Interesting Electrolyte Cases Joel M Topf M D






















- Slides: 125

Interesting Electrolyte Cases Joel M. Topf, M. D. Nephrology 248. 470. 8163 http: //PBFluids. blogspot. com

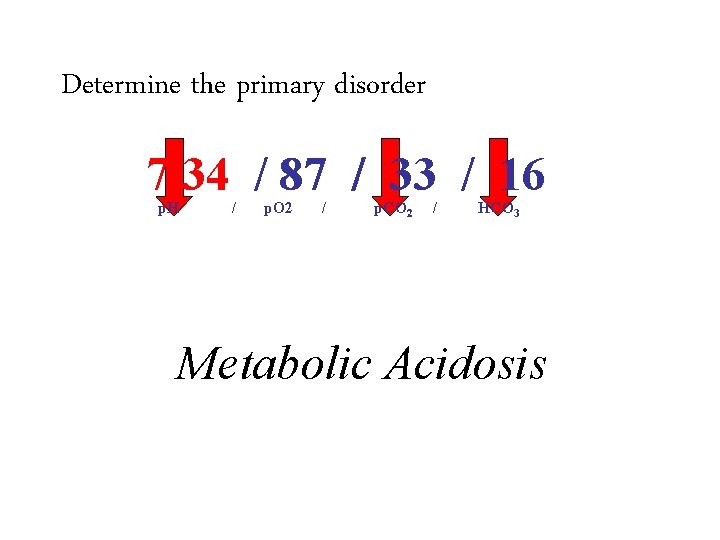
58 y. o. female with weakness and muscle aches 139 115 3. 1 17 16 1. 0 7. 34 / 87 / 33 / 17

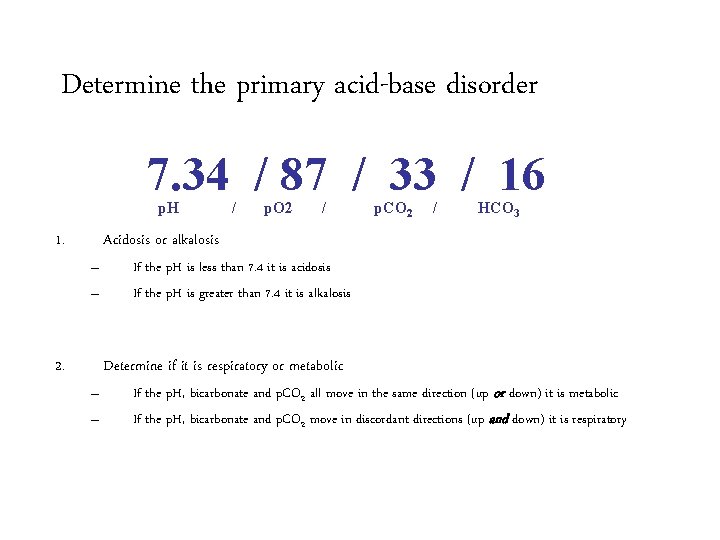
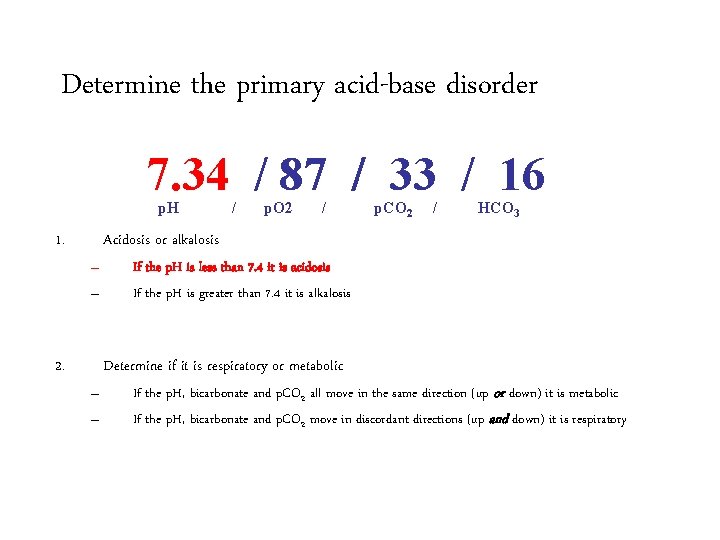
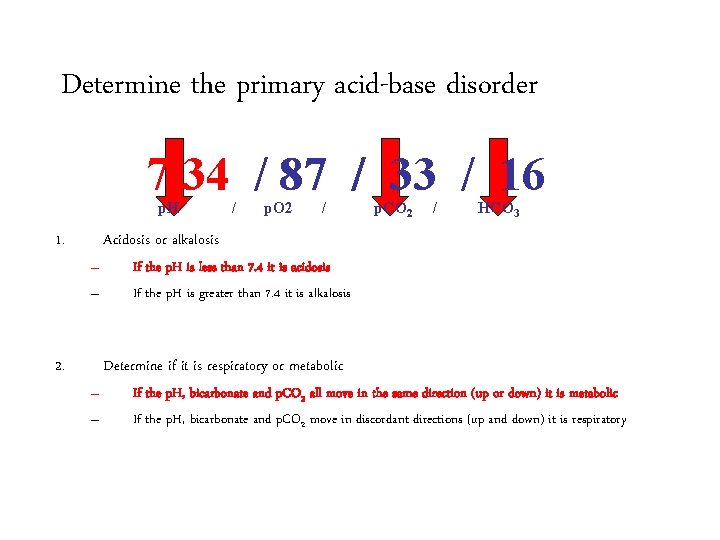
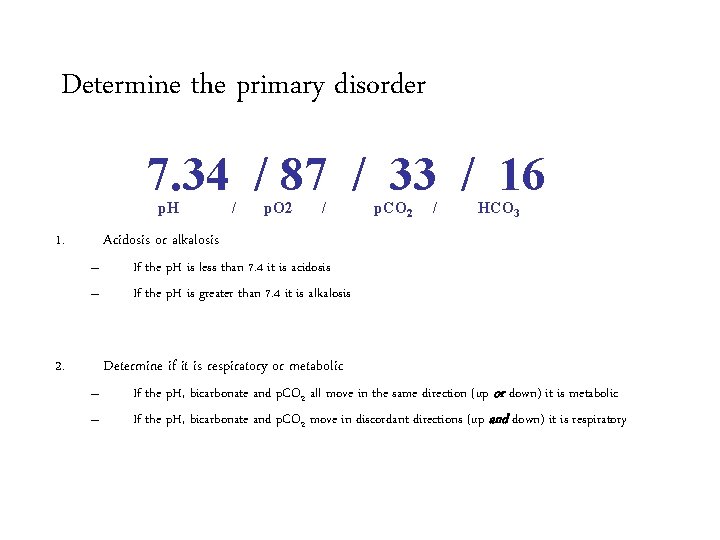
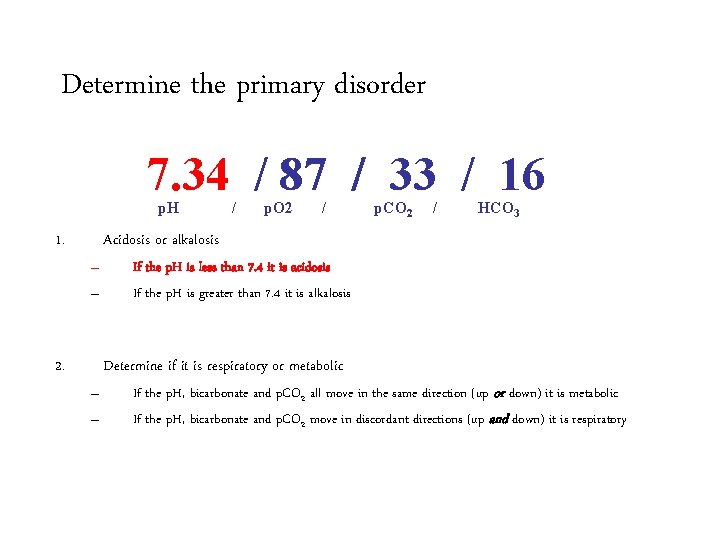
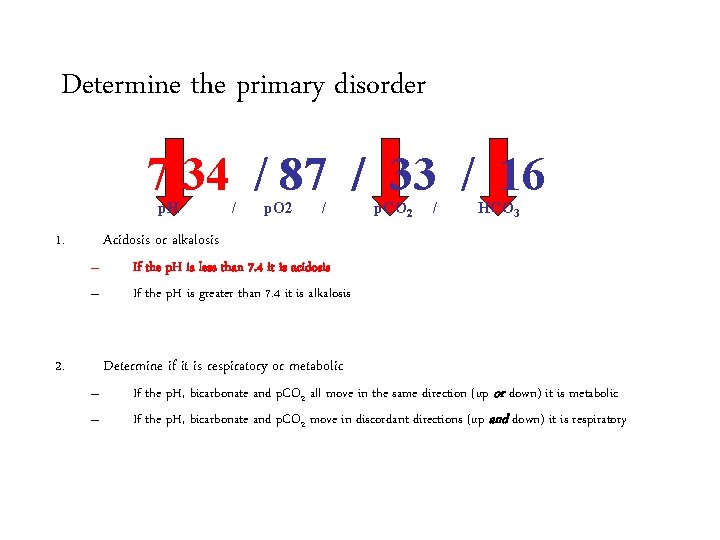
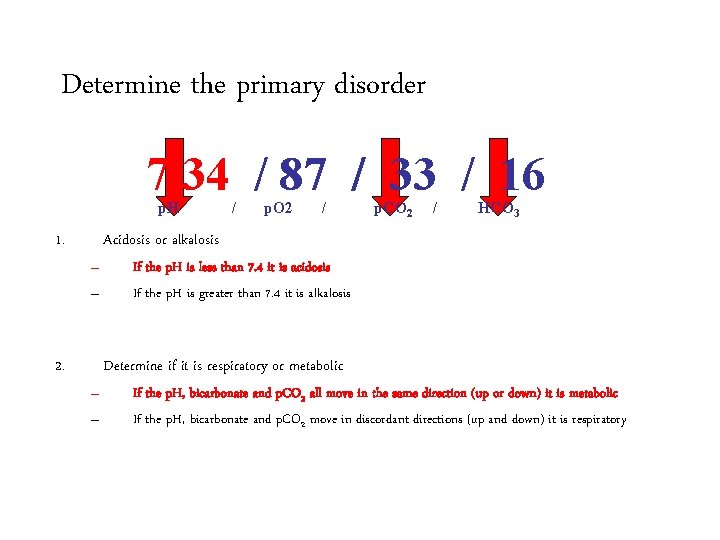
Determine the primary acid-base disorder 7. 34 / 87 / 33 / 16 p. H 1. / p. O 2 / p. CO 2 / HCO 3 Acidosis or alkalosis – – 2. If the p. H is less than 7. 4 it is acidosis If the p. H is greater than 7. 4 it is alkalosis Determine if it is respiratory or metabolic – – If the p. H, bicarbonate and p. CO 2 all move in the same direction (up or down) it is metabolic If the p. H, bicarbonate and p. CO 2 move in discordant directions (up and down) it is respiratory

Determine the primary acid-base disorder 7. 34 / 87 / 33 / 16 p. H 1. / p. O 2 / p. CO 2 / HCO 3 Acidosis or alkalosis – – 2. If the p. H is less than 7. 4 it is acidosis If the p. H is greater than 7. 4 it is alkalosis Determine if it is respiratory or metabolic – – If the p. H, bicarbonate and p. CO 2 all move in the same direction (up or down) it is metabolic If the p. H, bicarbonate and p. CO 2 move in discordant directions (up and down) it is respiratory

Determine the primary acid-base disorder 7. 34 / 87 / 33 / 16 p. H 1. / p. O 2 / p. CO 2 / HCO 3 Acidosis or alkalosis – – 2. If the p. H is less than 7. 4 it is acidosis If the p. H is greater than 7. 4 it is alkalosis Determine if it is respiratory or metabolic – – If the p. H, bicarbonate and p. CO 2 all move in the same direction (up or down) it is metabolic If the p. H, bicarbonate and p. CO 2 move in discordant directions (up and down) it is respiratory

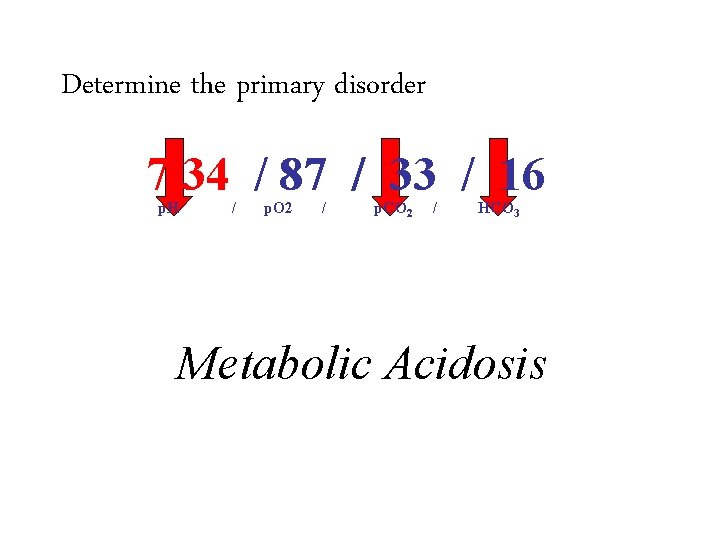
Determine the primary disorder 7. 34 / 87 / 33 / 16 p. H 1. / p. O 2 / p. CO 2 / HCO 3 Acidosis or alkalosis – – 2. If the p. H is less than 7. 4 it is acidosis If the p. H is greater than 7. 4 it is alkalosis Metabolic Acidosis Determine if it is respiratory or metabolic – – If the p. H, bicarbonate and p. CO 2 all move in the same direction (up or down) it is metabolic If the p. H, bicarbonate and p. CO 2 move in discordant directions (up and down) it is respiratory

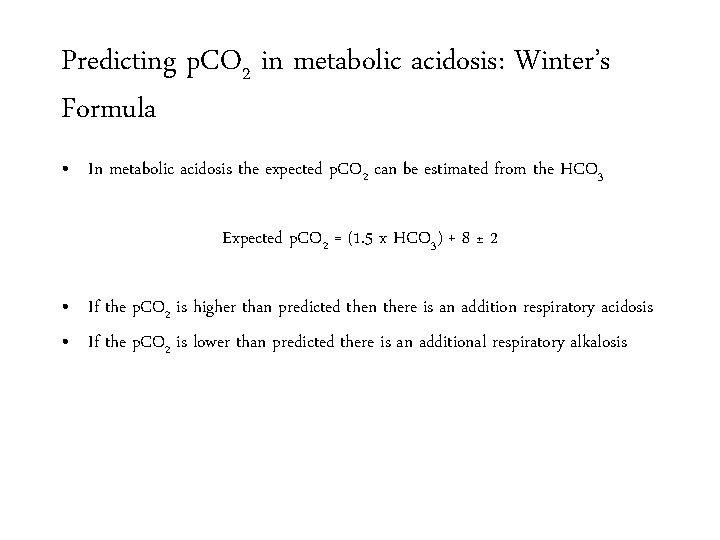
Predicting p. CO 2 in metabolic acidosis: Winter’s Formula • In metabolic acidosis the expected p. CO 2 can be estimated from the HCO 3 Expected p. CO 2 = (1. 5 x HCO 3) + 8 ± 2 • If the p. CO 2 is higher than predicted then there is an addition respiratory acidosis • If the p. CO 2 is lower than predicted there is an additional respiratory alkalosis

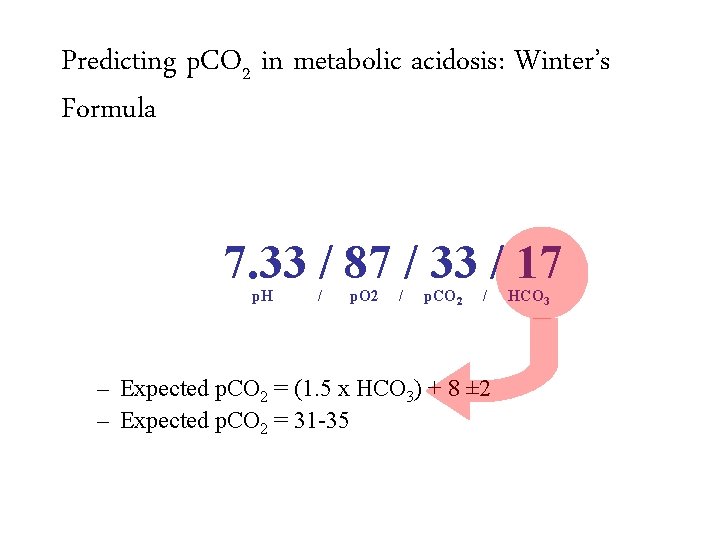
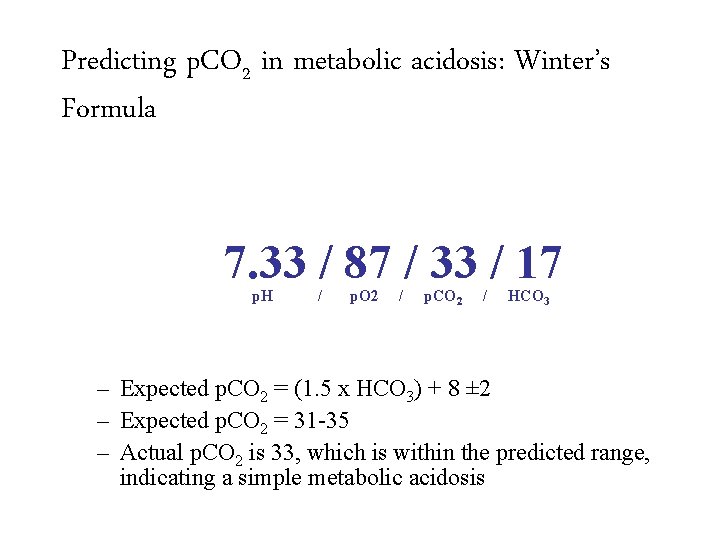
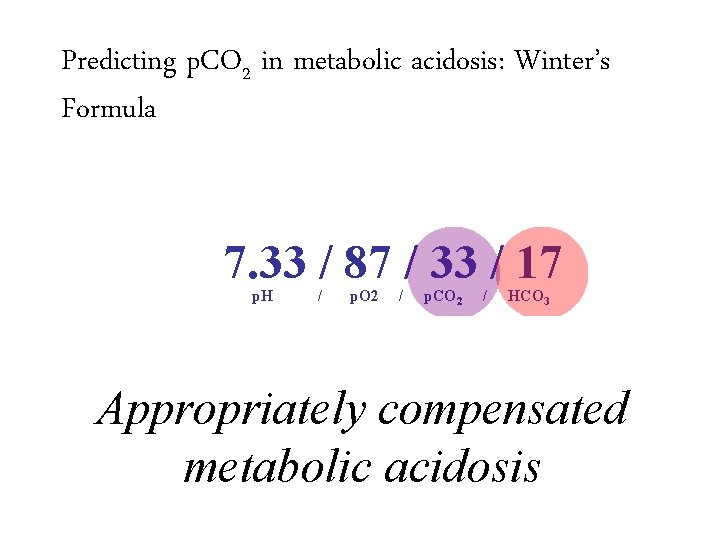
Predicting p. CO 2 in metabolic acidosis: Winter’s Formula 7. 33 / 87 / 33 / 17 p. H / p. O 2 / p. CO 2 / – Expected p. CO 2 = (1. 5 x HCO 3) + 8 ± 2 HCO 3

