Electrolytes and p H ELECTRICAL CONDUCTIVITY ELECTROLYTE Electrolyte










- Slides: 12

Electrolytes and p. H ELECTRICAL CONDUCTIVITY

ELECTROLYTE � Electrolyte: a substance that when dissolved in water allows an electric current to flow through the solution. Example: table salt � Some substances can be dissolved in water but do not conduct electricity called nonelectrolytes. Example: Sugar � Electrical conductivity of a solution is a measure of its ability to allow an electric current to flow through it.

ELECTROLYTIC SOLUTIONS � Solutions that contain electrolytes are electrolytic solutions. � Batteries are an example of an electric cell. � Containing an electrolytic solution that conducts electricity � Batteries conduct electricity between two poles

DISSOCIATION �A physical change; the separation of a dissolved compound into two ions of opposite charges � Example: Sodium chloride (Na. Cl) dissociates into Na+ and Cl� If sodium chloride dissolves in water, all of its properties are conserved � When a nonelectrolyte solution is dissolved, no ions are produced

SODIUM CHLORIDE EXAMPLE O + Na. Cl(s)H→Na + Cl (aq) 2 � (s)=solid � (aq)= aqueous solution � H 2 O indication that the solute was placed in water

CONT. . . � Ions conduct electricity � Electrodes connected to a power supply in an electrolyte solution � Positive ions migrate toward the negative electrode � Negative ions migrate toward the positive electrode

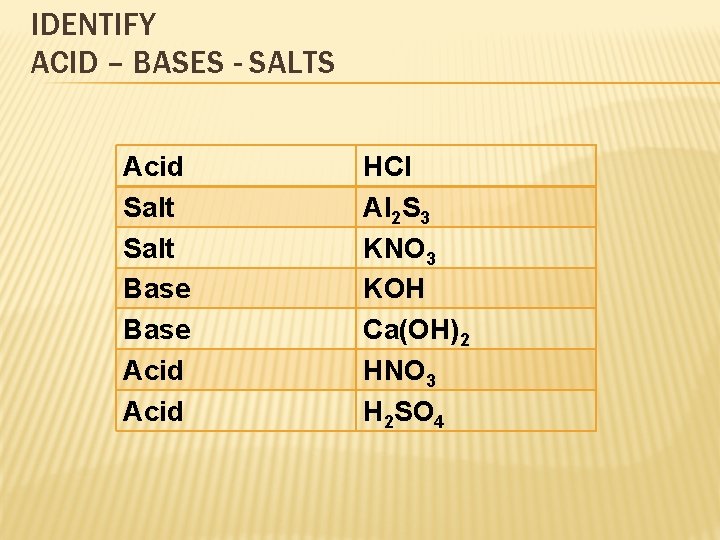
TYPES ACIDS – BASES - SALTS � Acids: A compound that produces H+ ion when dissolved in water. We can easily reconize acids because their molecular formulas start with “H” � Base: A compound that produces OH- ions when dissolved in water. We can easily recognize bases because their molecular formula ends with “OH” � A salt is an ionic compound that forms ions (other than H+ and OH-) when dissolved in water. We will work with neutral salts

ACIDS � Common acids: fruit juice, soft drinks, gastric juices � Exception to the rule acetic acid. . . vinegar! CH 3 COOH � Why? � Doesn`t start with Hydrogen

BASES � Common examples: heart burn medications, cleaning products, blood � Usually begins with a metal and ends with OH � Exception Ammonia: NH 3

SALTS � Salts made up of metal and one or more nonmetal � Not all salts dissolve easily in water � Electrolytic dissociation are important to us! � Vital to survival

IDENTIFY ACID – BASES - SALTS Acid Salt Base Acid HCl Al 2 S 3 KNO 3 KOH Ca(OH)2 HNO 3 H 2 SO 4

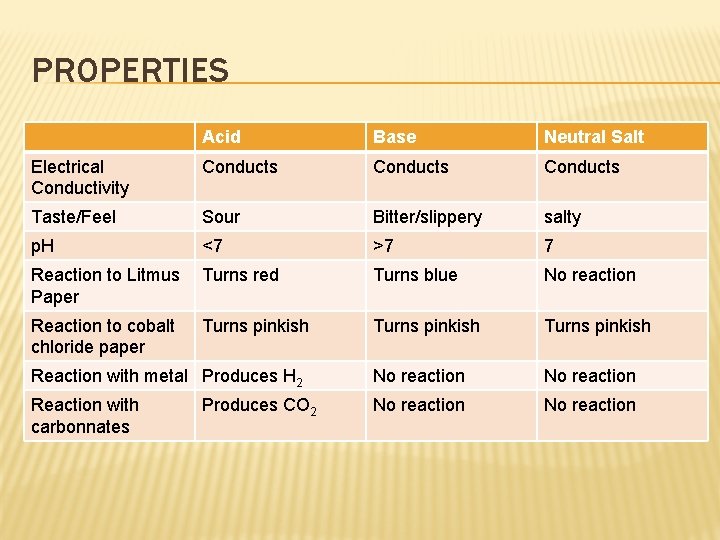
PROPERTIES Acid Base Neutral Salt Electrical Conductivity Conducts Taste/Feel Sour Bitter/slippery salty p. H <7 >7 7 Reaction to Litmus Paper Turns red Turns blue No reaction Reaction to cobalt chloride paper Turns pinkish Reaction with metal Produces H 2 No reaction Reaction with carbonnates No reaction Produces CO 2