Mass Cytometry Panel Design Mass Cytometry Panels Cy


- Slides: 24

Mass Cytometry Panel Design

Mass Cytometry Panels • Cy. TOF® is a single-cell analysis platform that enables simultaneous measurement of > 30 parameters. • A Cy. TOF panel is a set of metal-conjugated probes that identifies those parameters. • The design of such a panel requires an understanding of the sources of signal and noise in mass cytometry.

Panel Design Considerations • Signal – Antigen abundance – Sensitivity of the system to probes – Probe properties o Metal content o Epitope/ligand binding ability o Amplification schemes • Noise – Environmental – Measurement of probe in undesired channels (crosstalk) • Effective panel design: – Optimizes signal – Minimizes noise

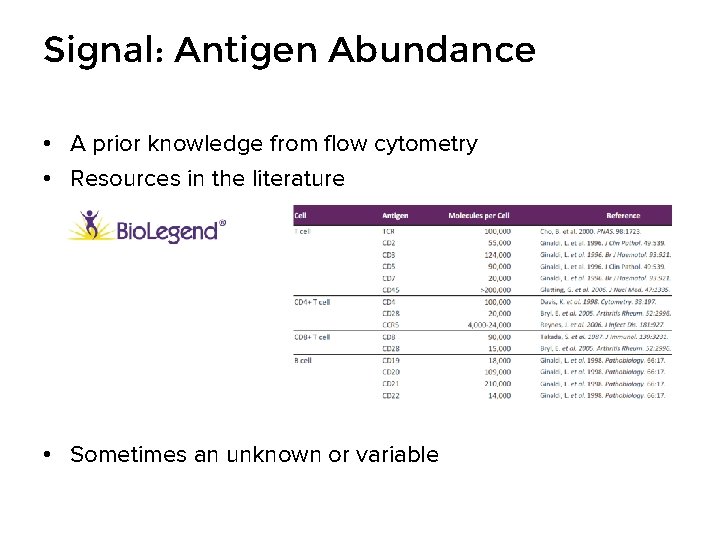
Signal: Antigen Abundance • A prior knowledge from flow cytometry • Resources in the literature • Sometimes an unknown or variable

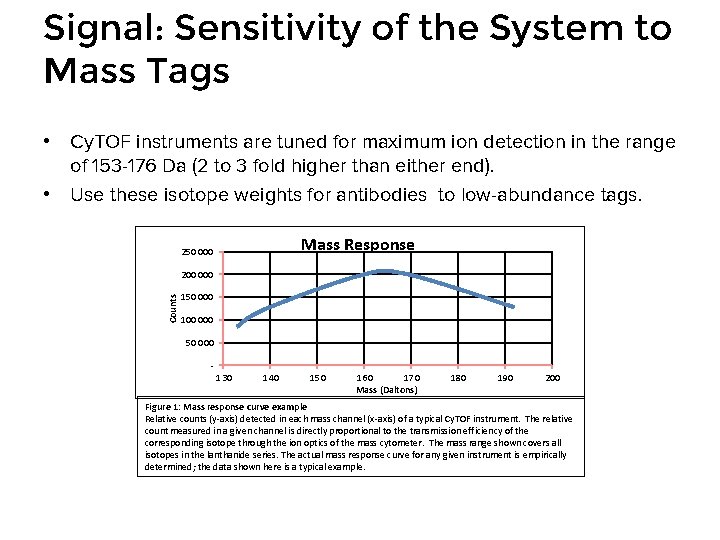
Signal: Sensitivity of the System to Mass Tags • Cy. TOF instruments are tuned for maximum ion detection in the range of 153 -176 Da (2 to 3 fold higher than either end). • Use these isotope weights for antibodies to low-abundance tags. Mass Response 250 000 Counts 200 000 150 000 100 000 50 000 130 140 150 160 170 Mass (Daltons) 180 190 200 Figure 1: Mass response curve example Relative counts (y-axis) detected in each mass channel (x-axis) of a typical Cy. TOF instrument. The relative count measured in a given channel is directly proportional to the transmission efficiency of the corresponding isotope through the ion optics of the mass cytometer. The mass range shown covers all isotopes in the lanthanide series. The actual mass response curve for any given instrument is empirically determined; the data shown here is a typical example.