Predicting p. CO 2 in metabolic acidosis: Winter’s Formula 7. 33 / 87 / 33 / 17 p. H / p. O 2 / p. CO 2 / – Expected p. CO 2 = (1. 5 x HCO 3) + 8 ± 2 – Expected p. CO 2 = 31 -35 HCO 3

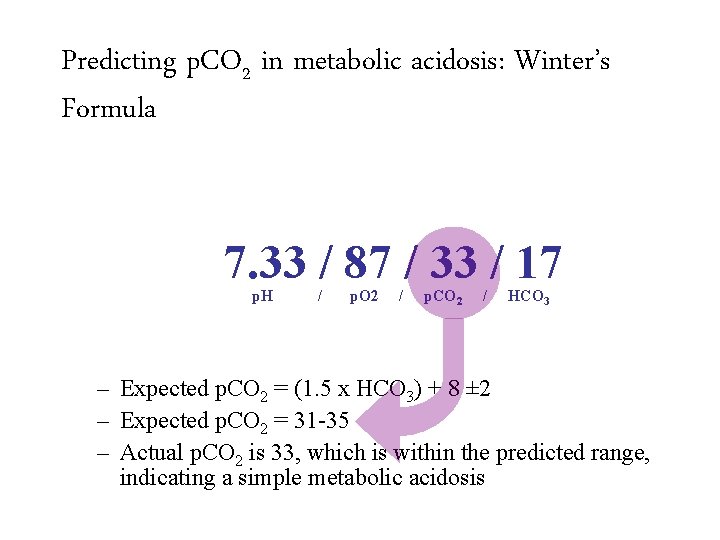
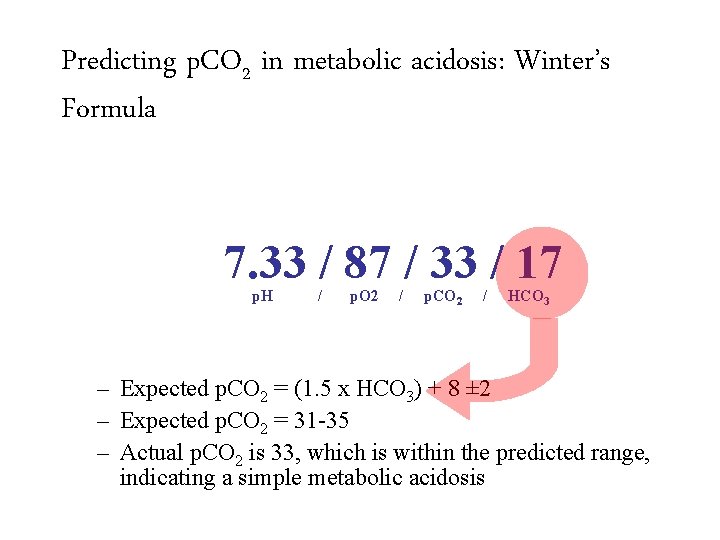
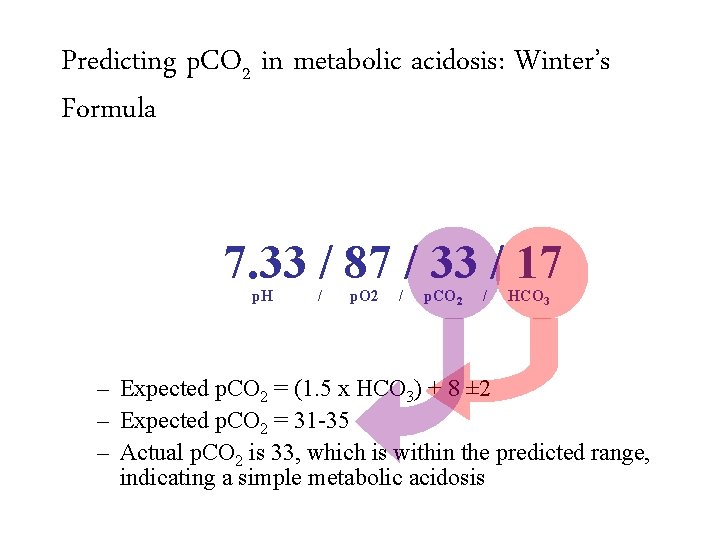
Predicting p. CO 2 in metabolic acidosis: Winter’s Formula 7. 33 / 87 / 33 / 17 p. H / p. O 2 / p. CO 2 / HCO 3 – Expected p. CO 2 = (1. 5 x HCO 3) + 8 ± 2 – Expected p. CO 2 = 31 -35 – Actual p. CO 2 is 33, which is within the predicted range, indicating a simple metabolic acidosis

Predicting p. CO 2 in metabolic acidosis: Winter’s Formula 7. 33 / 87 / 33 / 17 p. H / p. O 2 / p. CO 2 / HCO 3 – Expected p. CO 2 = (1. 5 x HCO 3) + 8 ± 2 – Expected p. CO 2 = 31 -35 – Actual p. CO 2 is 33, which is within the predicted range, indicating a simple metabolic acidosis Appropriately compensated metabolic acidosis

• Metabolic acidosis is further evaluated by determining the anion associated with the increased H+ cation

• Metabolic acidosis is further evaluated by determining the anion associated with the increased H+ cation It is either chloride Non-Anion Gap Metabolic Acidosis

• Metabolic acidosis is further evaluated by determining the anion associated with the increased H+ cation It is either chloride Non-Anion Gap Metabolic Acidosis Or it is not chloride Anion Gap Metabolic Acidosis

• Metabolic acidosis is further evaluated by determining the anion associated with the increased H+ cation It is either chloride Or it is not chloride Non-Anion Gap Metabolic Acidosis • These can be differentiated by measuring the anion gap. Anion Gap Metabolic Acidosis

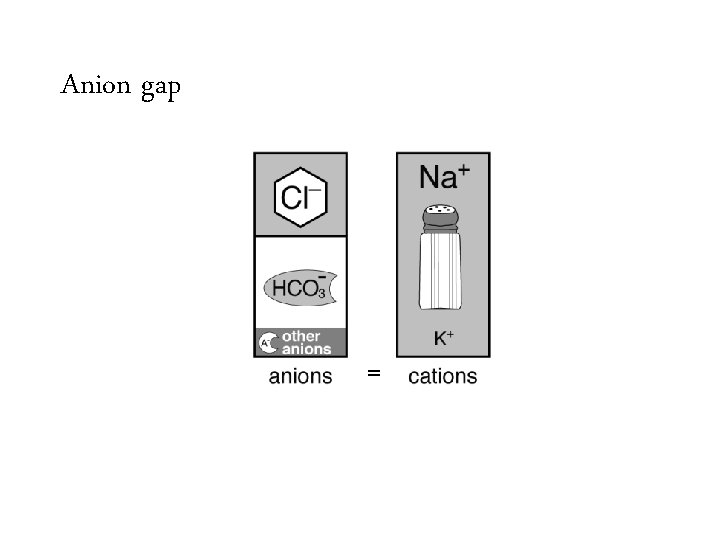
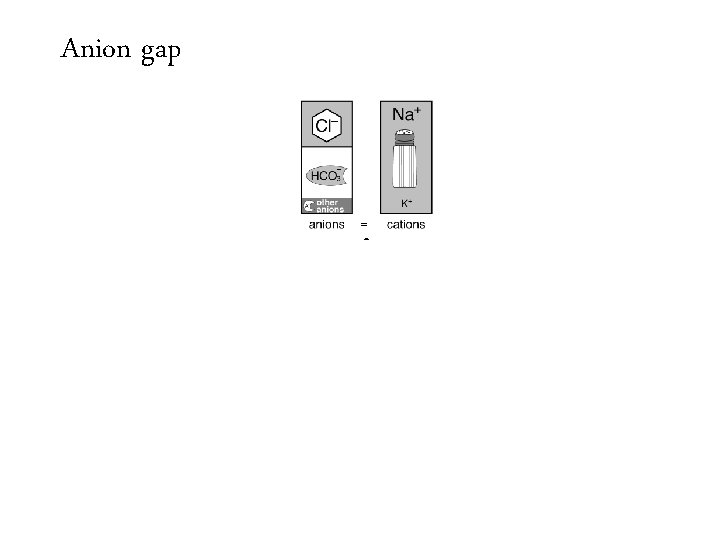
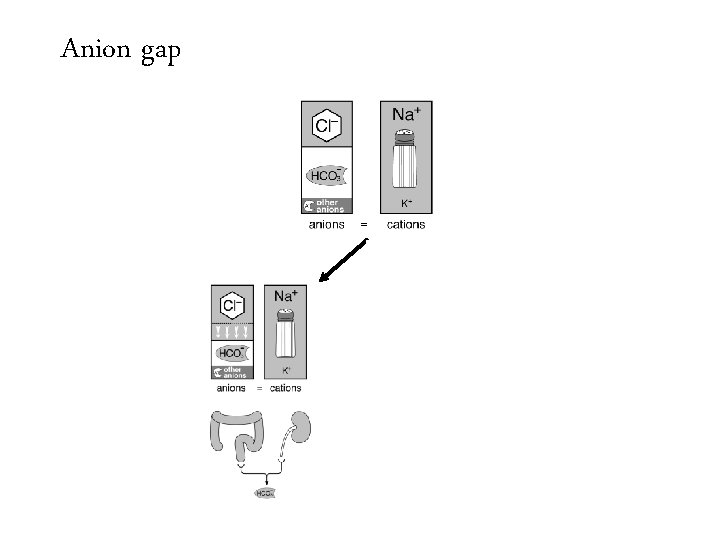
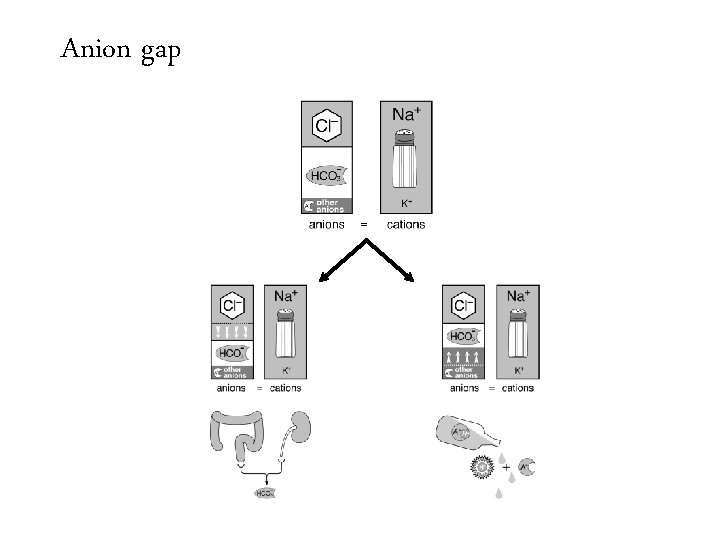
Anion gap =

Anion gap =

Anion gap =

Anion gap =

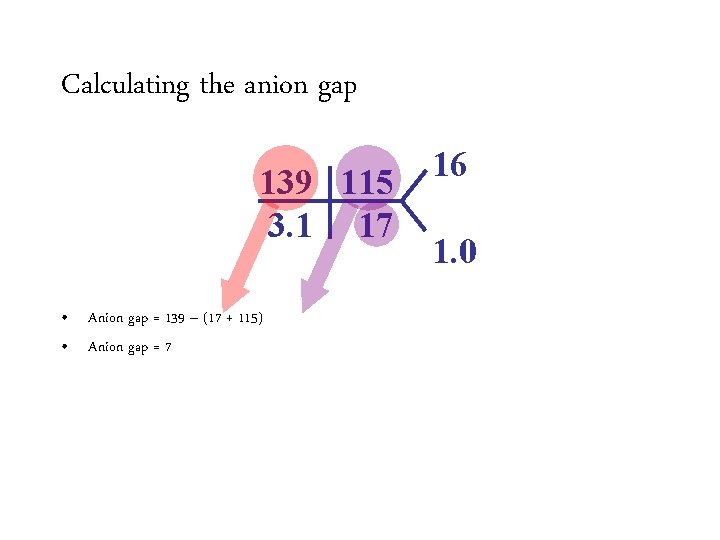
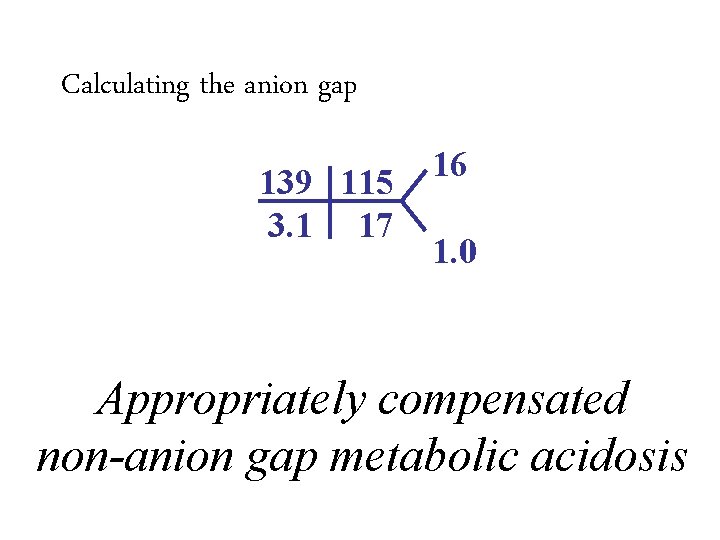
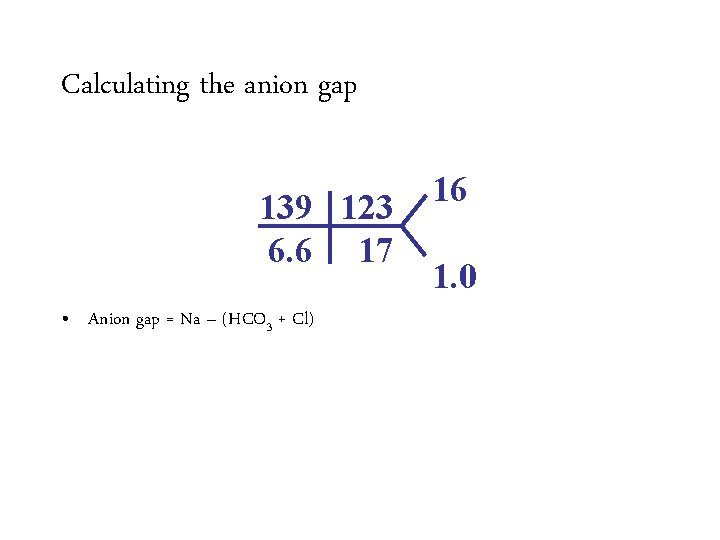
Calculating the anion gap 139 115 3. 1 17 • Anion gap = 139 Na –– (HCO (17 + 3115) + Cl) • Anion Normalgap is 12 =7 16 1. 0

Calculating the anion gap 139 115 3. 1 17 16 1. 0 • Anion gap = 139 Na –– (HCO (17 + 3115) + Cl) • Anion Normalgap is 12 =7 – Varies from lab to lab – Average anion gap in healthy controls is 6 ± 3 Appropriately compensated non-anion gap metabolic acidosis • Improving chloride assays have resulted in increased chloride levels and a decreased normal anion gap.

