 25% Hoechst red 100% of the gated side population is also Sca 1 + ; these are hematopoietic stem cells.

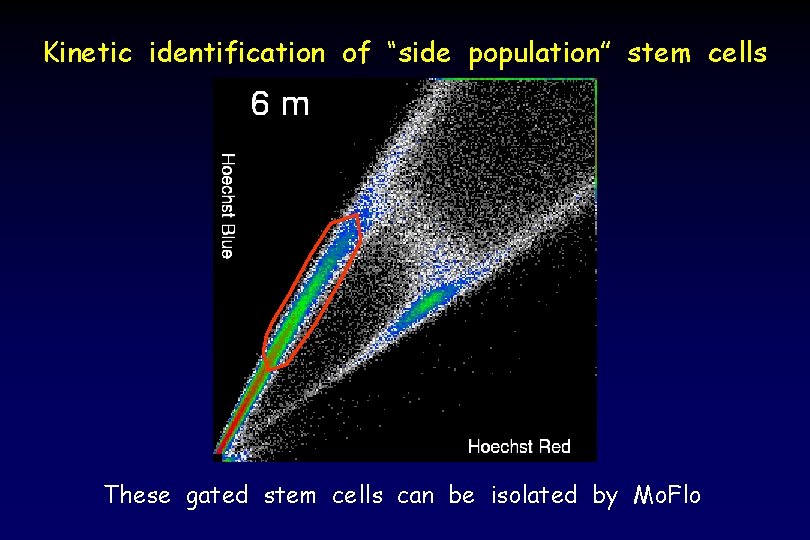
Kinetic identification of “side population” stem cells These gated stem cells can be isolated by Mo. Flo

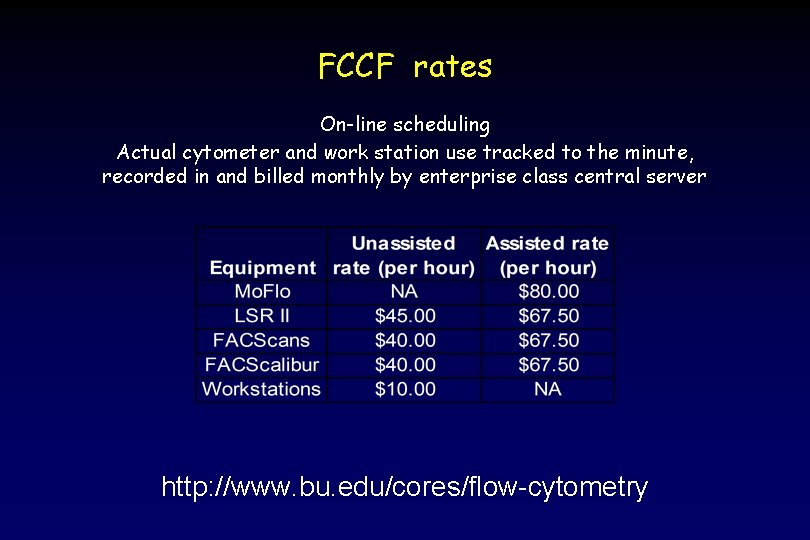
FCCF rates On-line scheduling Actual cytometer and work station use tracked to the minute, recorded in and billed monthly by enterprise class central server http: //www. bu. edu/cores/flow-cytometry

Help and training: Please sign up for basic training on FACScan or LSR 2 with Yan Deng (X 4 -5225), ydeng@bu. edu Problems during experiments: We can’t read minds. Please write a computer entry in the COMPLAINT LOG for each instrument. Or email us: flowcore@bu. edu We will contact you ASAP and usually can respond within 2 hours.
 Basic principles flow cytometry
Basic principles flow cytometry Purdue university flow cytometry
Purdue university flow cytometry Fluorochromes
Fluorochromes Olisambu uche
Olisambu uche Contrad flow cytometry
Contrad flow cytometry Flow cytometry simulator
Flow cytometry simulator Apd vs pmt flow cytometry
Apd vs pmt flow cytometry Fna
Fna Flow cytometry
Flow cytometry Boolean gating flow cytometry
Boolean gating flow cytometry Einstein flow cytometry
Einstein flow cytometry Einstein flow cytometry
Einstein flow cytometry Einstein flow cytometry
Einstein flow cytometry Flow cytometry lecture
Flow cytometry lecture Iccs 2016 cytometry
Iccs 2016 cytometry Fspos
Fspos Novell typiska drag
Novell typiska drag Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Vad står k.r.å.k.a.n för
Vad står k.r.å.k.a.n för Shingelfrisyren
Shingelfrisyren En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Kassaregister ideell förening
Kassaregister ideell förening Tidböcker
Tidböcker Sura för anatom
Sura för anatom Förklara densitet för barn
Förklara densitet för barn Datorkunskap för nybörjare
Datorkunskap för nybörjare Boverket ka
Boverket ka Debattartikel struktur
Debattartikel struktur För och nackdelar med firo
För och nackdelar med firo Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Kraft per area
Kraft per area Svenskt ramverk för digital samverkan
Svenskt ramverk för digital samverkan Bo bergman jag fryser om dina händer
Bo bergman jag fryser om dina händer Presentera för publik crossboss
Presentera för publik crossboss Teckenspråk minoritetsspråk argument
Teckenspråk minoritetsspråk argument Bat mitza
Bat mitza Treserva lathund
Treserva lathund Luftstrupen för medicinare
Luftstrupen för medicinare Bästa kameran för astrofoto
Bästa kameran för astrofoto Centrum för kunskap och säkerhet
Centrum för kunskap och säkerhet Lågenergihus nyproduktion
Lågenergihus nyproduktion Bra mat för unga idrottare
Bra mat för unga idrottare Verktyg för automatisering av utbetalningar
Verktyg för automatisering av utbetalningar Rutin för avvikelsehantering
Rutin för avvikelsehantering Smärtskolan kunskap för livet
Smärtskolan kunskap för livet Ministerstyre för och nackdelar
Ministerstyre för och nackdelar Tack för att ni har lyssnat
Tack för att ni har lyssnat Vad är referatmarkeringar
Vad är referatmarkeringar Redogör för vad psykologi är
Redogör för vad psykologi är Borstål, egenskaper
Borstål, egenskaper Tack för att ni har lyssnat
Tack för att ni har lyssnat Borra hål för knoppar
Borra hål för knoppar Vilken grundregel finns det för tronföljden i sverige?
Vilken grundregel finns det för tronföljden i sverige? Fr formel
Fr formel Tack för att ni har lyssnat
Tack för att ni har lyssnat Steg för steg rita
Steg för steg rita Vad är verksamhetsanalys
Vad är verksamhetsanalys Tobinskatten för och nackdelar
Tobinskatten för och nackdelar Blomman för dagen drog
Blomman för dagen drog