Noise: Environmental Background • Undesired signal derived from sources extrinsic to the instrument • Sources in mass cytometry – Environmental metals • Barium (130 -138) – ubiquitous, buffers, soaps, air • Mercury, lead, iodine, tin – buffers, lab equipment – Metals to which patient or animal has been exposed • Cisplatin – now used as viability indicator in mass cytometry! – Cells and debris from previous samples • Keep your instrument clean • Monitor with a “background template” – See “Sample Acquisition” presentation for details

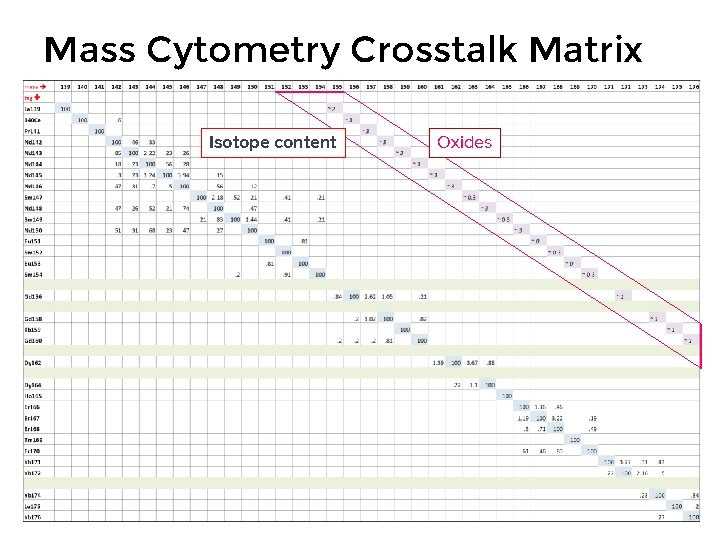
Noise: Crosstalk • Background intrinsic to mass cytometry and the metal probes used as labels • Sources in mass cytometry – Isotopic impurity – Oxide Formation – Abundance Sensitivity • Consider crosstalk when designing your panels

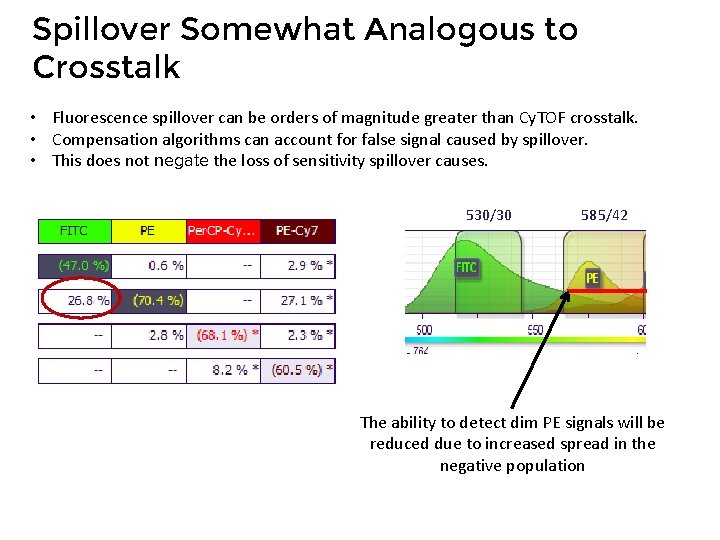
Spillover Somewhat Analogous to Crosstalk • Fluorescence spillover can be orders of magnitude greater than Cy. TOF crosstalk. • Compensation algorithms can account for false signal caused by spillover. • This does not negate the loss of sensitivity spillover causes. 530/30 585/42 The ability to detect dim PE signals will be reduced due to increased spread in the negative population