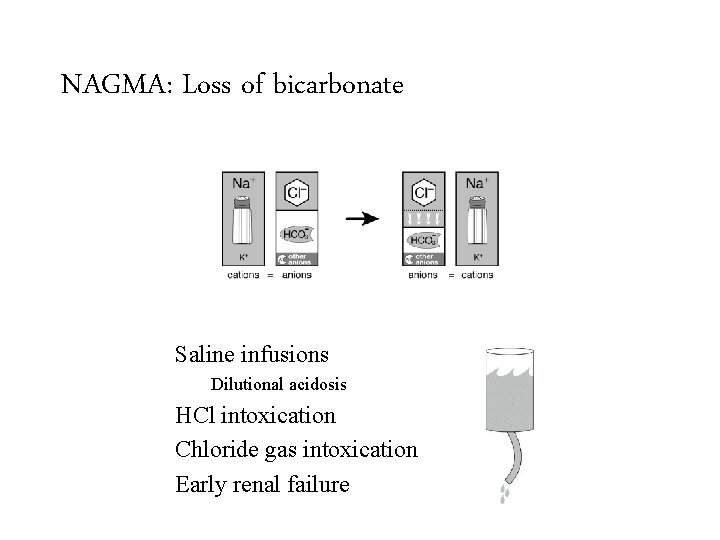
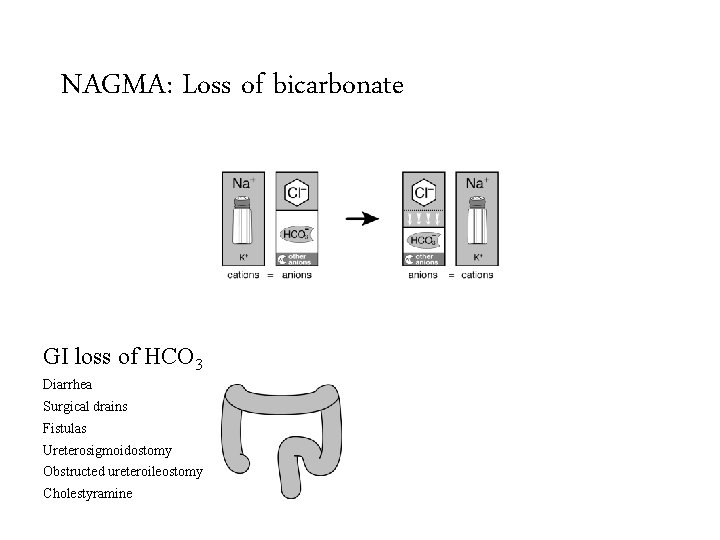
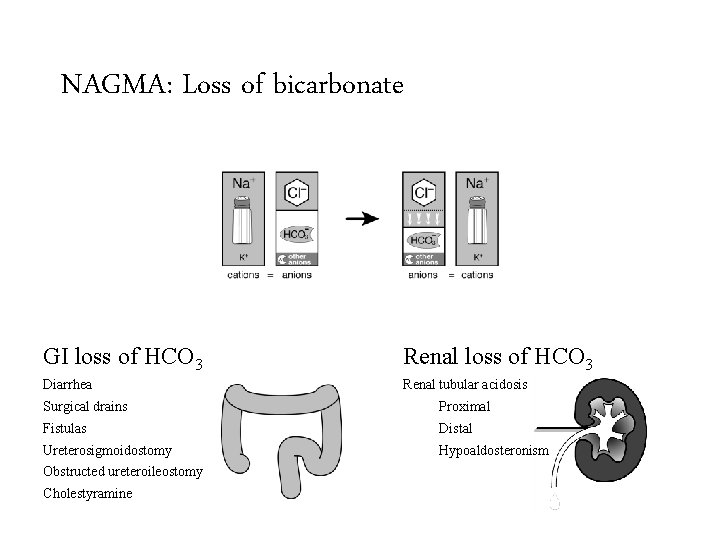
NAGMA: Loss of bicarbonate Saline infusions GI loss of HCO 3 Renal loss of HCO 3 Diarrhea Renal tubular acidosis Surgical drains Dilutional acidosis HCl intoxication Fistulas Ureterosigmoidostomy Chloride gas intoxication Obstructed ureteroileostomy Early renal failure Cholestyramine Proximal Distal Hypoaldosteronism

NAGMA: Loss of bicarbonate GI loss of HCO 3 Renal loss of HCO 3 Diarrhea Renal tubular acidosis Surgical drains Proximal Fistulas Distal Ureterosigmoidostomy Hypoaldosteronism Obstructed ureteroileostomy Cholestyramine

NAGMA: Loss of bicarbonate GI loss of HCO 3 Renal loss of HCO 3 Diarrhea Renal tubular acidosis Surgical drains Proximal Fistulas Distal Ureterosigmoidostomy Hypoaldosteronism Obstructed ureteroileostomy Cholestyramine

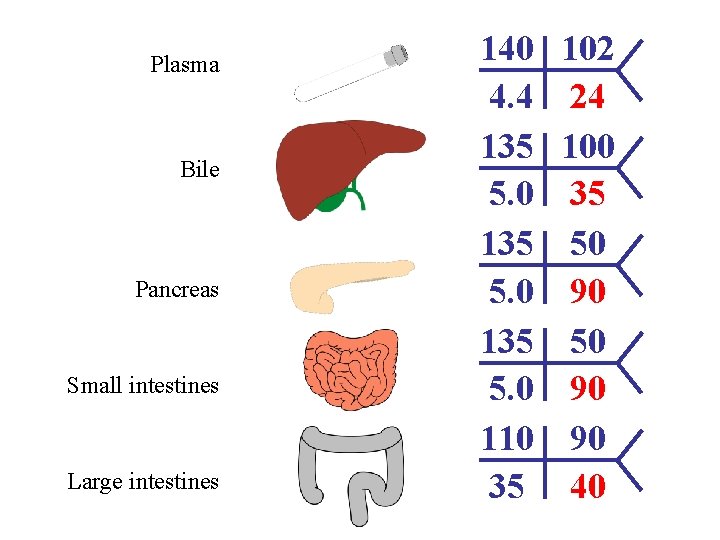
Plasma Bile Pancreas Small intestines Large intestines 140 4. 4 135 5. 0 110 35 102 24 100 35 50 90 90 40


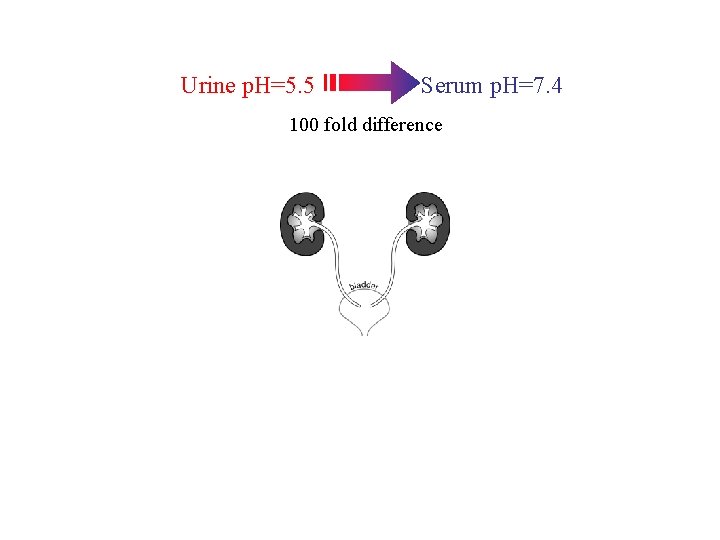
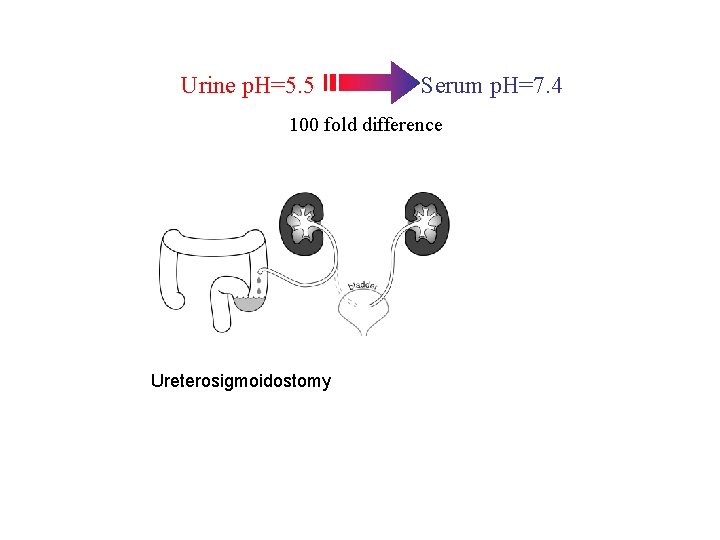
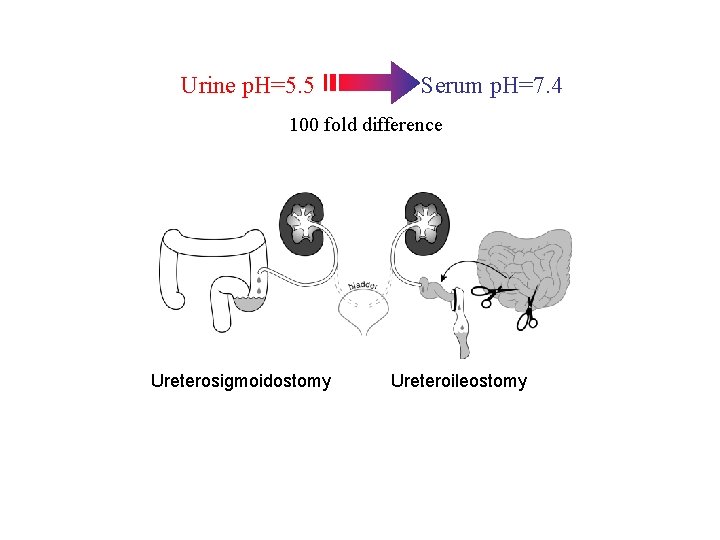
Urine p. H=5. 5 Serum p. H=7. 4 100 fold difference

Urine p. H=5. 5 Serum p. H=7. 4 100 fold difference Ureterosigmoidostomy

Urine p. H=5. 5 Serum p. H=7. 4 100 fold difference Ureterosigmoidostomy Ureteroileostomy

Renal causes of non-anion gap • Renal tubular acidosis is a failure of the kidney to reabsorb all of the filtered bicarbonate or synthesize new bicarbonate to keep up with metabolic demands. • Daily acid load – Protein metabolism consumes bicarbonate – This bicarbonate must be replaced – Generally equal to 1 mmol/kg

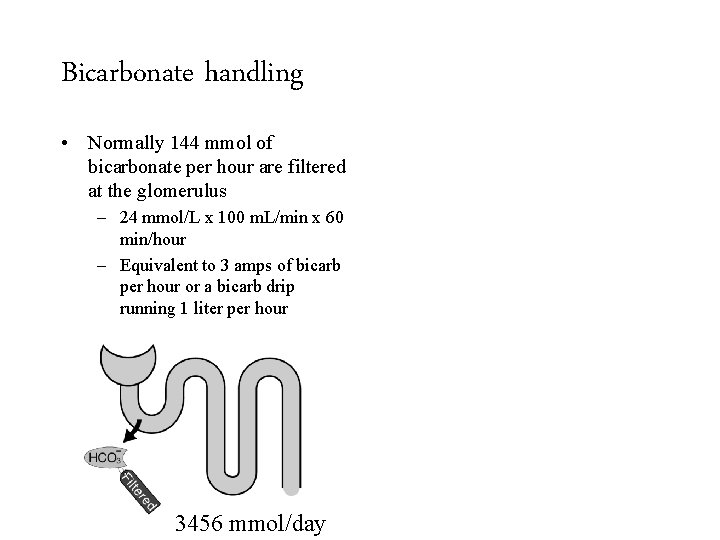
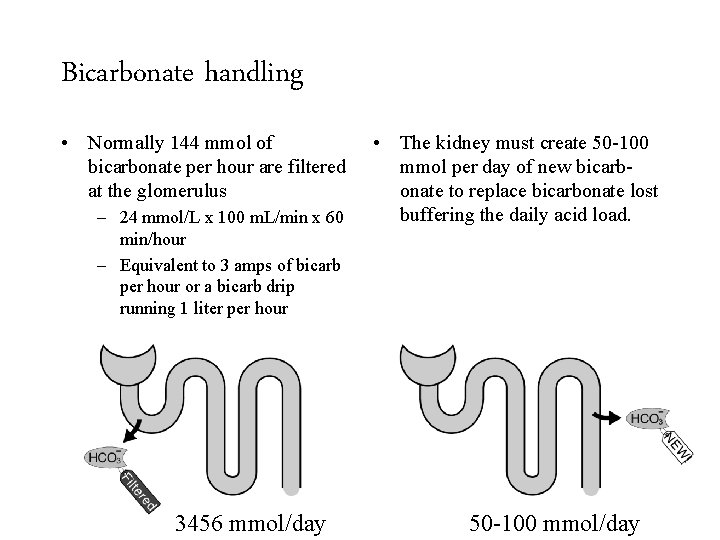
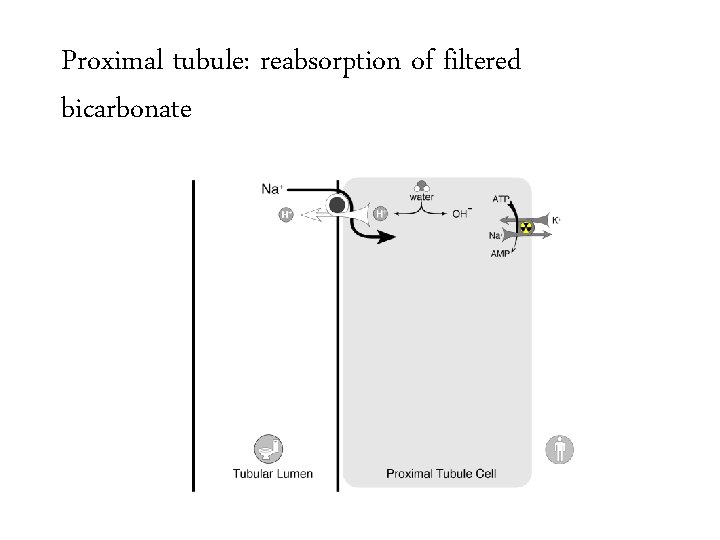
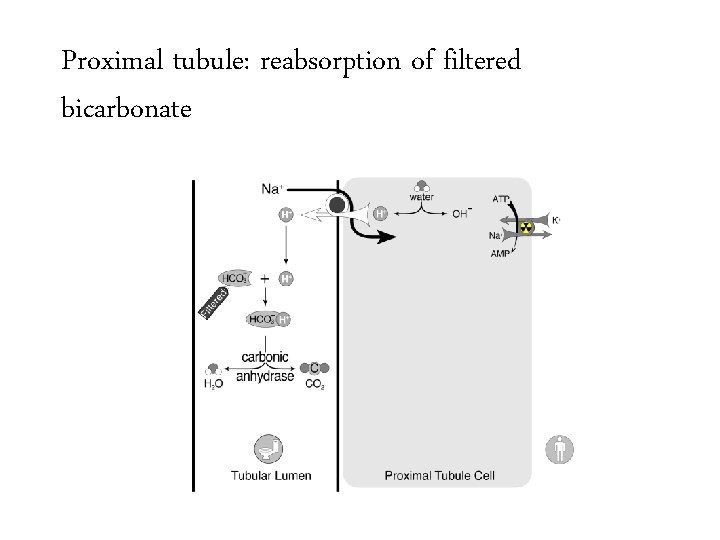
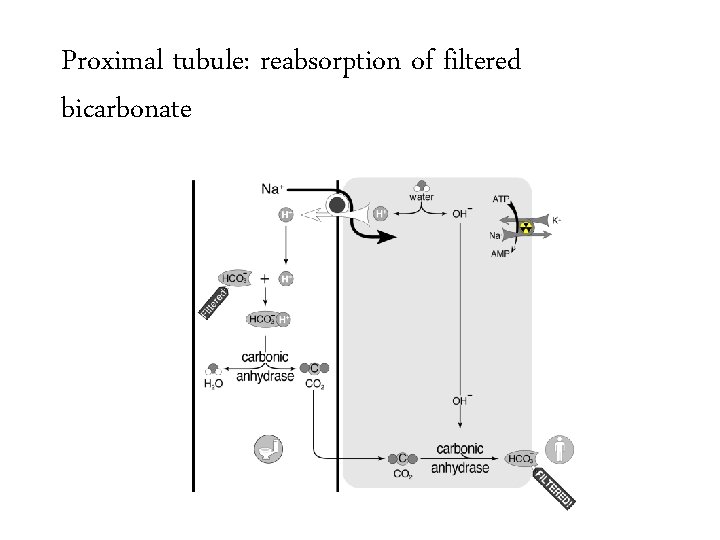
Bicarbonate handling • Normally 144 mmol of bicarbonate per hour are filtered at the glomerulus – 24 mmol/L x 100 m. L/min x 60 min/hour – Equivalent to 3 amps of bicarb per hour or a bicarb drip running 1 liter per hour 3456 mmol/day 50 -100 mmol/day

Bicarbonate handling • Normally 144 mmol of bicarbonate per hour are filtered at the glomerulus – 24 mmol/L x 100 m. L/min x 60 min/hour – Equivalent to 3 amps of bicarb per hour or a bicarb drip running 1 liter per hour 3456 mmol/day • The kidney must create 50 -100 mmol per day of new bicarbonate to replace bicarbonate lost buffering the daily acid load. 50 -100 mmol/day

Proximal tubule: reabsorption of filtered bicarbonate

Proximal tubule: reabsorption of filtered bicarbonate

Proximal tubule: reabsorption of filtered bicarbonate

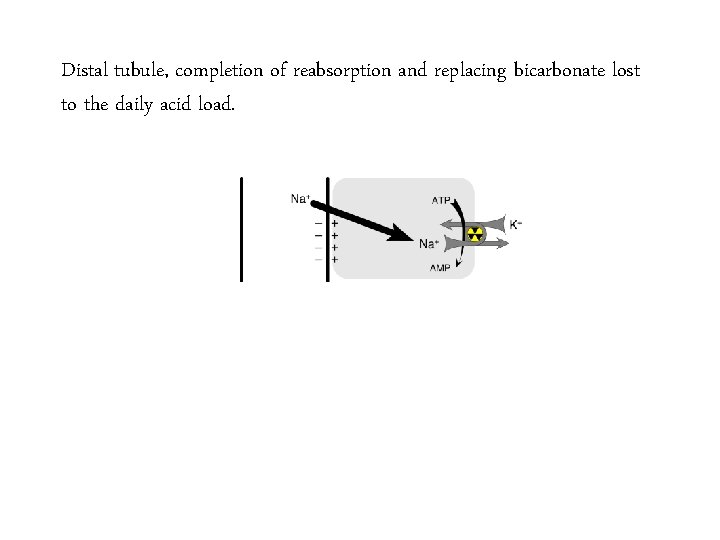
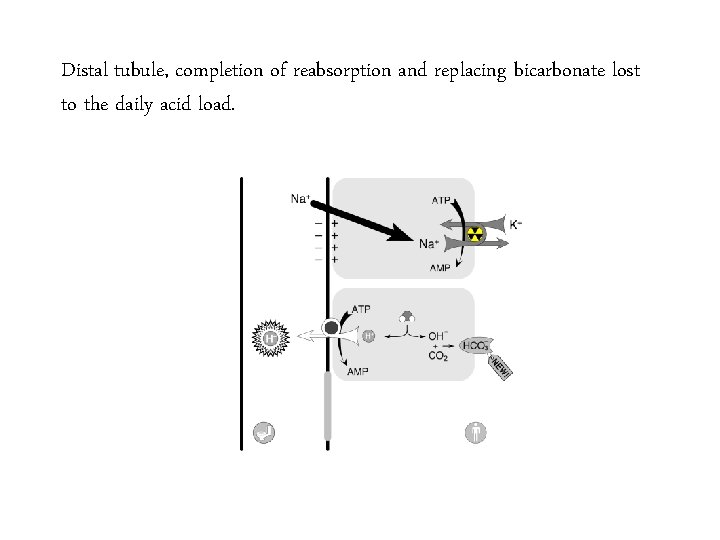
Distal tubule, completion of reabsorption and replacing bicarbonate lost to the daily acid load.

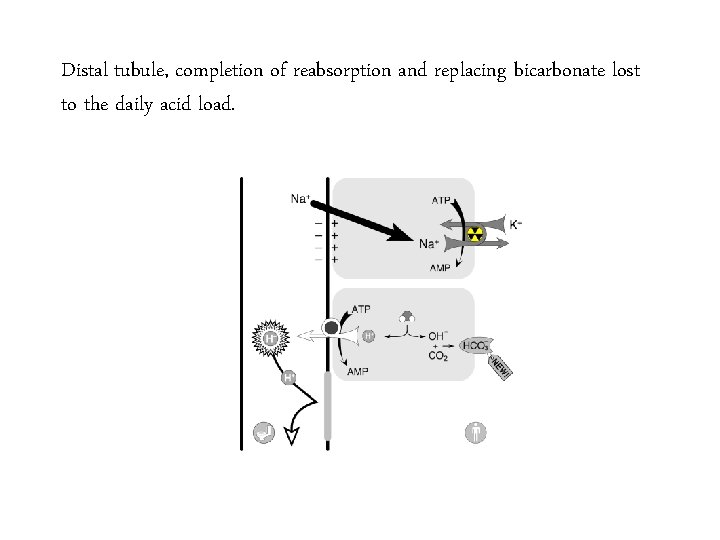
Distal tubule, completion of reabsorption and replacing bicarbonate lost to the daily acid load.

Distal tubule, completion of reabsorption and replacing bicarbonate lost to the daily acid load.

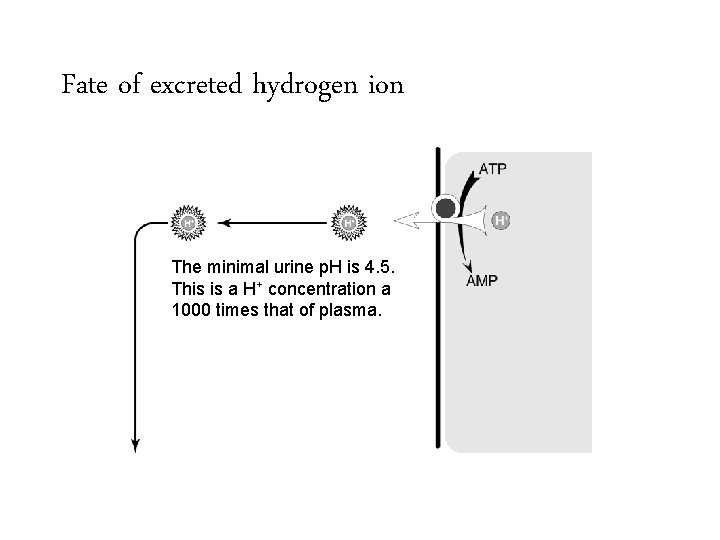
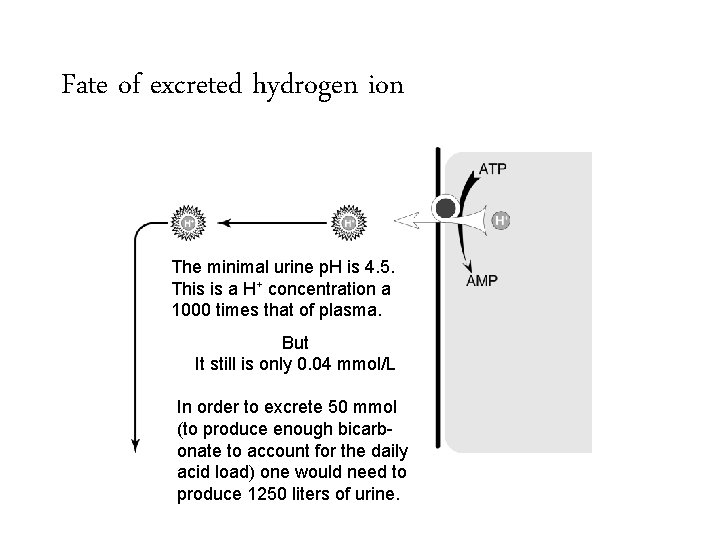
Fate of excreted hydrogen ion The minimal urine p. H is 4. 5. This is a H+ concentration a 1000 times that of plasma.

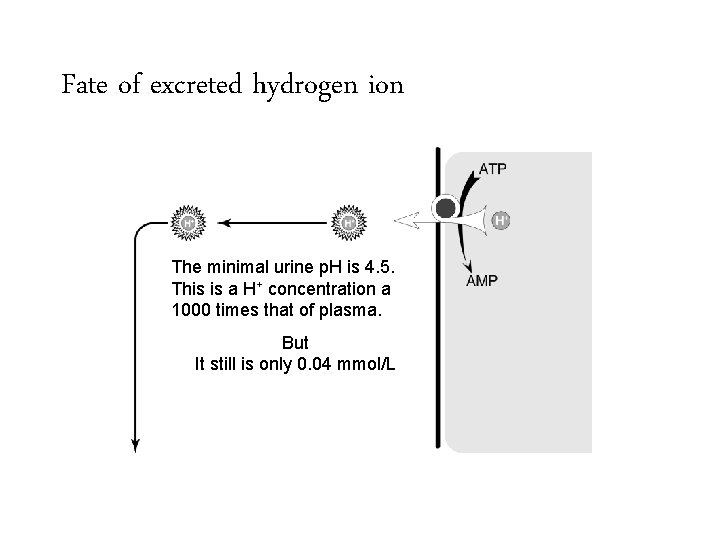
Fate of excreted hydrogen ion The minimal urine p. H is 4. 5. This is a H+ concentration a 1000 times that of plasma. But It still is only 0. 04 mmol/L

Fate of excreted hydrogen ion The minimal urine p. H is 4. 5. This is a H+ concentration a 1000 times that of plasma. But It still is only 0. 04 mmol/L In order to excrete 50 mmol (to produce enough bicarbonate to account for the daily acid load) one would need to produce 1250 liters of urine.

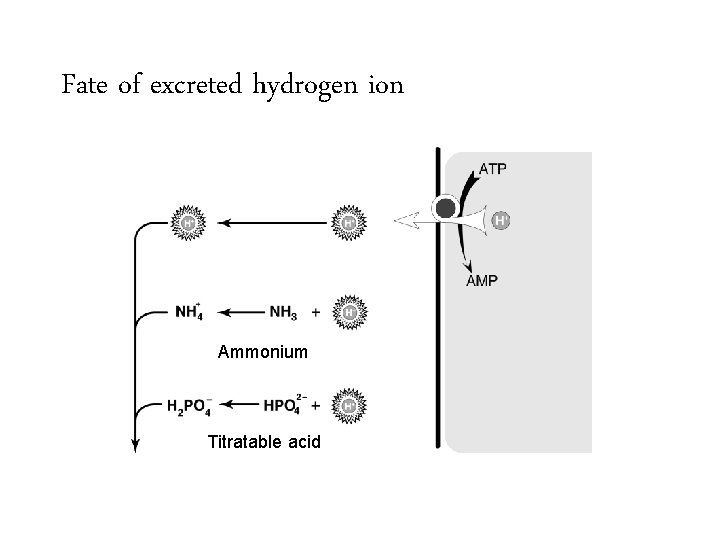
Fate of excreted hydrogen ion Ammonium Titratable acid

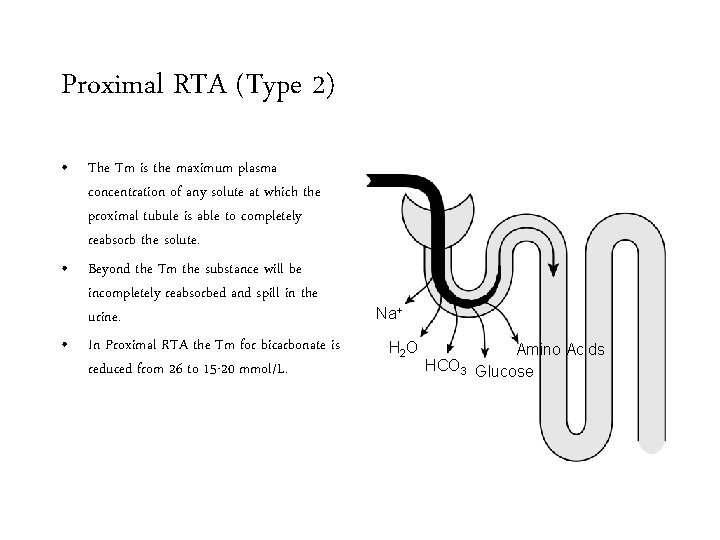
Proximal RTA (Type 2) • The Tm is the maximum plasma concentration of any solute at which the proximal tubule is able to completely reabsorb the solute. • Beyond the Tm the substance will be incompletely reabsorbed and spill in the urine. • In Proximal RTA the Tm for bicarbonate is reduced from 26 to 15 -20 mmol/L. Na+ H 2 O Amino Acids HCO 3 Glucose

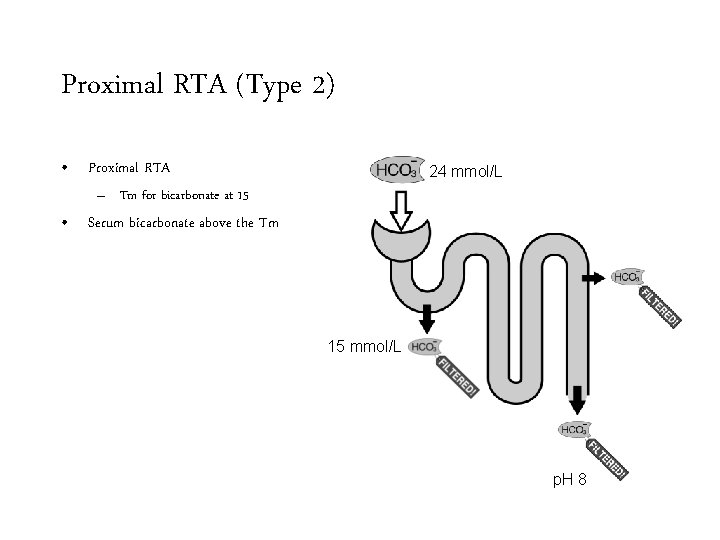
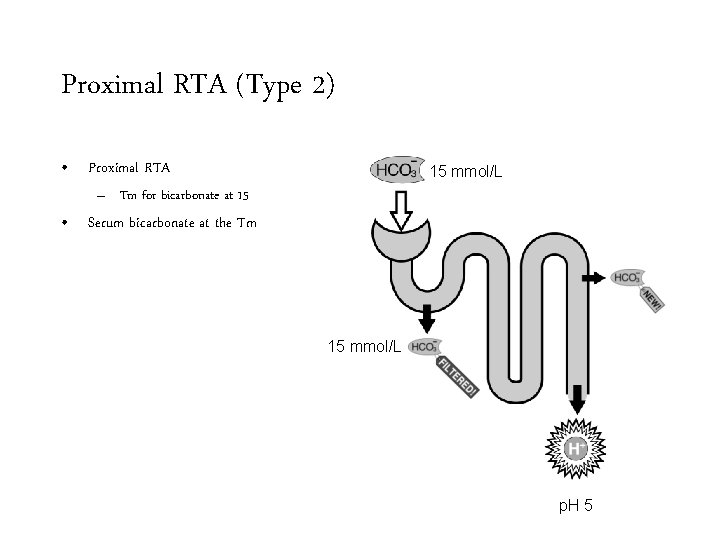
Proximal RTA (Type 2) • Proximal RTA 24 mmol/L – Tm for bicarbonate at 15 • Serum bicarbonate above the Tm 15 mmol/L p. H 8

Proximal RTA (Type 2) • Proximal RTA 15 mmol/L – Tm for bicarbonate at 15 • Serum bicarbonate at the Tm 15 mmol/L p. H 5

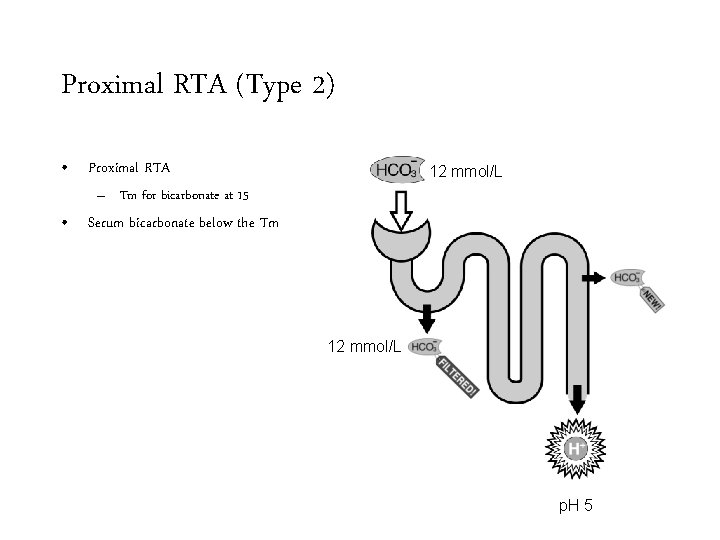
Proximal RTA (Type 2) • Proximal RTA 12 mmol/L – Tm for bicarbonate at 15 • Serum bicarbonate below the Tm 12 mmol/L p. H 5

Proximal RTA: etiologies • • Acquired – – – – – Acetylzolamide Ifosfamide Chronic hypocalcemia Multiple myeloma Cisplatin Lead toxicity Mercury poisoning Streptozocin Expired tetracycline – – Cystinosis Galactosemia Hereditary fructose intolerance Wilson’s disease Genetic • Hyperparathyroidism • Chronic hypocapnia – Intracellular alkalosis

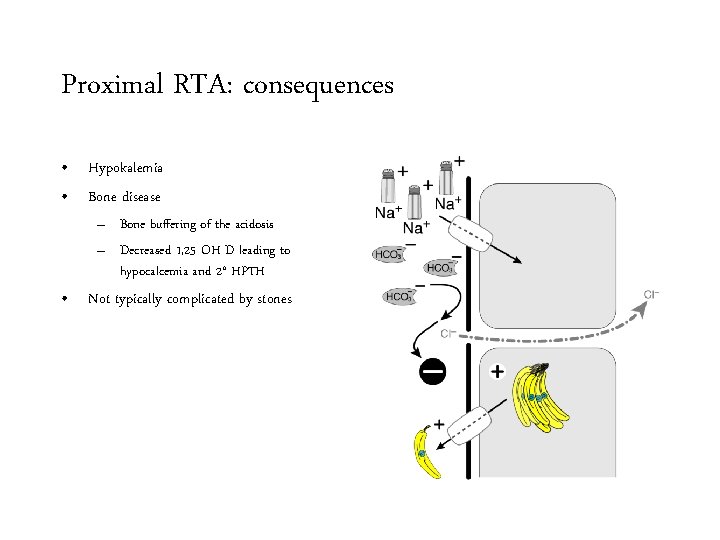
Proximal RTA: consequences • Hypokalemia • Bone disease – Bone buffering of the acidosis – Decreased 1, 25 OH D leading to hypocalcemia and 2° HPTH • Not typically complicated by stones

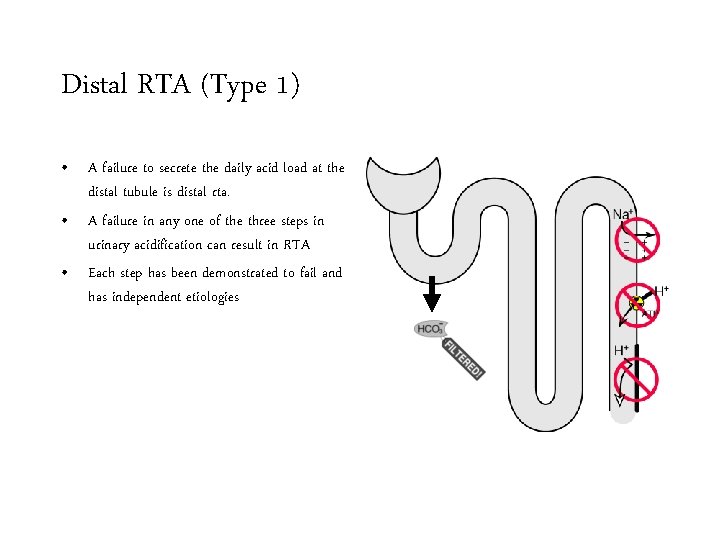
Distal RTA (Type 1) • A failure to secrete the daily acid load at the distal tubule is distal rta. • A failure in any one of the three steps in urinary acidification can result in RTA • Each step has been demonstrated to fail and has independent etiologies

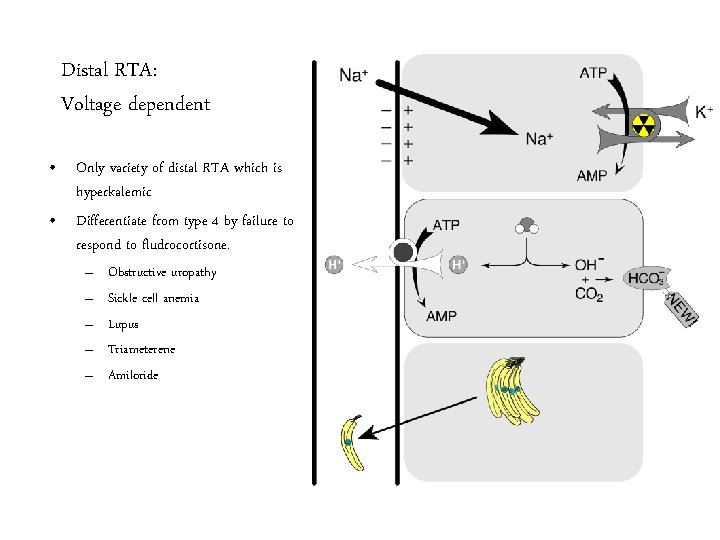
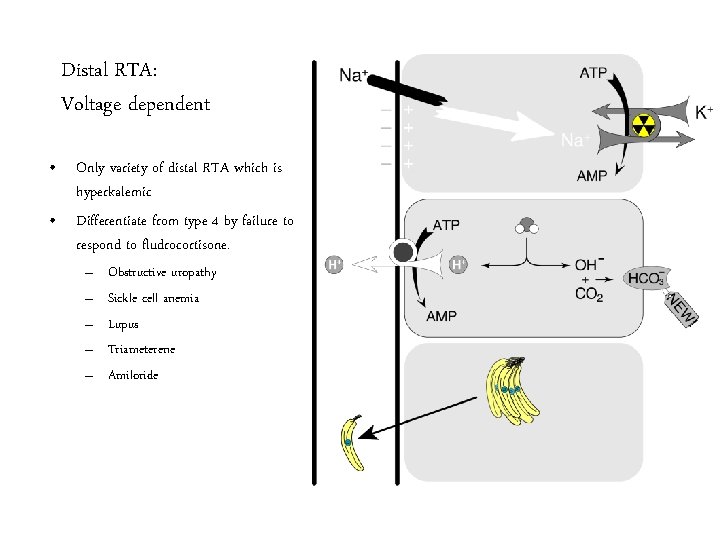
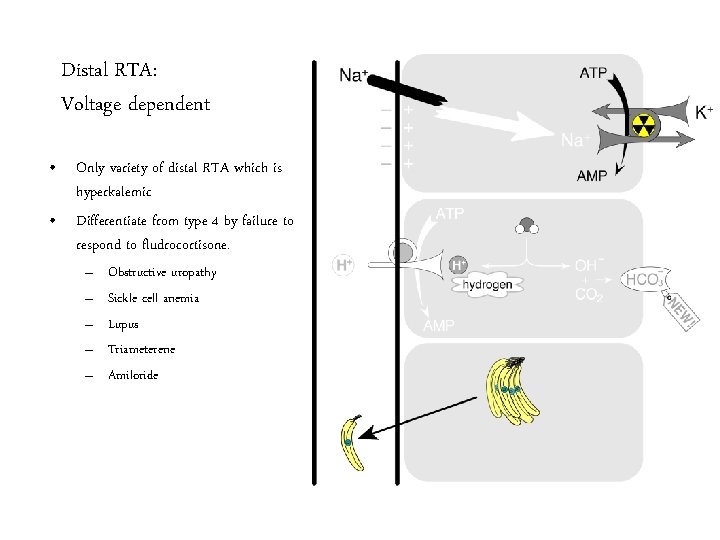
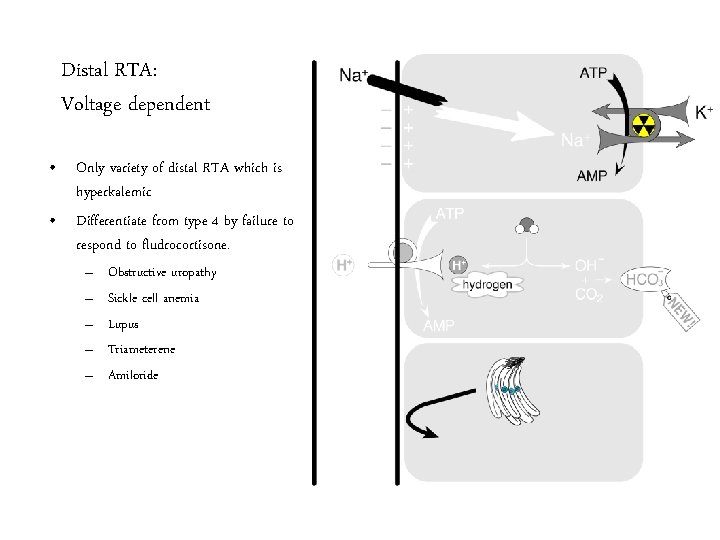
Distal RTA: Voltage dependent • Only variety of distal RTA which is hyperkalemic • Differentiate from type 4 by failure to respond to fludrocortisone. – – – Obstructive uropathy Sickle cell anemia Lupus Triameterene Amiloride