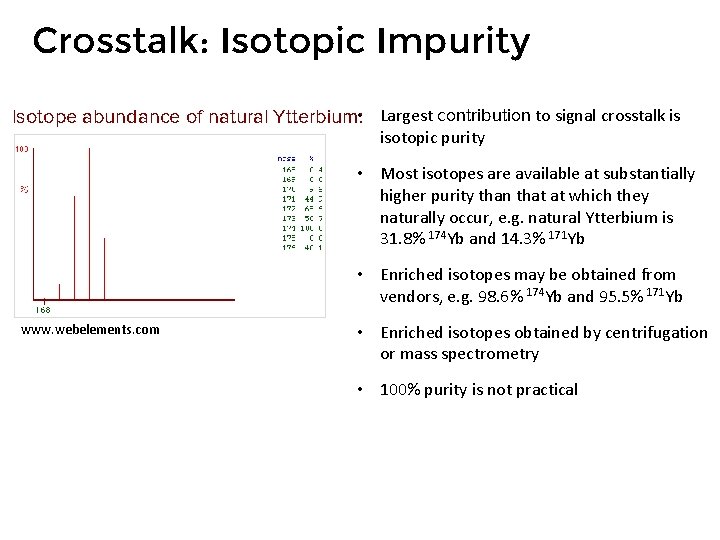
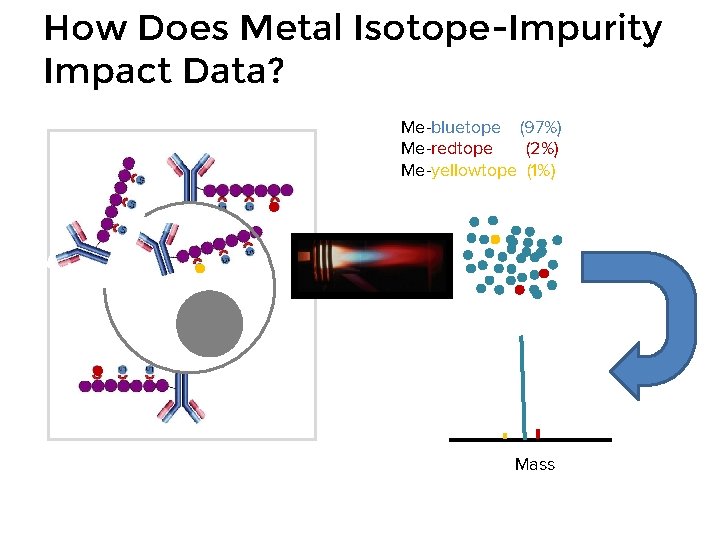
Crosstalk: Isotopic Impurity Isotope abundance of natural Ytterbium: • Largest contribution to signal crosstalk is isotopic purity • Most isotopes are available at substantially higher purity than that at which they naturally occur, e. g. natural Ytterbium is 31. 8% 174 Yb and 14. 3% 171 Yb • Enriched isotopes may be obtained from vendors, e. g. 98. 6% 174 Yb and 95. 5% 171 Yb www. webelements. com • Enriched isotopes obtained by centrifugation or mass spectrometry • 100% purity is not practical

How Does Metal Isotope-Impurity Impact Data? Me-bluetope (97%) Me-redtope (2%) Me-yellowtope (1%) Mass

Crosstalk: Oxide Formation Lanthanides: • Lesser contribution to signal spillover is oxide formation • Oxide ions appear 16 mass units above the atomic ion mass, e. g. 139 La will form an oxide with a mass of 155 • Oxide formation dependent on atom-oxygen bond strength: the higher the bond strength the more oxide formation occurring within the plasma • Oxide level tested during instrument QC: hot plasma reduces oxides • Only 7 metals may form significant oxides: La 139, Nd 142 -144, 148 and 150

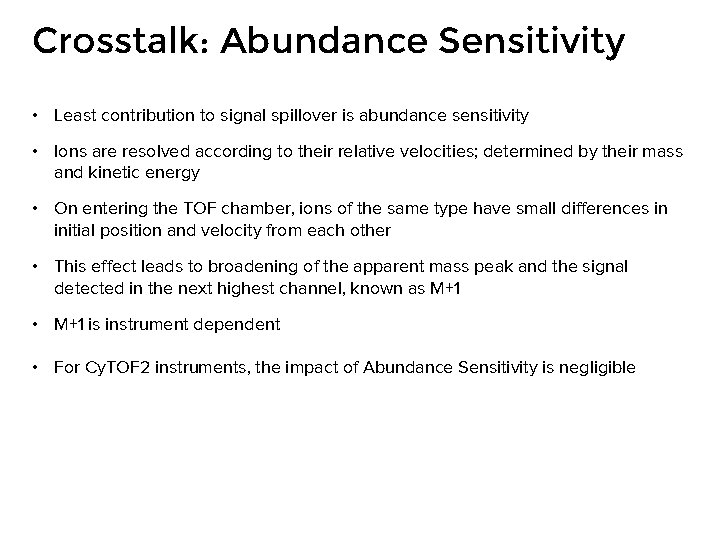
Crosstalk: Abundance Sensitivity • Least contribution to signal spillover is abundance sensitivity • Ions are resolved according to their relative velocities; determined by their mass and kinetic energy • On entering the TOF chamber, ions of the same type have small differences in initial position and velocity from each other • This effect leads to broadening of the apparent mass peak and the signal detected in the next highest channel, known as M+1 • M+1 is instrument dependent • For Cy. TOF 2 instruments, the impact of Abundance Sensitivity is negligible