Distal RTA: Voltage dependent • Only variety of distal RTA which is hyperkalemic • Differentiate from type 4 by failure to respond to fludrocortisone. – – – Obstructive uropathy Sickle cell anemia Lupus Triameterene Amiloride

Distal RTA: Voltage dependent • Only variety of distal RTA which is hyperkalemic • Differentiate from type 4 by failure to respond to fludrocortisone. – – – Obstructive uropathy Sickle cell anemia Lupus Triameterene Amiloride

Distal RTA: Voltage dependent • Only variety of distal RTA which is hyperkalemic • Differentiate from type 4 by failure to respond to fludrocortisone. – – – Obstructive uropathy Sickle cell anemia Lupus Triameterene Amiloride

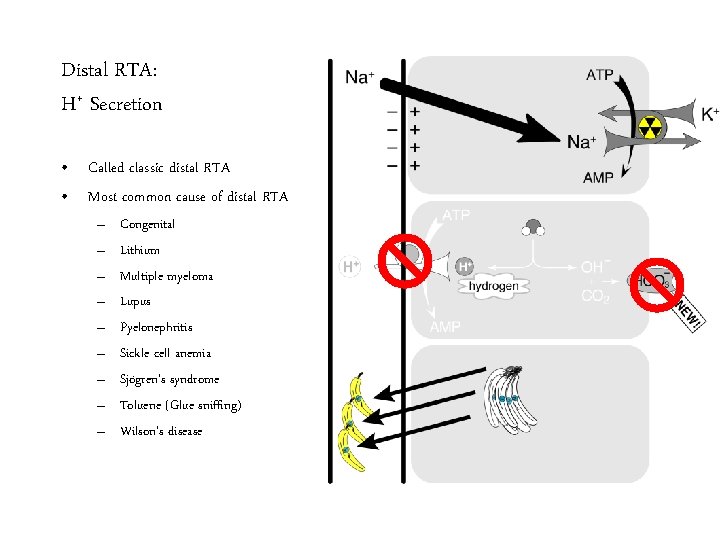
Distal RTA: H+ Secretion • Called classic distal RTA • Most common cause of distal RTA – – – – – Congenital Lithium Multiple myeloma Lupus Pyelonephritis Sickle cell anemia Sjögren’s syndrome Toluene (Glue sniffing) Wilson’s disease

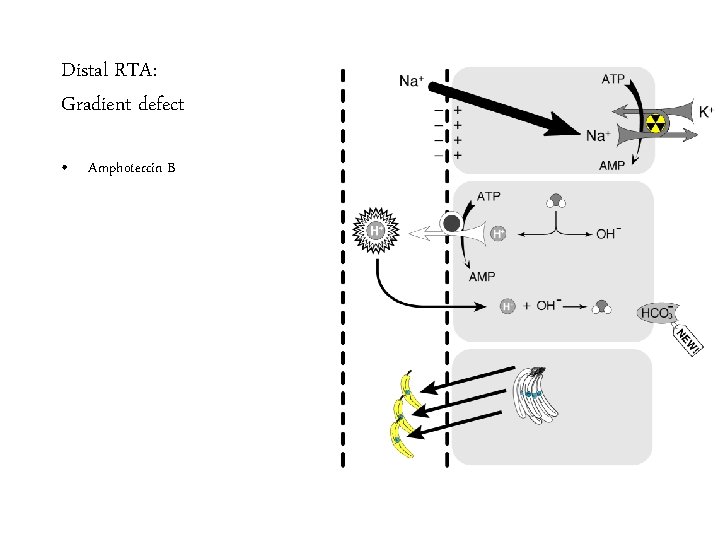
Distal RTA: Gradient defect • Amphotercin B

Distal RTA: consequences • Bones – Chronic metabolic acidosis results in bone buffering. • Bicarbonate • Phosphate • Calcium • Kidney stones – Calcium phosphate stones • Due to hypercalciuria • Increased urine p. H • Decreased urinary citrate Well Mr. Osborne, it may not be kidney stones after all.

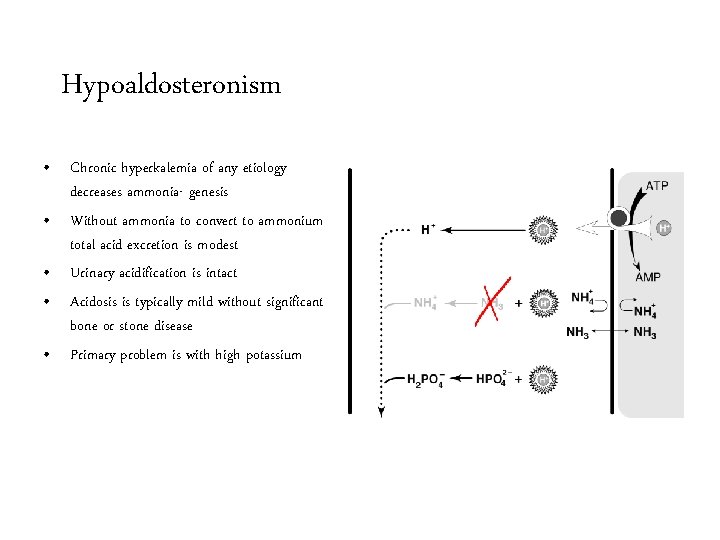
Hypoaldosteronism • Chronic hyperkalemia of any etiology decreases ammonia- genesis • Without ammonia to convert to ammonium total acid excretion is modest • Urinary acidification is intact • Acidosis is typically mild without significant bone or stone disease • Primary problem is with high potassium

Diagnosing the cause of: non-anion gap metabolic acidosis

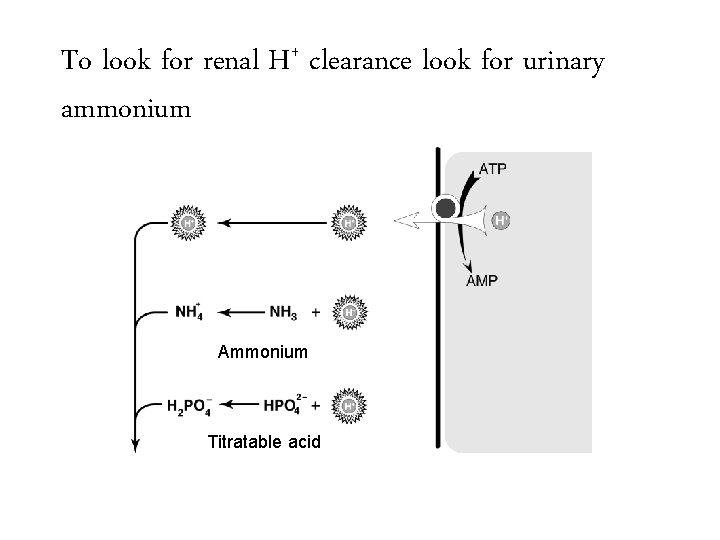
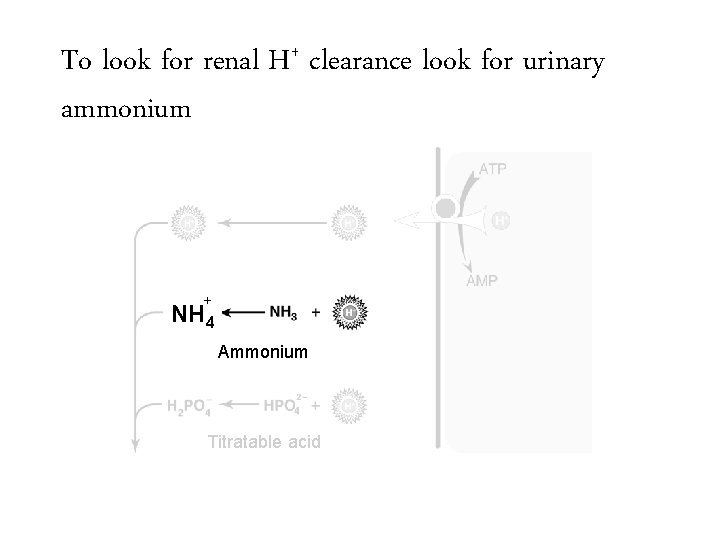
To look for renal H+ clearance look for urinary ammonium Ammonium Titratable acid

To look for renal H+ clearance look for urinary ammonium + NH 4 Ammonium Titratable acid

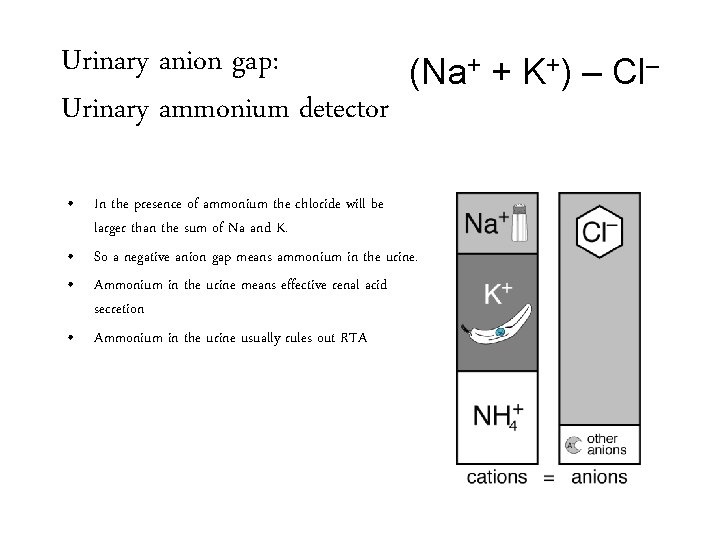
Urinary anion gap: (Na+ + K+) – Cl– Urinary ammonium detector • In the presence of ammonium the chloride will be larger than the sum of Na and K. • So a negative anion gap means ammonium in the urine. • Ammonium in the urine means effective renal acid secretion • Ammonium in the urine usually rules out RTA

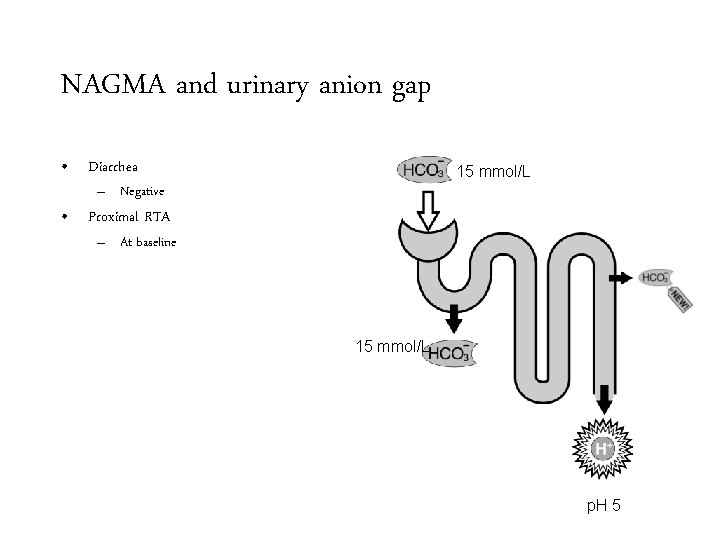
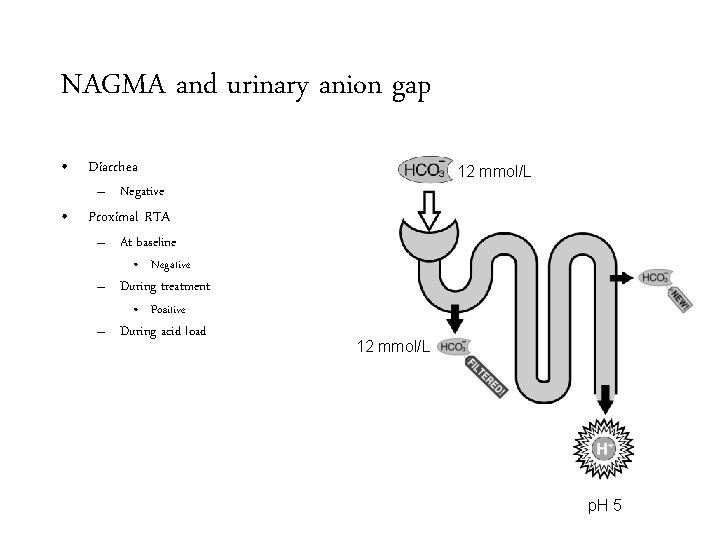
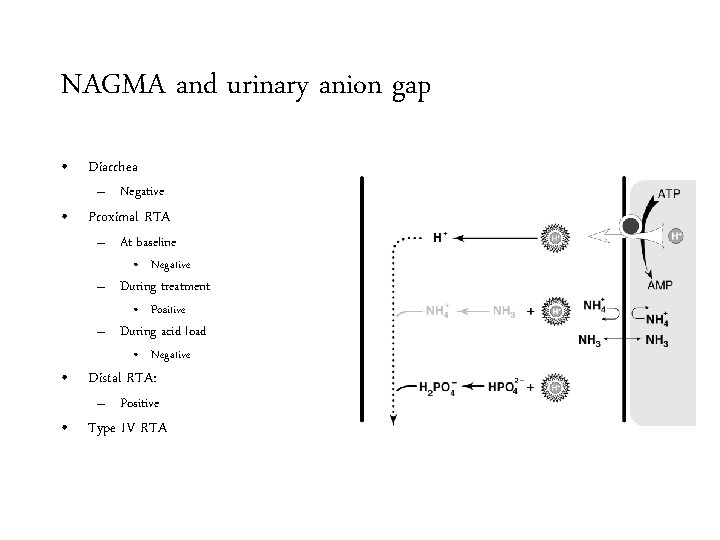
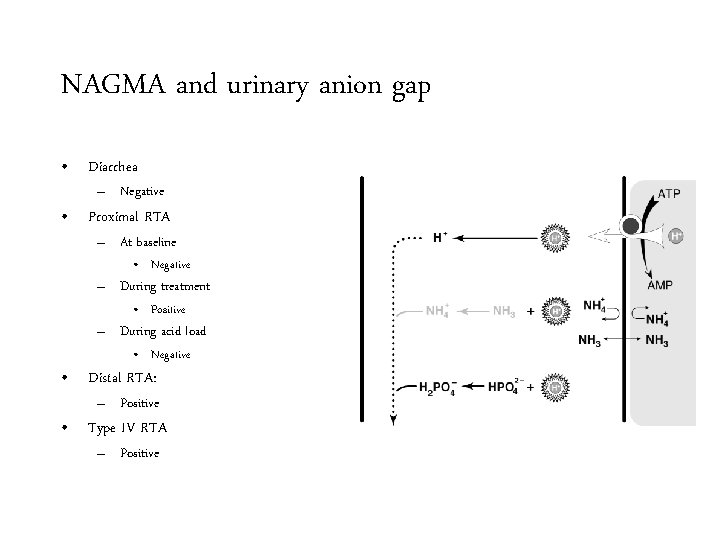
NAGMA and urinary anion gap • Diarrhea

NAGMA and urinary anion gap • Diarrhea – Negative

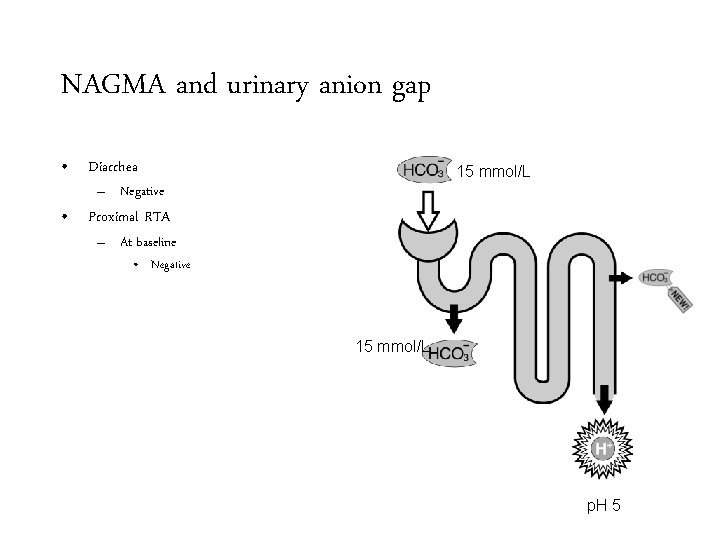
NAGMA and urinary anion gap • Diarrhea 15 mmol/L – Negative • Proximal RTA – At baseline 15 mmol/L p. H 5

NAGMA and urinary anion gap • Diarrhea 15 mmol/L – Negative • Proximal RTA – At baseline • Negative 15 mmol/L p. H 5

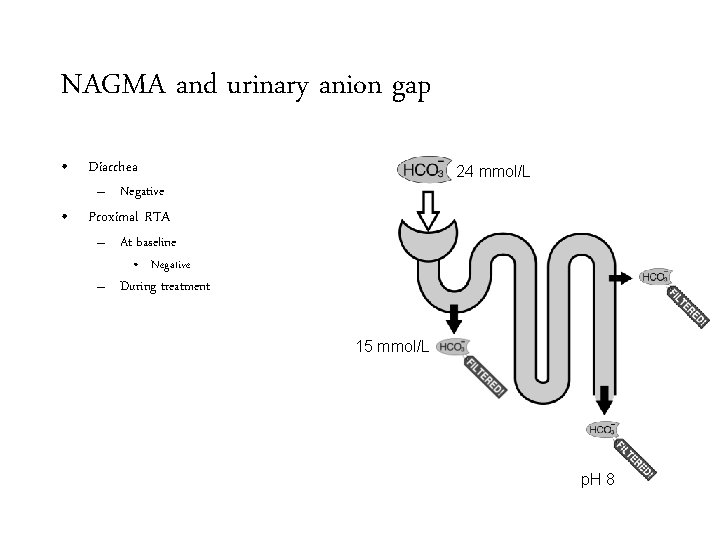
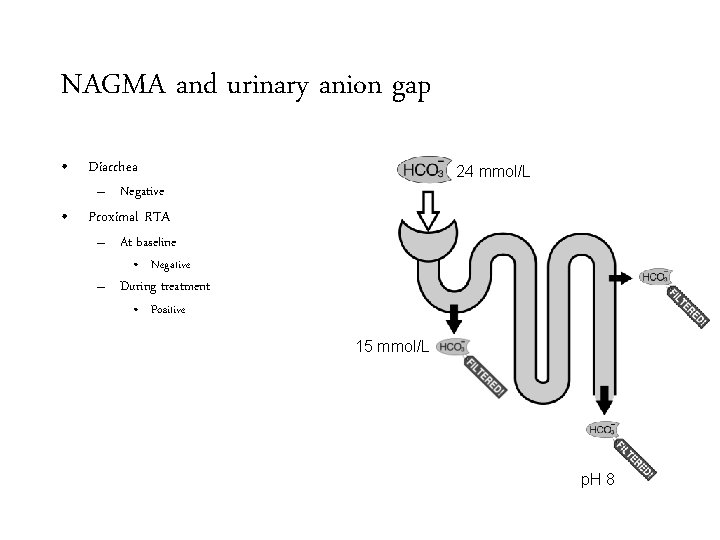
NAGMA and urinary anion gap • Diarrhea 24 mmol/L – Negative • Proximal RTA – At baseline • Negative – During treatment 15 mmol/L p. H 8

NAGMA and urinary anion gap • Diarrhea 24 mmol/L – Negative • Proximal RTA – At baseline • Negative – During treatment • Positive 15 mmol/L p. H 8

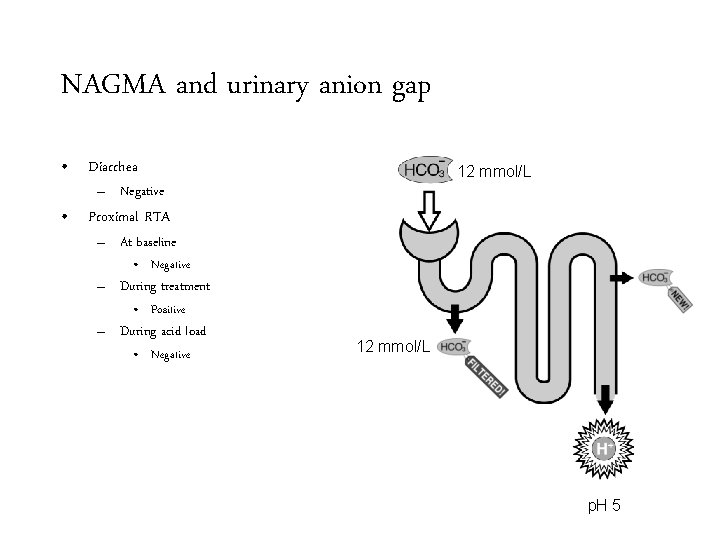
NAGMA and urinary anion gap • Diarrhea 12 mmol/L – Negative • Proximal RTA – At baseline • Negative – During treatment • Positive – During acid load 12 mmol/L p. H 5

NAGMA and urinary anion gap • Diarrhea 12 mmol/L – Negative • Proximal RTA – At baseline • Negative – During treatment • Positive – During acid load • Negative 12 mmol/L p. H 5

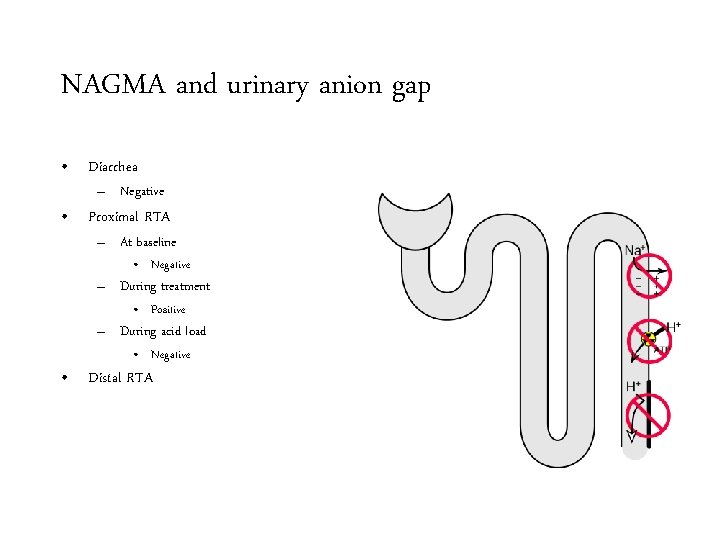
NAGMA and urinary anion gap • Diarrhea – Negative • Proximal RTA – At baseline • Negative – During treatment • Positive – During acid load • Negative • Distal RTA

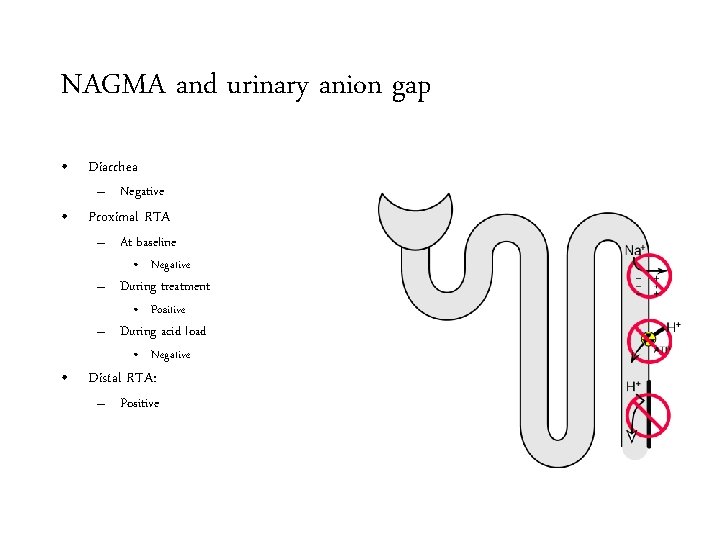
NAGMA and urinary anion gap • Diarrhea – Negative • Proximal RTA – At baseline • Negative – During treatment • Positive – During acid load • Negative • Distal RTA: – Positive

NAGMA and urinary anion gap • Diarrhea – Negative • Proximal RTA – At baseline • Negative – During treatment • Positive – During acid load • Negative • Distal RTA: – Positive • Type IV RTA

NAGMA and urinary anion gap • Diarrhea – Negative • Proximal RTA – At baseline • Negative – During treatment • Positive – During acid load • Negative • Distal RTA: – Positive • Type IV RTA – Positive

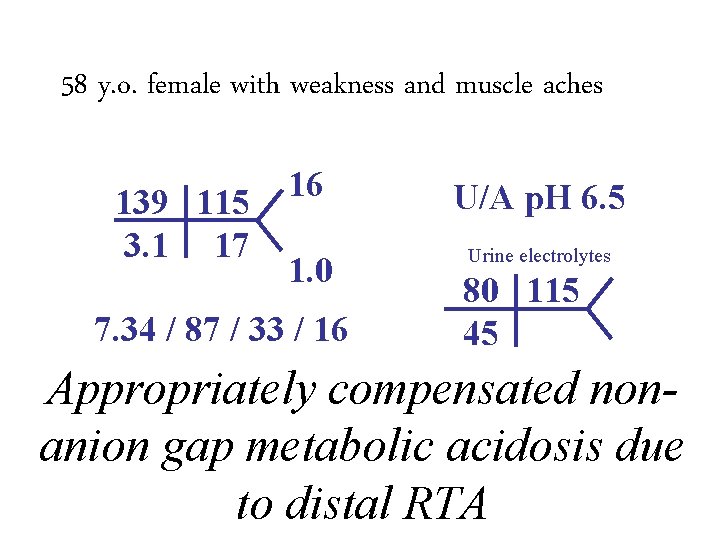
58 y. o. female with weakness and muscle aches 139 115 3. 1 17 16 1. 0 7. 34 / 87 / 33 / 16 U/A p. H 6. 5 Urine electrolytes 80 115 45

58 y. o. female with weakness and muscle aches 139 115 3. 1 17 16 1. 0 7. 34 / 87 / 33 / 16 U/A p. H 6. 5 Urine electrolytes 80 115 45 Appropriately compensated nonanion gap metabolic acidosis due to distal RTA

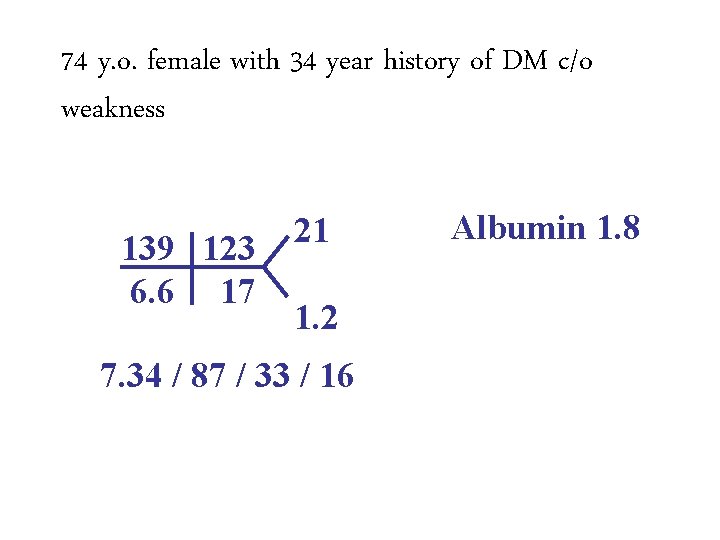
74 y. o. female with 34 year history of DM c/o weakness 139 123 6. 6 17 21 1. 2 7. 34 / 87 / 33 / 16 Albumin 1. 8

Determine the primary disorder 7. 34 / 87 / 33 / 16 p. H 1. / p. O 2 / p. CO 2 / HCO 3 Acidosis or alkalosis – – 2. If the p. H is less than 7. 4 it is acidosis If the p. H is greater than 7. 4 it is alkalosis Determine if it is respiratory or metabolic – – If the p. H, bicarbonate and p. CO 2 all move in the same direction (up or down) it is metabolic If the p. H, bicarbonate and p. CO 2 move in discordant directions (up and down) it is respiratory

Determine the primary disorder 7. 34 / 87 / 33 / 16 p. H 1. / p. O 2 / p. CO 2 / HCO 3 Acidosis or alkalosis – – 2. If the p. H is less than 7. 4 it is acidosis If the p. H is greater than 7. 4 it is alkalosis Determine if it is respiratory or metabolic – – If the p. H, bicarbonate and p. CO 2 all move in the same direction (up or down) it is metabolic If the p. H, bicarbonate and p. CO 2 move in discordant directions (up and down) it is respiratory

Determine the primary disorder 7. 34 / 87 / 33 / 16 p. H 1. / p. O 2 / p. CO 2 / HCO 3 Acidosis or alkalosis – – 2. If the p. H is less than 7. 4 it is acidosis If the p. H is greater than 7. 4 it is alkalosis Determine if it is respiratory or metabolic – – If the p. H, bicarbonate and p. CO 2 all move in the same direction (up or down) it is metabolic If the p. H, bicarbonate and p. CO 2 move in discordant directions (up and down) it is respiratory

Determine the primary disorder 7. 34 / 87 / 33 / 16 p. H 1. / p. O 2 / p. CO 2 / HCO 3 Acidosis or alkalosis – – 2. If the p. H is less than 7. 4 it is acidosis If the p. H is greater than 7. 4 it is alkalosis Determine if it is respiratory or metabolic – – If the p. H, bicarbonate and p. CO 2 all move in the same direction (up or down) it is metabolic If the p. H, bicarbonate and p. CO 2 move in discordant directions (up and down) it is respiratory

Determine the primary disorder 7. 34 / 87 / 33 / 16 p. H 1. / p. O 2 / p. CO 2 / HCO 3 Acidosis or alkalosis – – 2. If the p. H is less than 7. 4 it is acidosis If the p. H is greater than 7. 4 it is alkalosis Metabolic Acidosis Determine if it is respiratory or metabolic – – If the p. H, bicarbonate and p. CO 2 all move in the same direction (up or down) it is metabolic If the p. H, bicarbonate and p. CO 2 move in discordant directions (up and down) it is respiratory

Predicting p. CO 2 in metabolic acidosis: Winter’s Formula 7. 33 / 87 / 33 / 17 p. H / p. O 2 / p. CO 2 / HCO 3 – Expected p. CO 2 = (1. 5 x HCO 3) + 8 ± 2 – Expected p. CO 2 = 31 -35 – Actual p. CO 2 is 33, which is within the predicted range, indicating a simple metabolic acidosis

Predicting p. CO 2 in metabolic acidosis: Winter’s Formula 7. 33 / 87 / 33 / 17 p. H / p. O 2 / p. CO 2 / HCO 3 – Expected p. CO 2 = (1. 5 x HCO 3) + 8 ± 2 – Expected p. CO 2 = 31 -35 – Actual p. CO 2 is 33, which is within the predicted range, indicating a simple metabolic acidosis

Predicting p. CO 2 in metabolic acidosis: Winter’s Formula 7. 33 / 87 / 33 / 17 p. H / p. O 2 / p. CO 2 / HCO 3 – Expected p. CO 2 = (1. 5 x HCO 3) + 8 ± 2 – Expected p. CO 2 = 31 -35 – Actual p. CO 2 is 33, which is within the predicted range, indicating a simple metabolic acidosis

Predicting p. CO 2 in metabolic acidosis: Winter’s Formula 7. 33 / 87 / 33 / 17 p. H / p. O 2 / p. CO 2 / HCO 3 – Expected p. CO 2 = (1. 5 x HCO 3) + 8 ± 2 – Expected p. CO 2 = 31 -35 – Actual p. CO 2 is 33, which is within the predicted range, indicating a simple metabolic acidosis Appropriately compensated metabolic acidosis

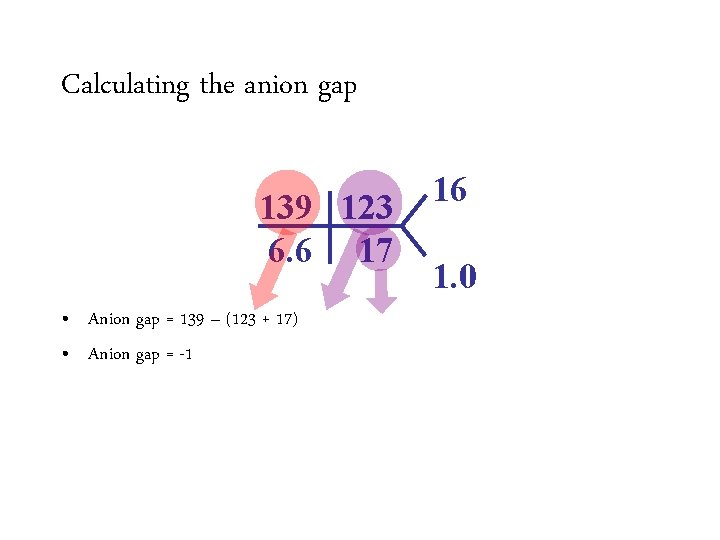
Calculating the anion gap 139 123 6. 6 17 • Anion gap = Na – (HCO 3 + Cl) 16 1. 0

Calculating the anion gap 139 123 6. 6 17 • Anion gap = 139 Na –– (HCO (123 +3 17) + Cl) • Anion gap = -1 16 1. 0

Calculating the anion gap 139 123 6. 6 17 • Anion gap = 139 Na –– (HCO (123 +3 17) + Cl) • Anion gap = -1 16 1. 0 A negative anion gap! That’s got to mean something!