Mass Cytometry Crosstalk Matrix Isotope content Oxides

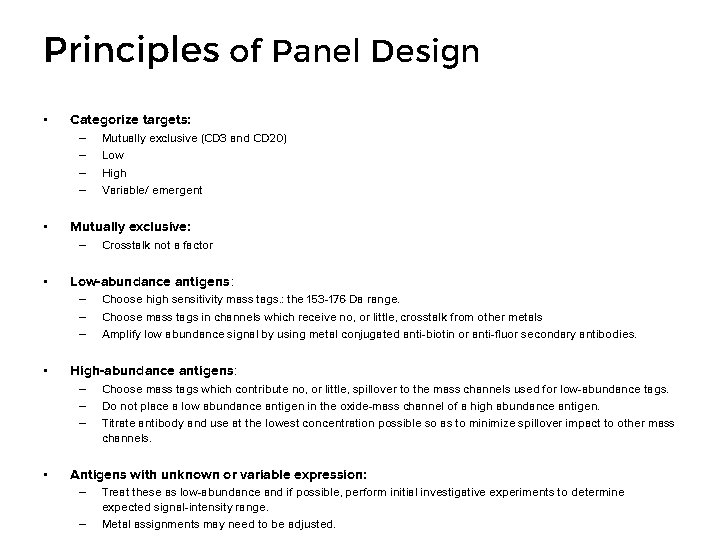
Principles of Panel Design • Categorize targets: – – • Mutually exclusive: – • Choose high sensitivity mass tags. : the 153 -176 Da range. Choose mass tags in channels which receive no, or little, crosstalk from other metals Amplify low abundance signal by using metal conjugated anti-biotin or anti-fluor secondary antibodies. High-abundance antigens: – – – • Crosstalk not a factor Low-abundance antigens: – – – • Mutually exclusive (CD 3 and CD 20) Low High Variable/ emergent Choose mass tags which contribute no, or little, spillover to the mass channels used for low-abundance tags. Do not place a low abundance antigen in the oxide-mass channel of a high abundance antigen. Titrate antibody and use at the lowest concentration possible so as to minimize spillover impact to other mass channels. Antigens with unknown or variable expression: – – Treat these as low-abundance and if possible, perform initial investigative experiments to determine expected signal-intensity range. Metal assignments may need to be adjusted.

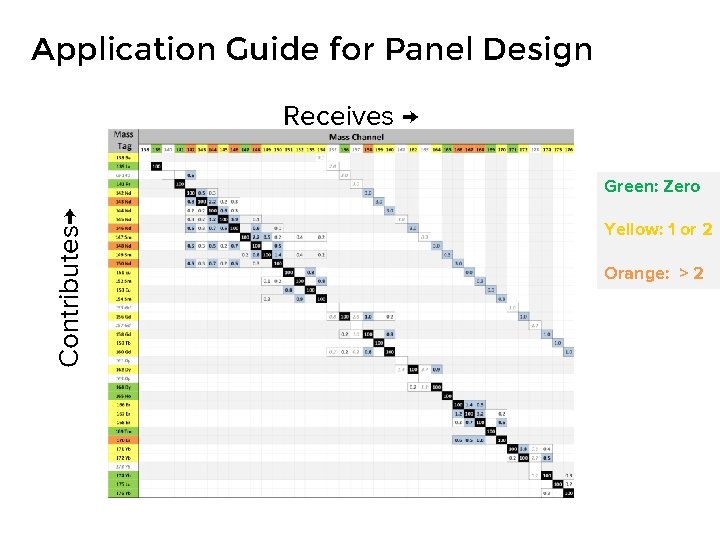
Application Guide for Panel Design Receives → Contributes→ Green: Zero Yellow: 1 or 2 Orange: > 2

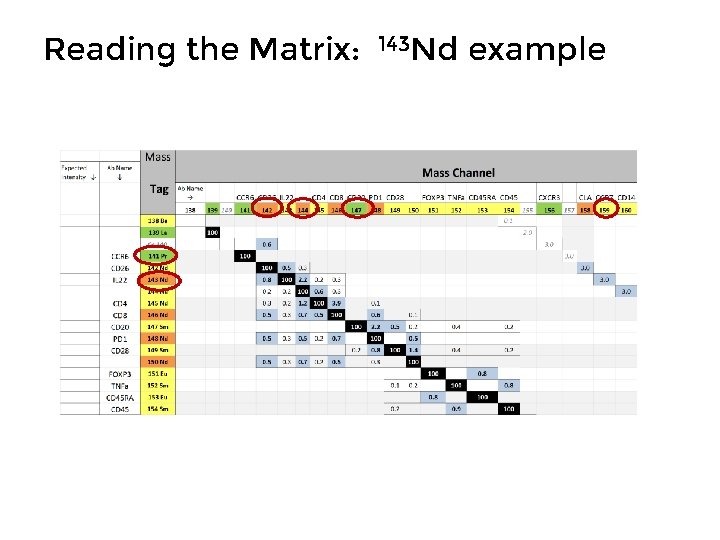
Reading the Matrix: 143 Nd example

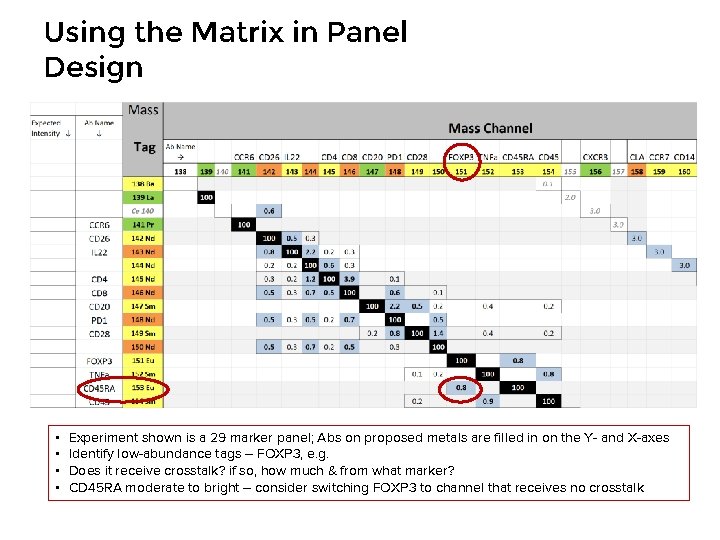
Using the Matrix in Panel Design • • Experiment shown is a 29 marker panel; Abs on proposed metals are filled in on the Y- and X-axes Identify low-abundance tags – FOXP 3, e. g. Does it receive crosstalk? if so, how much & from what marker? CD 45 RA moderate to bright – consider switching FOXP 3 to channel that receives no crosstalk

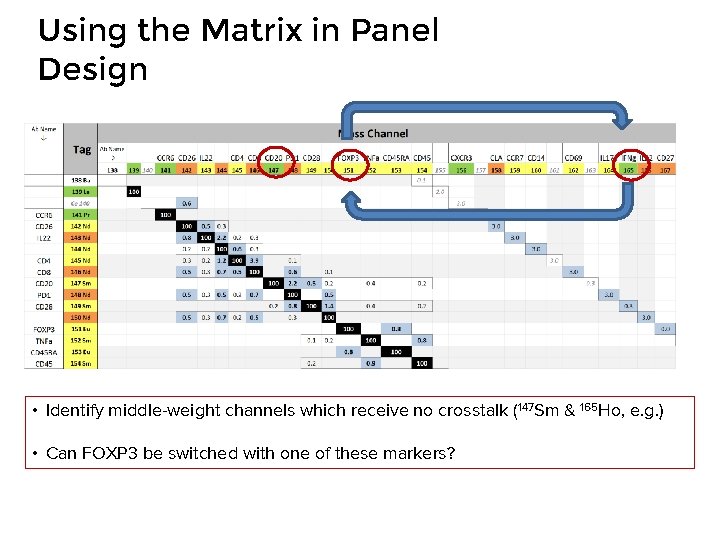
Using the Matrix in Panel Design • Identify middle-weight channels which receive no crosstalk (147 Sm & 165 Ho, e. g. ) • Can FOXP 3 be switched with one of these markers?