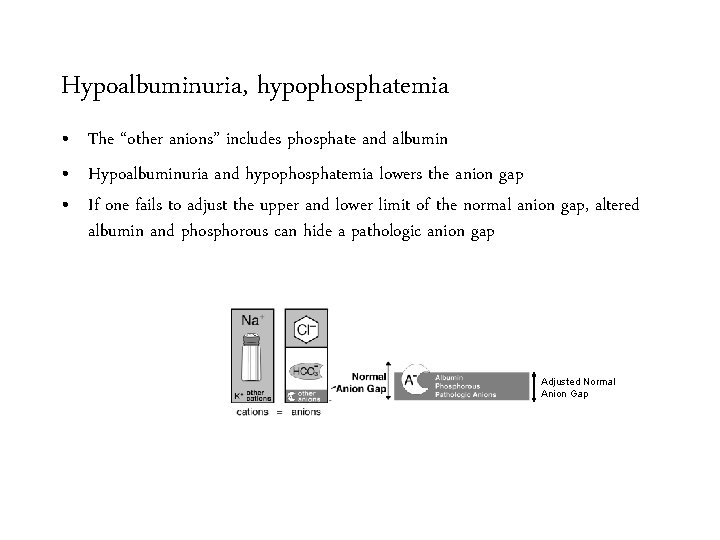
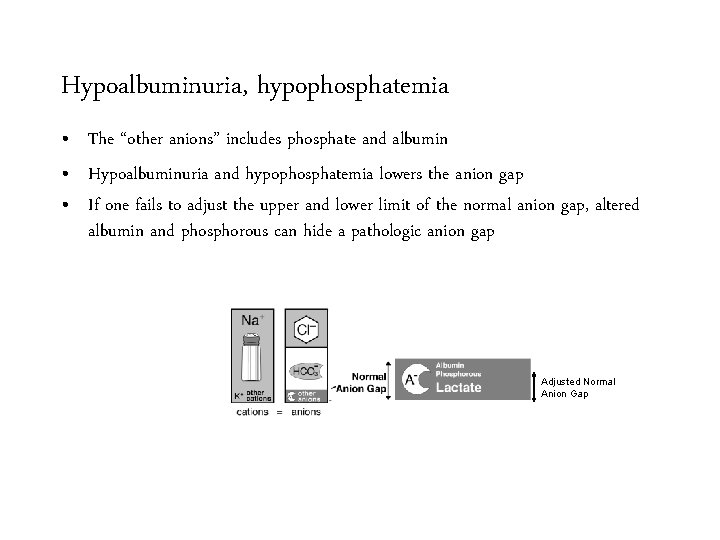
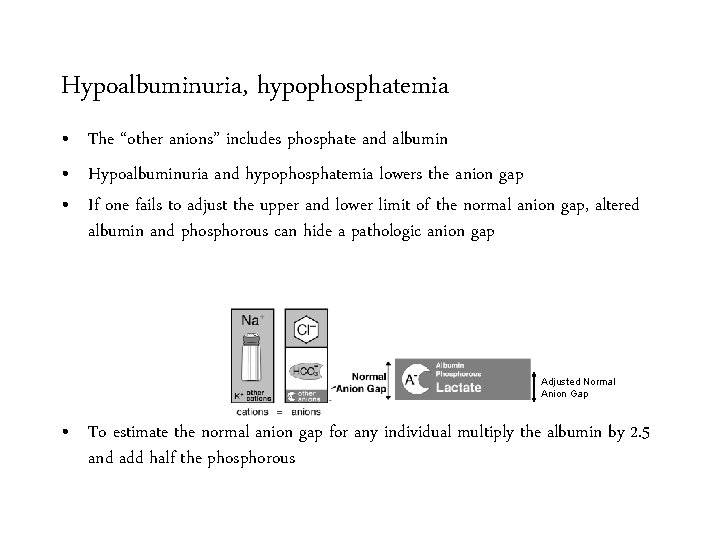
Hypoalbuminuria, hypophosphatemia • The “other anions” includes phosphate and albumin • Hypoalbuminuria and hypophosphatemia lowers the anion gap • If one fails to adjust the upper and lower limit of the normal anion gap, altered albumin and phosphorous can hide a pathologic anion gap

Hypoalbuminuria, hypophosphatemia • The “other anions” includes phosphate and albumin • Hypoalbuminuria and hypophosphatemia lowers the anion gap • If one fails to adjust the upper and lower limit of the normal anion gap, altered albumin and phosphorous can hide a pathologic anion gap

Hypoalbuminuria, hypophosphatemia • The “other anions” includes phosphate and albumin • Hypoalbuminuria and hypophosphatemia lowers the anion gap • If one fails to adjust the upper and lower limit of the normal anion gap, altered albumin and phosphorous can hide a pathologic anion gap Adjusted Normal Anion Gap

Hypoalbuminuria, hypophosphatemia • The “other anions” includes phosphate and albumin • Hypoalbuminuria and hypophosphatemia lowers the anion gap • If one fails to adjust the upper and lower limit of the normal anion gap, altered albumin and phosphorous can hide a pathologic anion gap Adjusted Normal Anion Gap

Hypoalbuminuria, hypophosphatemia • The “other anions” includes phosphate and albumin • Hypoalbuminuria and hypophosphatemia lowers the anion gap • If one fails to adjust the upper and lower limit of the normal anion gap, altered albumin and phosphorous can hide a pathologic anion gap Adjusted Normal Anion Gap • To estimate the normal anion gap for any individual multiply the albumin by 2. 5 and add half the phosphorous

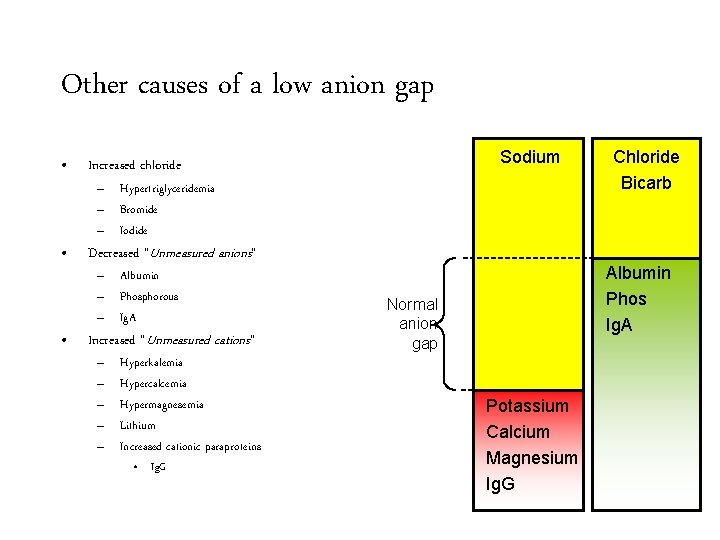
Other causes of a low anion gap • Increased chloride • Decreased “Unmeasured anions” • Sodium – Hypertriglyceridemia – Bromide – Iodide – Albumin – Phosphorous – Ig. A Increased “Unmeasured cations” – – – Hyperkalemia Hypercalcemia Hypermagnesemia Lithium Increased cationic paraproteins • Ig. G Chloride Bicarb Albumin Phos Ig. A Normal anion gap Potassium Calcium Magnesium Ig. G

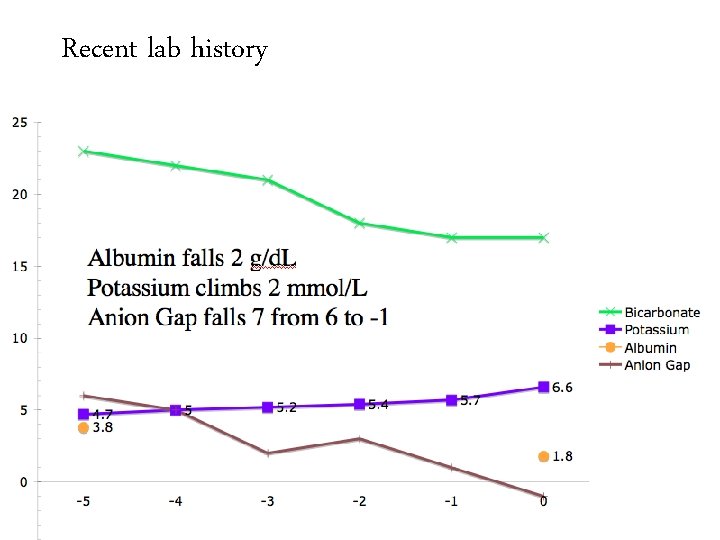
Recent lab history
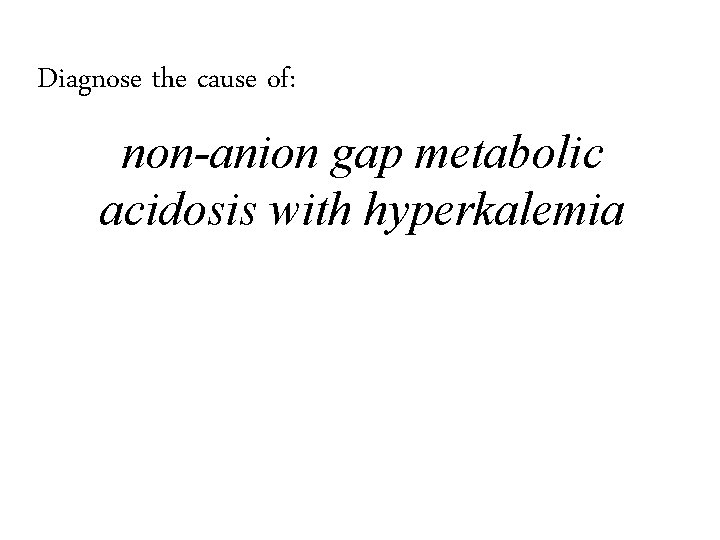
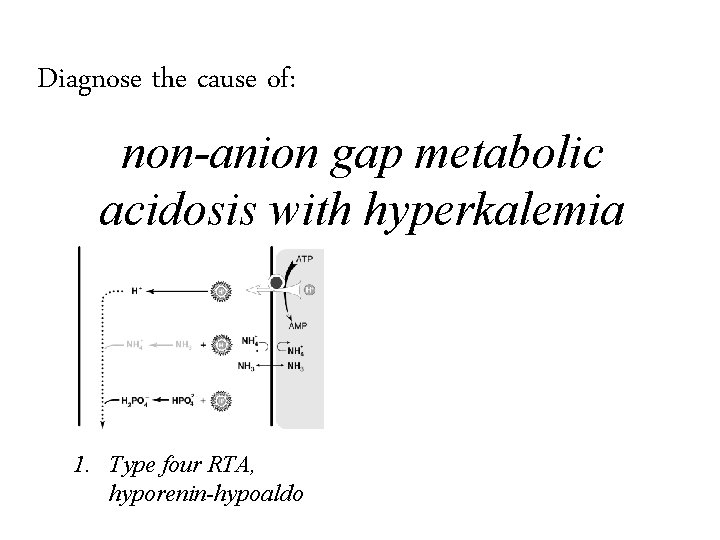
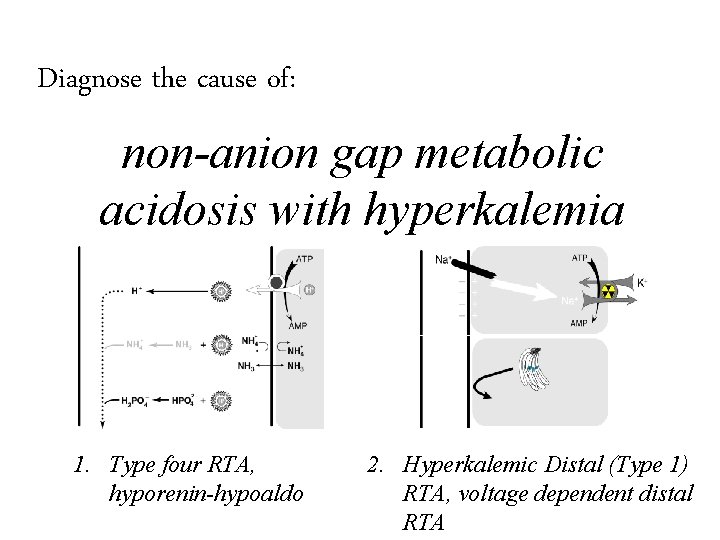
Diagnose the cause of: non-anion gap metabolic acidosis with hyperkalemia

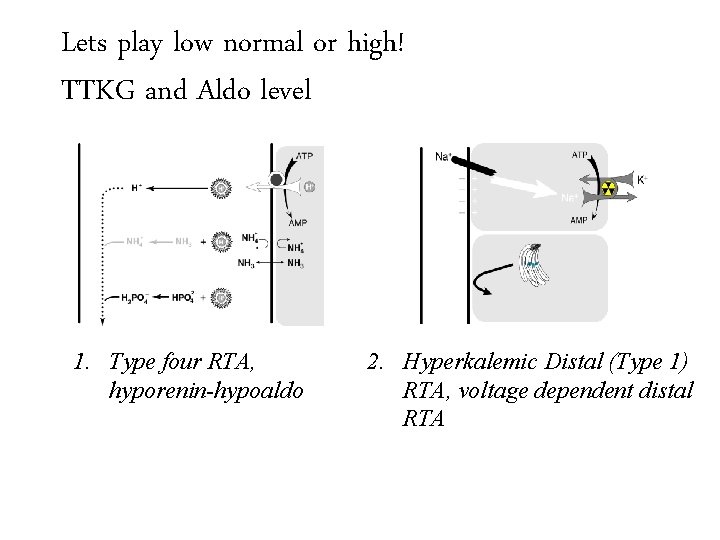
Diagnose the cause of: non-anion gap metabolic acidosis with hyperkalemia 1. Type four RTA, hyporenin-hypoaldo

Diagnose the cause of: non-anion gap metabolic acidosis with hyperkalemia 1. Type four RTA, hyporenin-hypoaldo 2. Hyperkalemic Distal (Type 1) RTA, voltage dependent distal RTA

A dipstick for aldosterone activity: The trans-tubular potassium gradient The TTKG

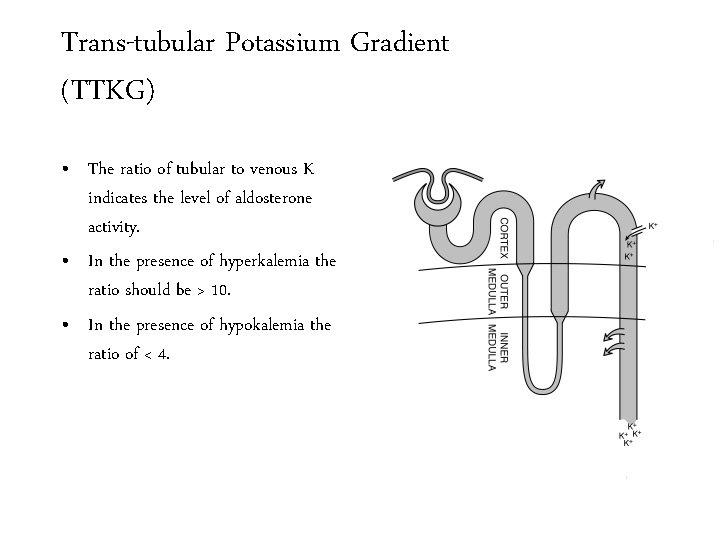
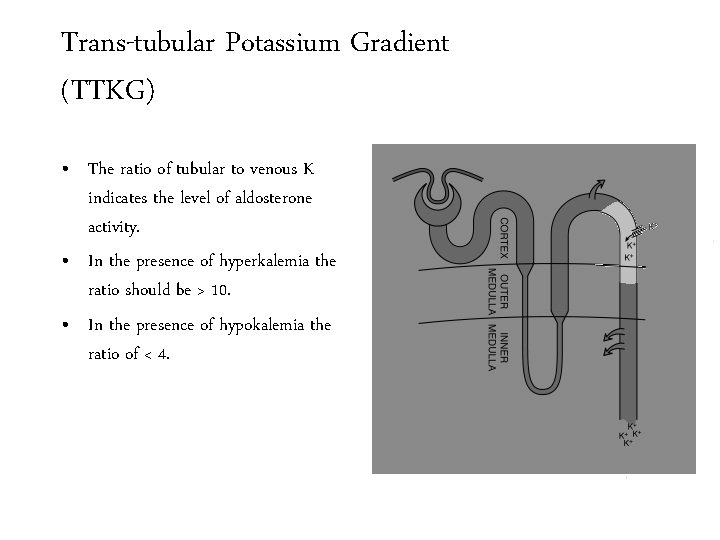
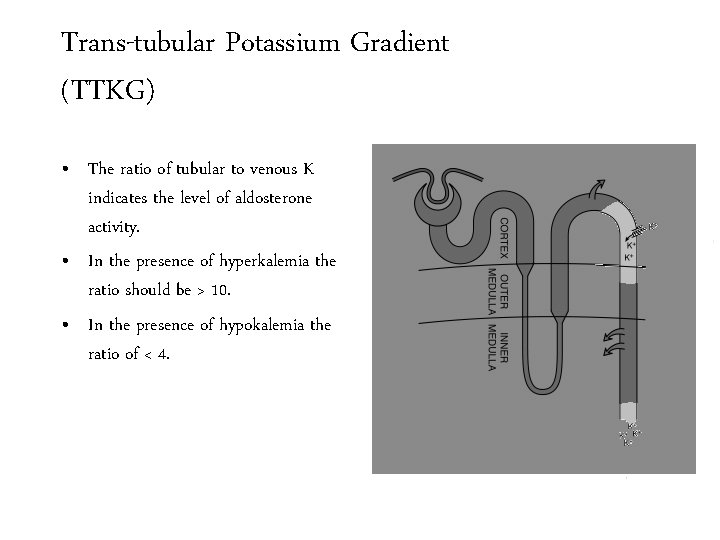
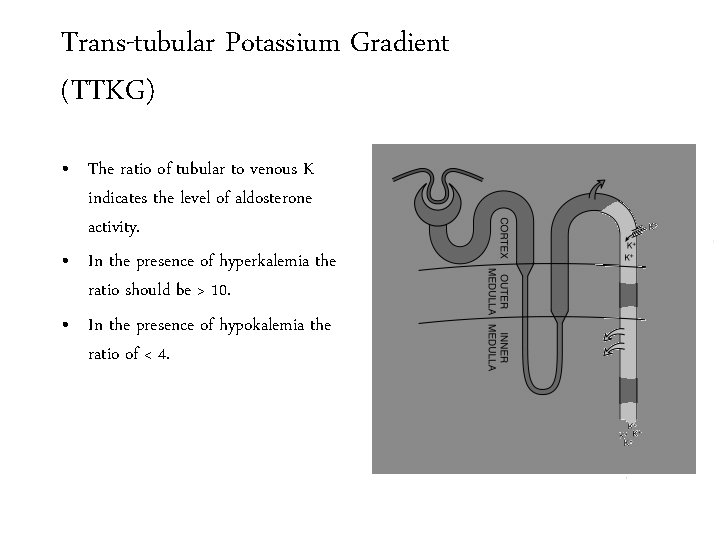
Trans-tubular Potassium Gradient (TTKG) • The ratio of tubular to venous K indicates the level of aldosterone activity. • In the presence of hyperkalemia the ratio should be > 10. • In the presence of hypokalemia the ratio of < 4.

Trans-tubular Potassium Gradient (TTKG) • The ratio of tubular to venous K indicates the level of aldosterone activity. • In the presence of hyperkalemia the ratio should be > 10. • In the presence of hypokalemia the ratio of < 4.

Trans-tubular Potassium Gradient (TTKG) • The ratio of tubular to venous K indicates the level of aldosterone activity. • In the presence of hyperkalemia the ratio should be > 10. • In the presence of hypokalemia the ratio of < 4.

Trans-tubular Potassium Gradient (TTKG) • The ratio of tubular to venous K indicates the level of aldosterone activity. • In the presence of hyperkalemia the ratio should be > 10. • In the presence of hypokalemia the ratio of < 4.