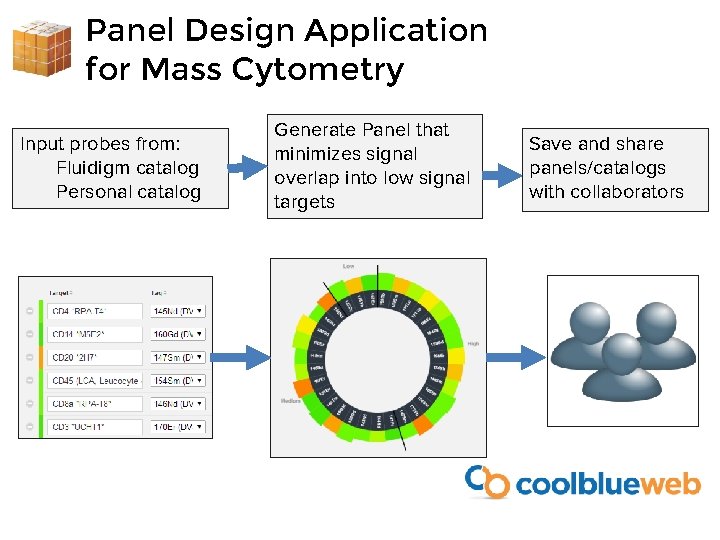
Panel Design Application for Mass Cytometry Input probes from: Fluidigm catalog Personal catalog Generate Panel that minimizes signal overlap into low signal targets Save and share panels/catalogs with collaborators

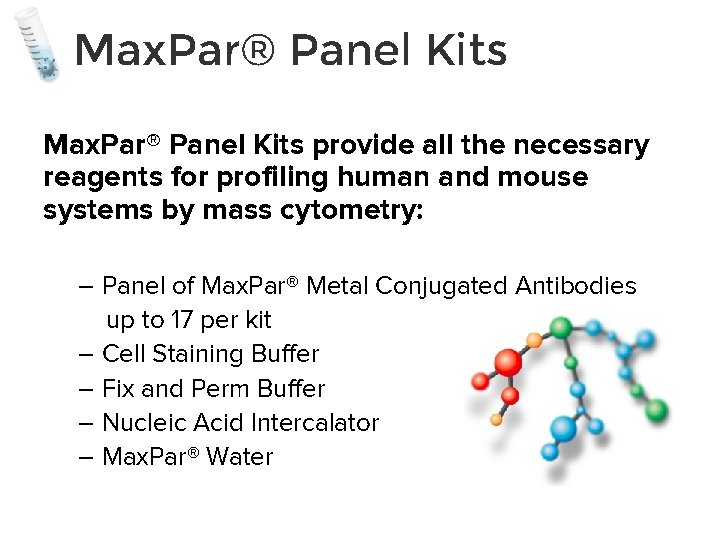
Max. Par® Panel Kits provide all the necessary reagents for profiling human and mouse systems by mass cytometry: – Panel of Max. Par® Metal Conjugated Antibodies up to 17 per kit – Cell Staining Buffer – Fix and Perm Buffer – Nucleic Acid Intercalator – Max. Par® Water

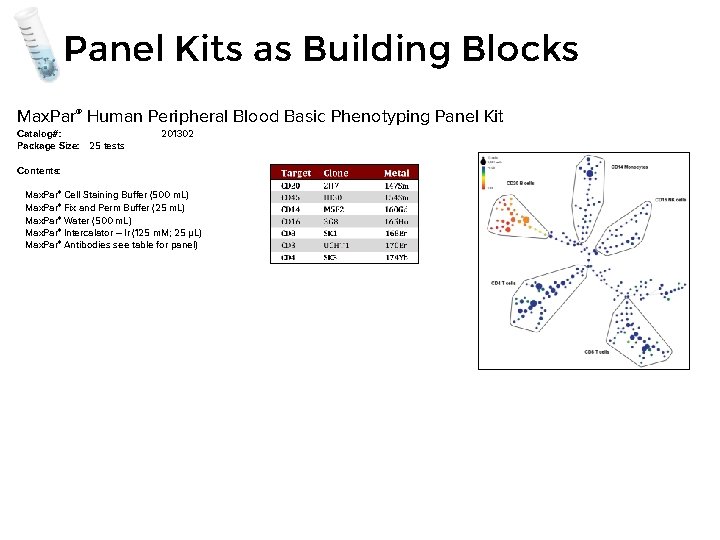
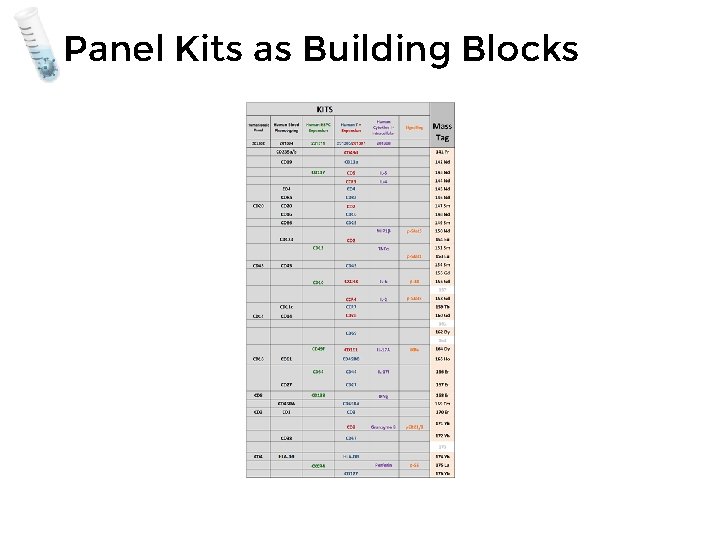
Panel Kits as Building Blocks Max. Par® Human Peripheral Blood Basic Phenotyping Panel Kit Catalog#: Package Size: 25 tests 201302 Contents: Max. Par® Cell Staining Buffer (500 m. L) Max. Par® Fix and Perm Buffer (25 m. L) Max. Par® Water (500 m. L) Max. Par® Intercalator – Ir (125 m. M; 25 µL) Max. Par® Antibodies see table for panel)

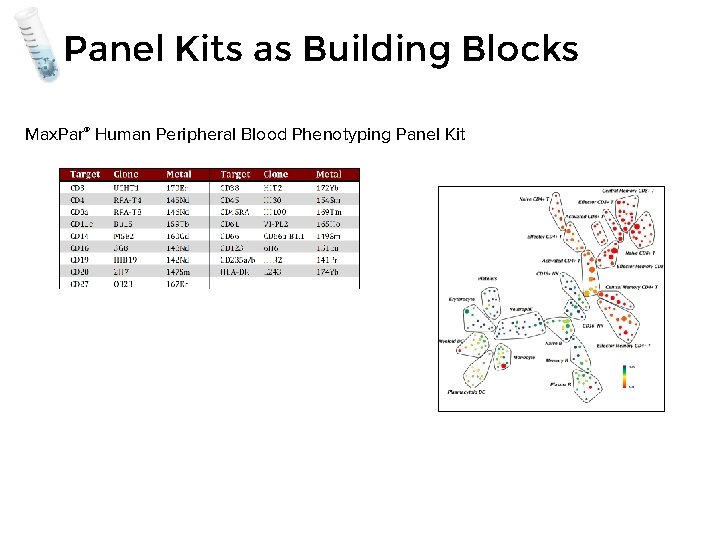
Panel Kits as Building Blocks Max. Par® Human Peripheral Blood Phenotyping Panel Kit

Panel Kits as Building Blocks

Mass Cytometry Panel Design • Mass cytometry uniquely isolates signal from over 30 probes into single channels with minimal signal overlap, thereby enabling system-wide single-cell proteomic studies • Sources of noise are very small in Mass cytometry but do need to be considered for optimal panel design • Optimal panel design utilizes a strategy that: – Maximizes signal and minimizes crosstalk into channels assigned to low abundance targets – Minimizes crosstalk from channels for variable expression targets