Trans-tubular Potassium Gradient (TTKG) • The trans-tubular potassium gradient adjusts the urine potassium for water loss in the collecting ducts. • This allows the use of urinary potassium to calculate the ratio of potassium from the tubule to the interstitium in the CCD.

Trans-tubular Potassium Gradient (TTKG) • The trans-tubular potassium gradient adjusts the urine potassium for water loss in the collecting ducts. • This allows the use of urinary potassium to calculate the ratio of potassium from the tubule to the interstitium in the CCD.

Trans-tubular Potassium Gradient (TTKG) • The trans-tubular potassium gradient adjusts the urine potassium for water loss in the collecting ducts. • This allows the use of urinary potassium to calculate the ratio of potassium from the tubule to the interstitium in the CCD.

Trans-tubular Potassium Gradient (TTKG) • The trans-tubular potassium gradient adjusts the urine potassium for water loss in the collecting ducts. • This allows the use of urinary potassium to calculate the ratio of potassium from the tubule to the interstitium in the CCD.

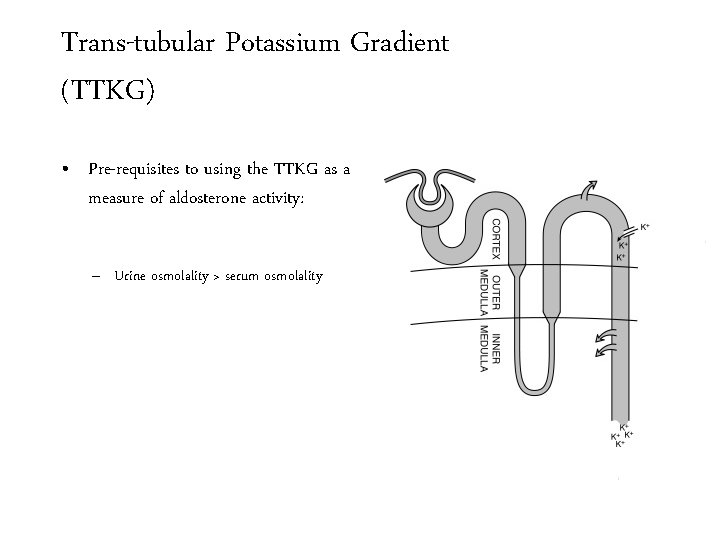
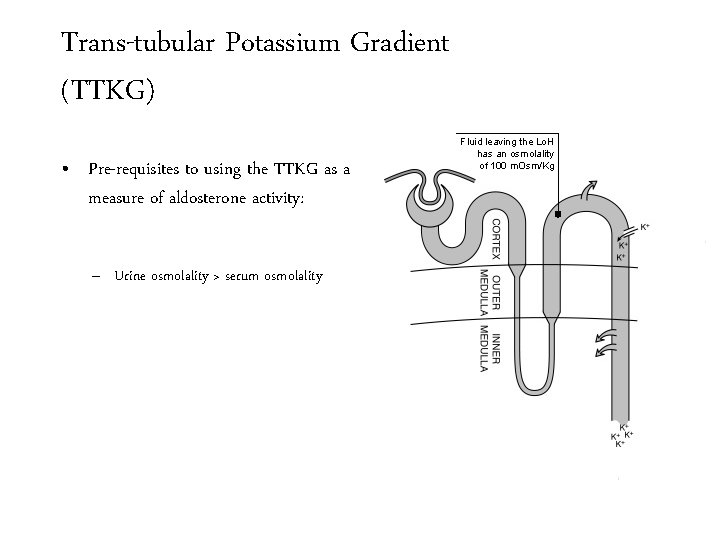
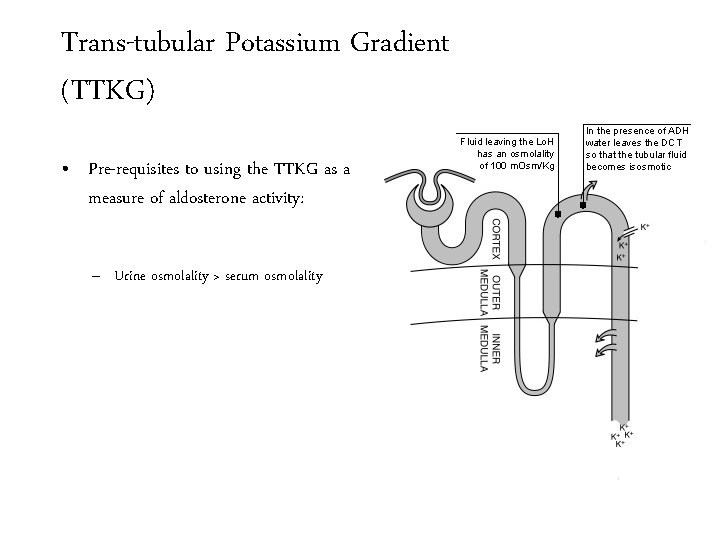
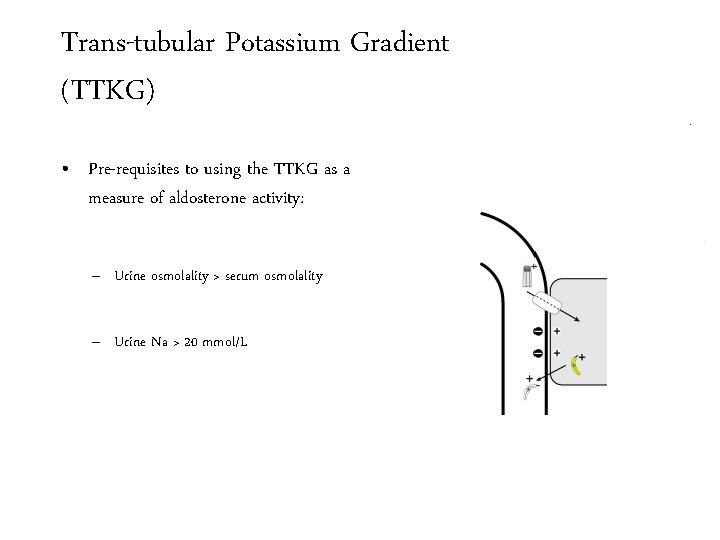
Trans-tubular Potassium Gradient (TTKG) • Pre-requisites to using the TTKG as a measure of aldosterone activity: – Urine osmolality > serum osmolality

Trans-tubular Potassium Gradient (TTKG) • Pre-requisites to using the TTKG as a measure of aldosterone activity: – Urine osmolality > serum osmolality Fluid leaving the Lo. H has an osmolality of 100 m. Osm/Kg

Trans-tubular Potassium Gradient (TTKG) • Pre-requisites to using the TTKG as a measure of aldosterone activity: – Urine osmolality > serum osmolality Fluid leaving the Lo. H has an osmolality of 100 m. Osm/Kg In the presence of ADH water leaves the DCT so that the tubular fluid becomes isosmotic

Trans-tubular Potassium Gradient (TTKG) • Pre-requisites to using the TTKG as a measure of aldosterone activity: – Urine osmolality > serum osmolality – Urine Na > 20 mmol/L Fluid leaving the Lo. H has an osmolality of 100 m. Osm/Kg In the presence of ADH water leaves the DCT so that the tubular fluid becomes isosmotic

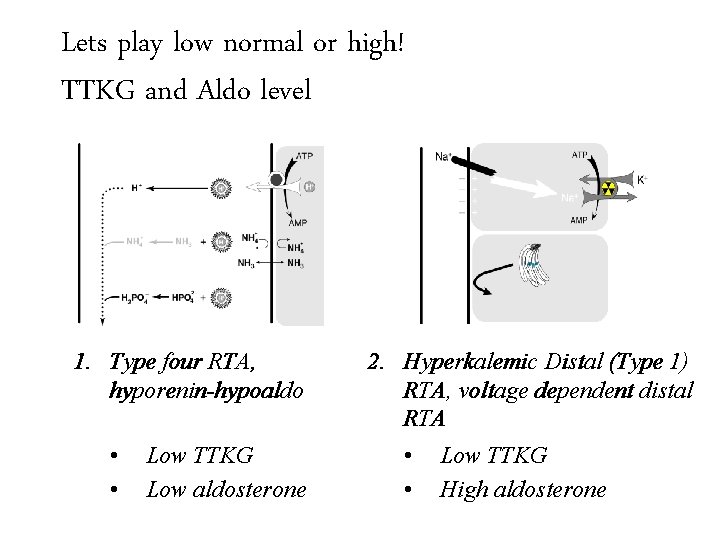
Lets play low normal Trans-tubular Potassium or high! Gradient (TTKG)and Aldo level TTKG 1. Type four RTA, hyporenin-hypoaldo 2. Hyperkalemic Distal (Type 1) RTA, voltage dependent distal RTA

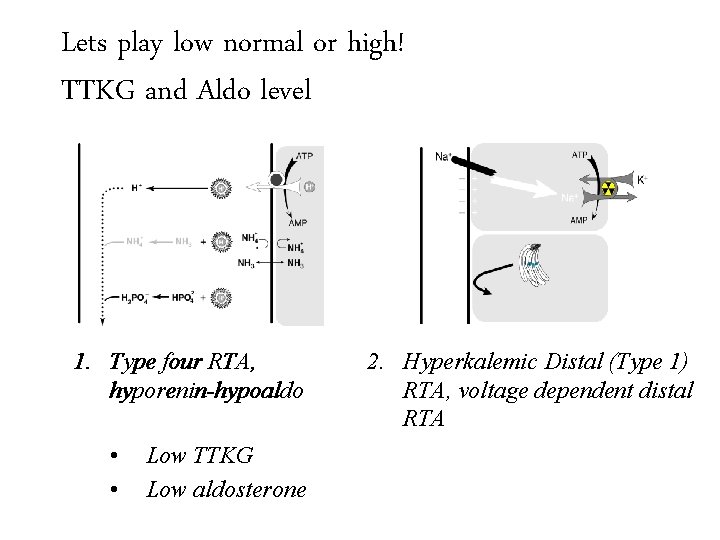
Lets play low normal Trans-tubular Potassium or high! Gradient (TTKG)and Aldo level TTKG 1. Type four RTA, hyporenin-hypoaldo • • Low TTKG Low aldosterone 2. Hyperkalemic Distal (Type 1) RTA, voltage dependent distal RTA

Lets play low normal Trans-tubular Potassium or high! Gradient (TTKG)and Aldo level TTKG 1. Type four RTA, hyporenin-hypoaldo • • Low TTKG Low aldosterone 2. Hyperkalemic Distal (Type 1) RTA, voltage dependent distal RTA • Low TTKG • High aldosterone

58 y. o. female with weakness and muscle aches

58 y. o. female with weakness and muscle aches Both the bicarbonate and potassium were normal at admission. This is hospital acquired RTA (type 1 or 4)

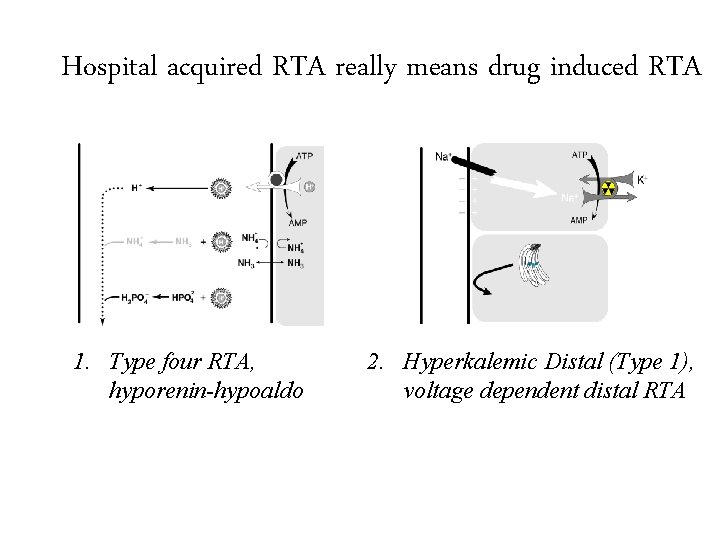
Hospital acquired RTA really means drug induced RTA 1. Type four RTA, hyporenin-hypoaldo 2. Hyperkalemic Distal (Type 1), voltage dependent distal RTA

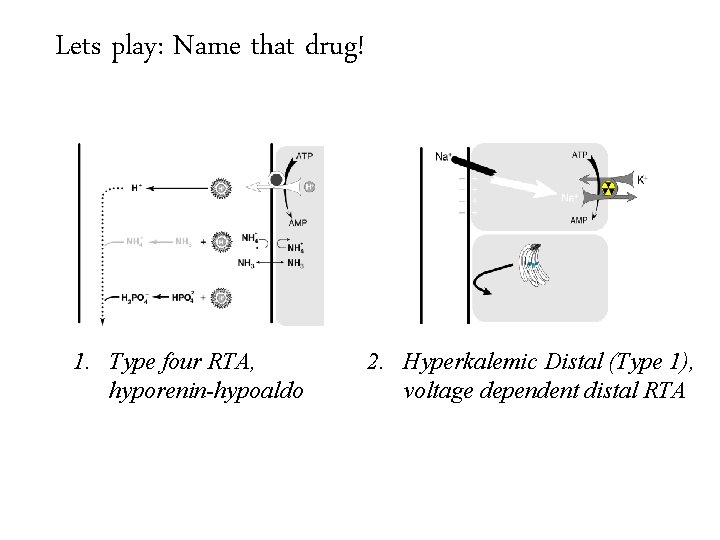
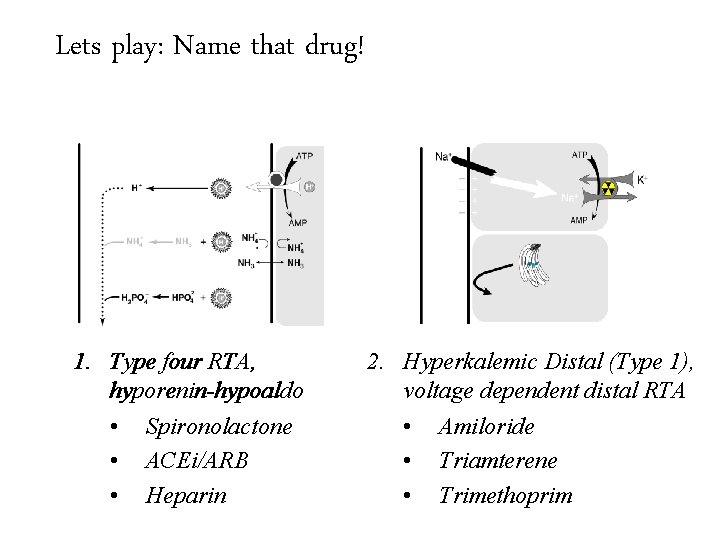
Lets play: Name that drug! Hospital acquired RTA really means drug induced RTA 1. Type four RTA, hyporenin-hypoaldo 2. Hyperkalemic Distal (Type 1), voltage dependent distal RTA

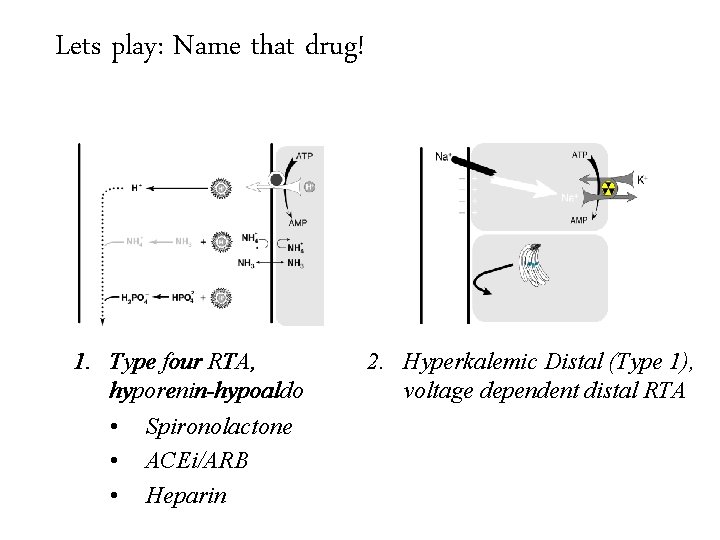
Lets play: Name that drug! Hospital acquired RTA really means drug induced RTA 1. Type four RTA, hyporenin-hypoaldo • Spironolactone • ACEi/ARB • Heparin 2. Hyperkalemic Distal (Type 1), voltage dependent distal RTA

Lets play: Name that drug! Hospital acquired RTA really means drug induced RTA 1. Type four RTA, hyporenin-hypoaldo • Spironolactone • ACEi/ARB • Heparin 2. Hyperkalemic Distal (Type 1), voltage dependent distal RTA • Amiloride • Triamterene • Trimethoprim

58 y. o. female with weakness and muscle aches 1. Type four RTA, hyporenin-hypoaldo 2. Hyperkalemic Distal (Type 1), voltage dependent distal RTA

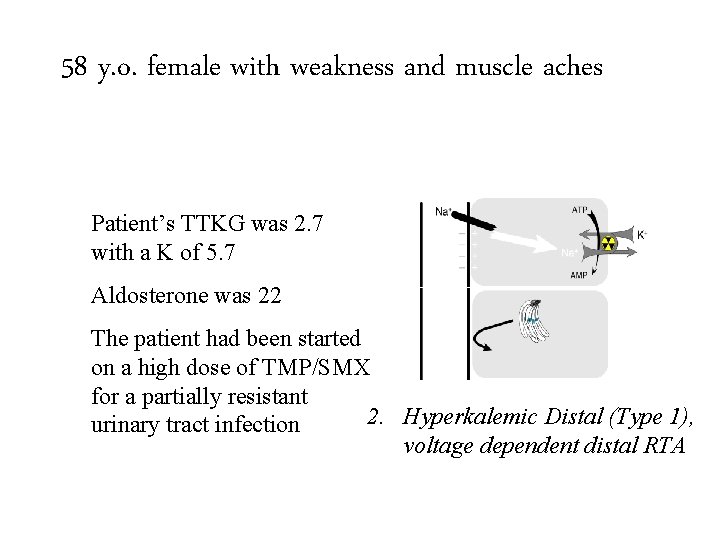
58 y. o. female with weakness and muscle aches Patient’s TTKG was 2. 7 with a K of 5. 7 Aldosterone was 22 The patient had been started on a high dose of TMP/SMX for a partially resistant 1. urinary Type four 2. Hyperkalemic Distal (Type 1), tract. RTA, infection hyporenin-hypoaldo voltage dependent distal RTA

Two women with non-anion gap metabolic acidosis One with hypokalemia One with hyperkalemia Both with distal RTA

Fin